Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application
Abstract
:1. Introduction
2. Conventional Methods of Fat Modification
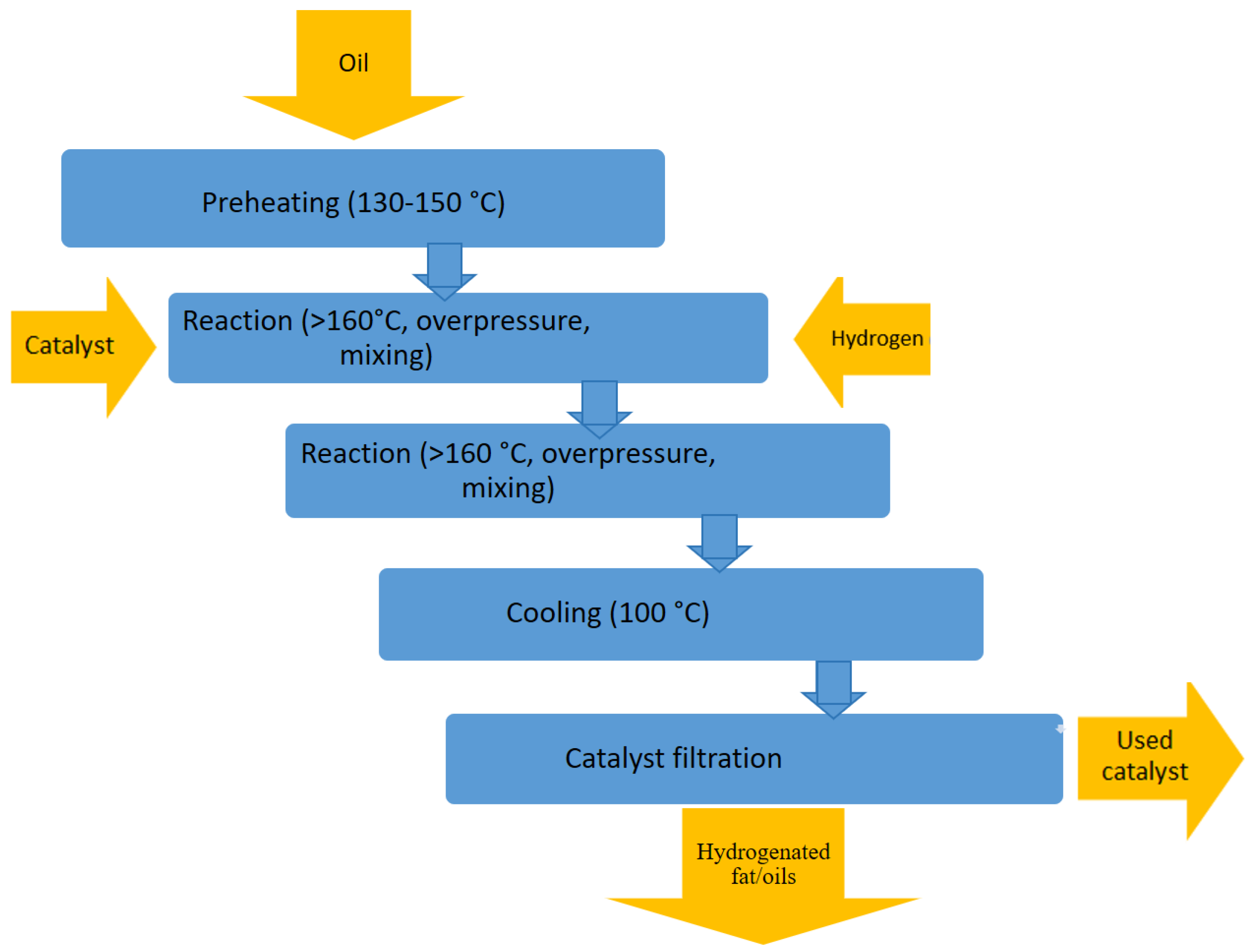
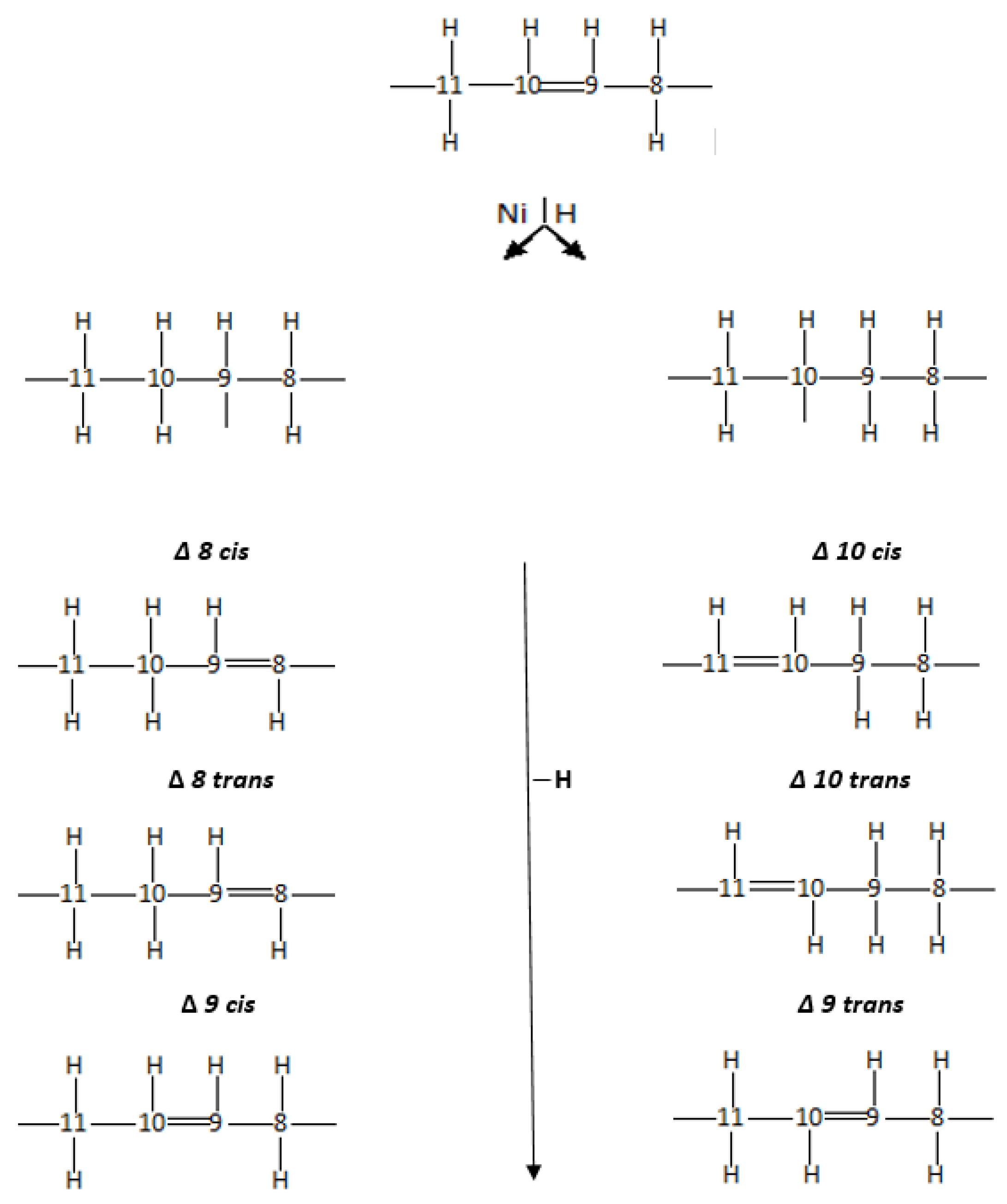
2.1. Hydrogenation
2.2. Fractionation
- -
- Dry fractionation (named crystallisation from the melt) is fractional crystallisation in this simple form. It is an economic and ecological technique [28,32]. This modification is a cheaper process than hydrogenation or interesterification [25,31]. The principle of dry fractionation is simple. The fat to be fractionated is heated above its melting point. Then it is cooled to the separation temperature, and the fractions are separated from each other. The cooling rate depends on the characteristics of the end products. The crystal mass (stearin) suspended in the oil is separated, which must be performed quickly to avoid partial remelting of the crystals. In large fractionation lines, crystal nuclei are formed in a precooling step in a big vessel that feeds several small vessels (crystallisation vessels) where the crystals are allowed to grow. Thus, one achieves higher efficiency by separating the sensitive step of nucleus formation from the time-consuming step of crystal growth [33,34].
- -
- Solvent fractionation involves using an organic solvent (hexane, acetone, or isopropyl) to let the high-melting molecules crystallise in a low viscosity solvent. This method gives much purer solid fractions than can be obtained with vacuum filtration. However, it is a more expensive process and is thus less common than dry fractionation, and only comes into the picture when the added value of the resulting fractions makes up for the high cost [33,34].
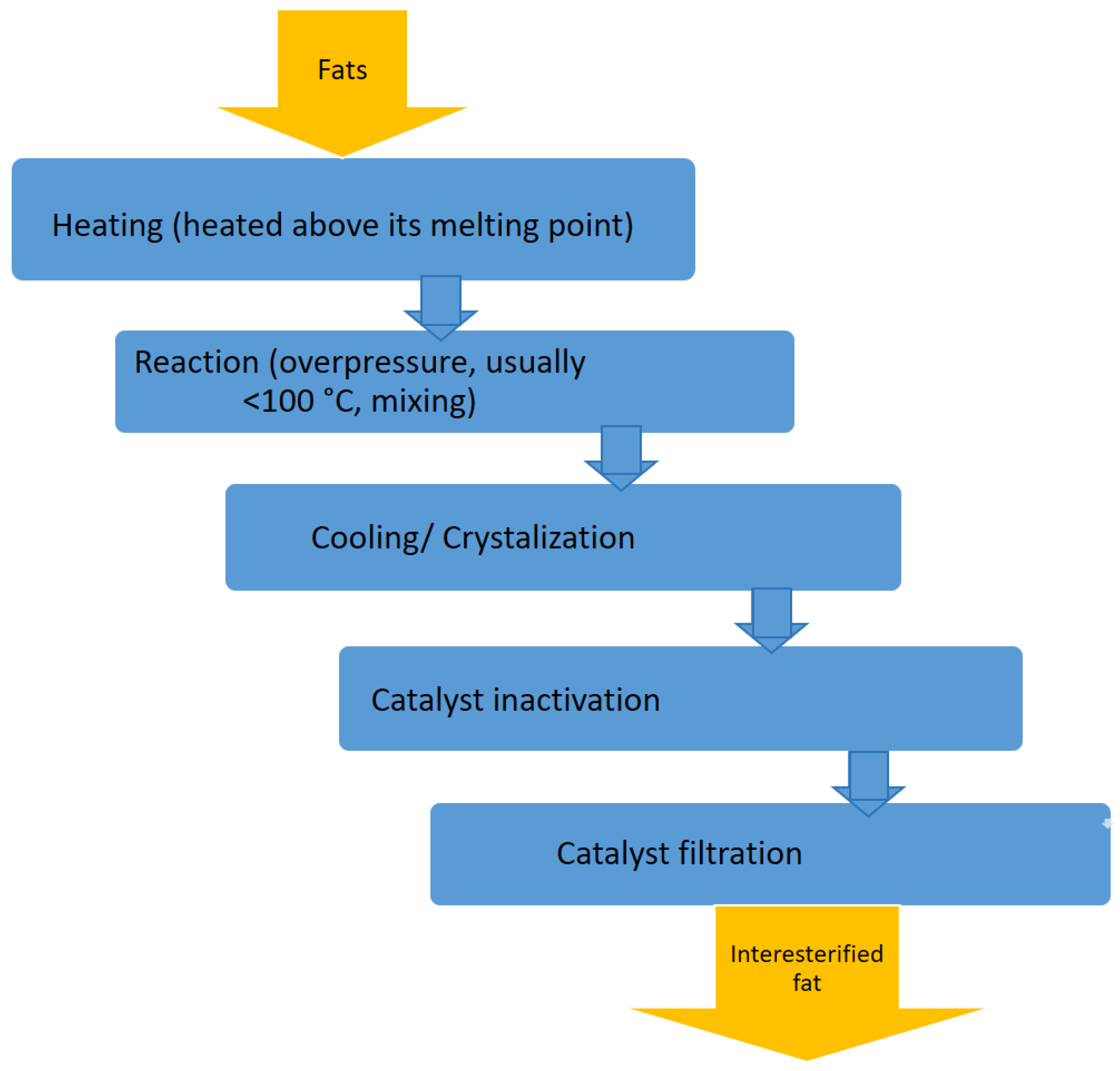
2.3. Interesterification
3. Trends in Fat Modification Technology
3.1. Enzymic Interesterification
3.2. Oleogelation
3.2.1. Oleogelators
Monoglycerides
Waxes
Phytosterols
Polysaccharides
Proteins
3.2.2. Effect of Type of Oil on the Properties of Oleogels
3.2.3. Oleogels as an Alternative to Hydrogenated Fats
| Products—The Source of Saturated Fatty Acids and a Potential Source of Hydrogenated Fat (in Commercial Products) | Optimal Replacement of Solid Fat by Oleogels | Type of Oleogels (Oleogelator and Type of Oil) | References | |
|---|---|---|---|---|
| Shortenings / bakery fats in bakery products | Cookies | 40% | 3% candelilla wax in canola oil and 70% replacement of shortening by 6% candelilla wax in canola oil | Mert&Demirkesen [105] |
| 100% | 10% carnauba wax–insect oil (Tenebrio molitor) | Kim&Oh [113] | ||
| 70% | 6% rice bran wax- rice bran oil oleogel | Pang et al. [114] | ||
| 100% | 3% candelilla wax—high oleic rapeseed oil oleogel and 5% monoglycerides—high oleic rapeseed oil oleogel | Onacik-Gur&Zbikowska, [99] | ||
| Muffins | 100% | monoglycerides -high oleic sunflower oil oleogel | Giacomozzi et al. [115] | |
| 50% | 1% HPMC—sunflower oil oleogel | Oh &Lee [106] | ||
| 100% | Rapseed oil oleogels structured by 5% of candelilla, sunflower, yellow and white beeswax | Kupiec et al. [100] | ||
| Bread | 100% | 10% of monoglycerides or rice bran wax—high oleic soybean oil oleogel | Zhao et al. [116] | |
| Buns | 100% | HPMC+ xanthan gum—olive oil or high olei sunflower oil oleogel | Bascuas et al. [117] | |
| French pastry | 50% | HPMC—high oleic sunflower oil oleogel | Espert et al. [118] | |
| Chocolate spreads | 50% | HPMC—olive/sunflower oil oleogel | Bascuas et al. [119] | |
| 100% | 20% Glycerol monostearate—corn oil oleogel (45% water in oleogel emultion) | Tirgarian et al. [120] | ||
| Margarines | 100% replacement of partially hydrogenated palm fat | 10% beeswax-sunflower oil oleogel hydrocolloid based oleogel (3.15% sodium caseinate, 0.5% guar gum, 0.22% xanthan gum) | Abdolmaleki et al. [108] | |
| Creams and fillings | 100% | monoglycerides–high oleic sunflower oil oleogel (100 g/kg) | Palla et al. [121] | |
| Ice cream | 50% | 6% carnauba wax—soybean oil oleogel | Airoldi et al. [122] | |
| 100% | 7% beeswax—camellia oil oleogel | Jing et al. [123] | ||
| Frying medium | 100% | 3% an 8% beeswax—sunflower oil oleogel | Gunser et al. [112] | |
| 100% | carnauba wax—canola oil (5 g/100 g and 10 g/100 g) oleogel | Adrah et al. [111] | ||
| 100% | carnauba wax—soybean oil (5 g/100 g and 10 g/100 g) oleogel | Lim et al. [110] | ||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witkamp, R.F.; van Norren, K. Let thy food be thy medicine.... when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, A. Generation and role of trans fatty acids—A review. Pol. J. Food Nutr. Sci. 2010, 1, 113–117. [Google Scholar]
- Żbikowska, A.; Kupiec, M.; Marciniak-Łukasiak, K.; Kowalska, M. Oleogels—Perspectives on applying them to food. Food Sci.-Technol.-Qual. 2017, 3, 5–13. [Google Scholar] [CrossRef]
- Zbikowska, A.; Kowalska, M.; Stauffer, C.E. Fats and oils in bakery products. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Amico, A.; Wootan, M.G.; Jacobson, M.F.; Leung, C.; Willett, W. The demise of artificial trans fat: A history of a public health achievement. Milbank Q. 2021, 99, 746–770. [Google Scholar] [CrossRef]
- WHO World Health Organization. Draft Guidelines: Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children; WHO: Geneva, Switzerland, 2018.
- Health Canada. Canada’s Dietary Guidelines for Health Professionals and Policy Makers; Health Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- EFSA-European Food Safety Authority. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to nutrition claims concerning omega-3 fatty acids, monounsaturated fat, polyunsaturated fat and unsaturated fat. EFSA J. 2005, 253, 1–29. [Google Scholar]
- EU Regulation: Commission Regulation (EU) 2019/649 of 24 April 2019 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Fat of Animal. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32019R0649 (accessed on 2 March 2023).
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef]
- Rasmussen, B.M.; Vessby, B.; Uusitupa, M.; Berglund, L.; Pedersen, E.; Riccardi, G. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am. J. Clin. Nutr. 2006, 83, 221–226. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Clarke, R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur. J. Clin. Nut. 2009, 63, 22–33. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Puşcaş, A.; Mureşan, V.; Socaciu, C.; Muste, S. Oleogels in food: A review of current and potential applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Singh, M.; Lee, S. Properties of Cookies Made with NaturalWax-Vegetable Oil Organogels. J. Food Sci. 2016, 81, C1045–C1054. [Google Scholar] [CrossRef] [PubMed]
- Ntsomboh-Ntsefong, G.; Ngalle-Bille, H.; Ajambang, W.; Likeng-Li-Ngue, B.C.; Kingsley, T.M.; Bell, J.M.; Youmbi, E. Brief review on the controversies around oil palm Elaeis Guineensis Jacq.production and palm oil consumption. IJRD 2016, 3, 61–75. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Food Sci. Hum. Well. 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Bana’s, K.; Harasym, J. Natural Gums as Oleogelators. Int. J. Mol. Sci. 2021, 22, 12977. [Google Scholar] [CrossRef]
- DeMan, J.M. Lipids. In Principles of Food Chemistry, 3rd ed.; Springer Science & Business Media: Berlin, Germany, 2013; pp. 33–107. [Google Scholar]
- Farajzadeh, A.D.; Naeli, M.H.; Naderi, M.; Jafari, S.M.; Tavakoli, H.R. Production of Trans-free fats by chemical interesterified blends of palm stearin and sunflower oil. Food Sci. Nutr. 2019, 7, 3722–3730. [Google Scholar] [CrossRef]
- Senanayake, N.; Shahidi, F. Modification of Fats and Oils via Chemical and Enzymatic Methods. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1–29. [Google Scholar] [CrossRef]
- Kellens, M.; Calliauw, G. Oil Modification Processes (Chapter 6). In Edible Oil Processing, 2nd ed.; Hamm, W., Hamilton, R.J., Calliauw, G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 171–191. [Google Scholar] [CrossRef]
- Al-Ismail, K.M.; Takruri, H.R.; Tayyem, R.F.; Al-Dabbas, M.M.; Abdelrahim, D.N. Trans fatty acids content of sweets and appetisers traditionally consumed in Jordan. RISG 2021, XCVIII, 129–135. Available online: www.innovhub-ssi.it/kdocs/2012987/2021_vol._982_-_art._06_-_al-ismail.pdf (accessed on 3 March 2023).
- Dijkstra, A.J. Kinetics and mechanism of the hydrogenation process—The state of the art. Eur. J. Lipid Sci. Technol. 2012, 114, 985–998. [Google Scholar] [CrossRef]
- Timms, R.E. Fractional crystallisation—The fat modification process for the 21st century. Eur. J. Lipid Sci. Technol. 2005, 107, 48–57. [Google Scholar] [CrossRef]
- Rossell, J.B. Fractionation of lauric oils. JAOCS 1985, 62, 385–389. [Google Scholar] [CrossRef]
- Hishamuddin, E.; Huey, S.M. The role of liquid entrainment and its effect on separation efficiency in palm oil fractionation. Palm Oil Eng. Bull. 2021, 137, 23–29. [Google Scholar]
- Ramli, M.R.; Siew, W.L.; Cheah, K.Y. Production of high oleic palm oils on a pilot scale. JAOCS 2009, 86, 587–594. [Google Scholar] [CrossRef]
- Mulyono, M.E.; Lubis, E.S.; Yudanto, B.G.; Panjaitan, F.R.; Rizki, I.F.; Bajra1, B.D. International Application of dry fractional crystallisation on High-Oleic Low-Palmitic red palm super olein production. JFST 2023, 58, 3402–3409. [Google Scholar] [CrossRef]
- Calliauw, G.H. Dry Fractionation. In Edible Oil Processing; Dijkstra, A.J., Ed.; AOCS Lipid Library: Champaign, IL, USA, 2019; Available online: www.lipidlibrary.aocs.org/edible-oil-processing (accessed on 2 March 2023).
- Kellens, M.; Gibon, V.; Hendrix, M.; De Greyt, W. Palm oil fractionation. Eur. J. Lipid Sci. Technol. 2007, 109, 336–349. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Interesterification. In The Lipids Handbook, 3rd ed.; Gunstone, F.D., Harwood, J.L., Dijkstra, A.J., Eds.; Taylor & Francis Group LLC.: Boca Raton, FL, USA, 2007; pp. 285–300. [Google Scholar]
- Rousseau, D.; Ghazani, S.M.; Marangoni, A.G. Chemical interesterification of food lipids: Chemistry, nutrition, and biotechnology. In Food Lipids, 4th ed.; Akoh, C.C., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 149–184. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Interesterification revisited. In Bailey Award Address Presented at the 100th AOCS Annual Meeting; Alton, E., Ed.; Expo: Orlando, FL, USA, 2009. [Google Scholar] [CrossRef]
- Kadhum, A.A.H.; Shamma, M.N. Edible lipids modification processes: A review. Crit. Rev. Food Sci. 2017, 57, 48–58. [Google Scholar] [CrossRef]
- Tarazona-Lopez, E.M.; Barrutia-Mauricio, V.H.; Castaneda-Olivera, C.A.; Valverde-Flores, J.W.; Benites, E.; Valdiviezo, L. Comparison of biodiesel extracted from pork and duck fat. CET 2021, 87, 505–510. [Google Scholar] [CrossRef]
- Toldra-Reig, F.; Mora, L.; Toldra, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. LWT 2020, 132, 109880. [Google Scholar] [CrossRef]
- Kowalska, M.; Krzton-Maziopa, M.; Krzton-Maziopa, A.; Zbikowska, A.; Szakiel, J. Rheological Characterization and Quality of Emulsions Based on Fats Produced during the Reaction Catalyzed by Immobilized Lipase from Rhizomucor Miehei. Catalysts 2022, 12, 649. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, W.; Wang, X.; Jin, Q. Biosynthesis of structured lipids enriched with medium and long-chain triacylglycerols for human milk fat substitute. LWT 2020, 128, 109255. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Xu, W.; Miu, Z.; Jin, Q.; Wang, X. Enzymatic synthesis of structured triacylglycerols rich in 1,3-dioleoyl-2-palmitoylglycerol and 1-oleoyl-2-palmitoyl-3-linoleoylglycerol in a solvent—Free system. LWT 2020, 118, 108798. [Google Scholar] [CrossRef]
- Kowalska, M.; Krztoń-Maziopa, A.; Babut, M.; Mitrosz, P. Rheological and physical analysis of oil-water emulsion based on enzymatic structured fat. Rheol. Acta 2020, 59, 717–726. [Google Scholar] [CrossRef]
- FDA—U.S. Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018. Available online: www.fda.gov/food/food-additives-petitions/final-determination-regarding-partially-hydrogenated-oils-removing-trans-fat (accessed on 15 May 2023).
- Azimah, K.N.; Zaliha, O. Influence of enzymatic and chemical interesterification on crystallisation properties of refined, bleached and deodourised (RBD) palm oil and RBD palm kernel oil blends. Food Res. Int. 2018, 106, 982–991. [Google Scholar] [CrossRef]
- Moore, M.A.; Akoh, C.C. Enzymatic interesterification of coconut and high oleic sunflower oils for edible film application. JAOCS 2017, 94, 567–576. [Google Scholar] [CrossRef]
- Utama, Q.D.; Sitanggang, A.B.; Adawiyah, D.R.; Hariyadi, P. Lipase-catalysed interesterification for the synthesis of medium-long-medium (MLM) structured lipids—A review. Food Technol. Biotechnol. 2019, 57, 305–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, W.J.; Sun, X.; Wang, Y. Enzymatic interesterification of palm olein in a continuous packed bed reactor: Effect of process parameters on the properties of fats and immobilised Thermomyces lanuginosus lipase. LWT 2022, 162, 113459. [Google Scholar] [CrossRef]
- Kowalska, M.; Żbikowska, A.; Tarnowska, K. Stability of emulsions containing interesterified fats based on mutton tallow and walnut oil. JAOCS 2015, 92, 993–1002. [Google Scholar] [CrossRef]
- Kowalska, M.; Żbikowska, A. Wykorzystanie biokatalizatorów w procesie przeestryfikowania enzymatycznego. Postępy Tech. Przetwórstwa Spożywczego 2011, 1, 66–70. [Google Scholar]
- Kowalska, M.; Woźniak, M.; Tavernier, S.; Żbikowska, A.; Pazdur, L. Assessment of the effectiveness of synthetic diacylglycerols as emulsifiers in dispersion systems containing interesterified turkey fat. Eur. Food Res. Technol. 2018, 244, 1665–1674. [Google Scholar] [CrossRef]
- Aktas, A.B.; Ozen, B.; Alamprese, C. Effects of processing parameters on chemical and physical properties of enzymatically interesterified beef tallow–corn oil blends. J. Food Process. Preserv. 2021, 45, e14587. [Google Scholar] [CrossRef]
- Pang, M.; Ge, Y.; Cao, L.; Cheng, J.; Jiang, S. Physicochemical properties, crystallisation behavior and oxidative stabilities of enzymatic interesterified fats of beef tallow, palm stearin and camellia oil blends. J. Oleo Sci. 2019, 68, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Trpin, S.M.; Hoy, A.J.; Brown, R.D.; Rudaz, C.G.; Honeyman, J.; Matzaris, M.; Watt, M.J. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 2011, 54, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Shen, J.R.; Xiong, C.Y.; Zhu, X.M.; Deng, Z.Y. Investigation of lipid metabolism by a new structured lipid with medium- and long-chain triacylglycerols from Cinnamomum camphora seed oil in healthy c57bl/6j mice. J. Agric. Food Chem. 2018, 66, 1990–1998. [Google Scholar] [CrossRef]
- Miura, S.; Ogawa, A.; Konishi, H. A rapid method for enzymatic synthesis and purification of structured triacylglycerol, 1,3-dilauroyl-2-oleoyl-glycerol. JAOCS 1999, 76, 927–931. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.N.; Parmon, V.N. Prospects for application of enzymatic interesterification of oils in the production of modified fats. Catal. Ind. 2016, 8, 348–353. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilised enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Roy, S.S.; Bhattacharyya, D.K. Comparative nutritional study of enzymatically and chemically interesterified palm oil products. JAOCS 1995, 72, 327–330. [Google Scholar] [CrossRef]
- Kleiner, L.; Akoh, C.C. Applications of structured lipids in selected food market segments and their evolving consumer demands. In Lipid Modification by Enzymes and Engineered Microbes; Bornscheuer, U., Ed.; eBook; AOCS Press: Champaign, IL, USA, 2018; pp. 179–202. ISBN 9780128131688. [Google Scholar]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilised enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Walsh, M.K. Immobilised enzyme technology for food applications. In Novel Enzyme Technology for Food Applications; Rastall, R., Ed.; eBook; Woodhead Publishing: Cambridge, UK, 2007; pp. 60–84. ISBN 9781845693718. [Google Scholar]
- Zhou, J.; Lee, Y.Y.; Mao, Y.; Wang, Y.; Zhang, Z. Future of structured lipids: Enzymatic synthesis and their new applications in food systems. Foods 2022, 11, 2400. [Google Scholar] [CrossRef]
- Law, M. Plant sterol and stanol margarines and health. BMJ 2000, 320, 861–864. [Google Scholar] [CrossRef]
- Rohm, H.; Schäper, C.; Zahn, S. Interesterified fats in chocolate and bakery products: A concise review. LWT 2018, 87, 379–384. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A.; Kowalska, M.K.; Przybysz, M. Assessment of physical properties of structured oils and palm fat. Mater. Plast. 2017, 54, 800–805. [Google Scholar] [CrossRef]
- Kupiec, M.; Żbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Sowiński, M.; Szymańska, I.; Zbikowska, K.; Marciniak-Łukasiak, K.; Werpachowski, W. Analysis of stability, rheological and structural properties of oleogels obtained from peanut oil structured with yellow beeswax. Gels 2022, 8, 448. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of oleogels based on edible lipid materials. COCIS 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Rogers, M.A.; Strober, T.; Bot, A.; Toro-Vazquez, J.F.; Stortz, T.; Marangoni, A.G. Edible oleogels in molecular gastronomy. Int. J. Gastron. Food Sci. 2014, 2, 22–31. [Google Scholar] [CrossRef]
- Mallia, V.A.; George, M.; Blair, D.L.; Weiss, R.G. Robust Organogels from Nitrogen-containing derivatives of (R)-12-hydroxustearic acid gelators: Comparisons with gels from stearic acid derivatives. Langmuir 2009, 25, 8615–8625. [Google Scholar] [CrossRef]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The influence of edible oils’ composition on the properties of beeswax-based oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef]
- Scharfe, M.; Flöter, E. Oloeogelation: From Scientific feasibility to applicability in food. EJLST 2020, 122, 2000213. [Google Scholar] [CrossRef]
- Vereecken, J.; Meeussen, W.; Foubert, I.; Lesaffer, A.; Wouters, J.; Dewettinck, K. Comparing the crystallisation and polymorphic behaviour of saturated and unsaturated monoglycerides. Food Res. Int. 2009, 42, 1415–1425. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Tang, C.; Li, Y. Crystallization behavior and physical properties of monoglycerides-based oleogels as function of oleogelator concentration. Foods 2023, 12, 345. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charo-Alonso, M.; Alonzo-Macias, M.; Gonzalez-Chavez, M.M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. JAOCS 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Yılmaz, E.; Ögütcü, M. Properties and stability of hazelnut oil organogels with beeswax and monoglyceride. JAOCS 2014, 91, 1007–1017. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. JFST 2019, 57, 1609–1618. [Google Scholar] [CrossRef]
- Hwang, H.S.; Winkler-Moser, J.K. Properties of margarines prepared from soybean oil oleogels with mixtures of candelilla wax and beeswax. J. Food Sci. 2020, 85, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Scharfe, M.; Prange, D.; Flöter, E. The composition of edible oils modifies sitosterol/oryzanol oleogels. Part I: Stripped triglyceride oils. JAOCS 2021, 99, 43–56. [Google Scholar] [CrossRef]
- Scharfe, M.; Ahmane, Y.; Seilert, J.; Keim, J.; Flöter, E. On the effect of minor oil components on β-sitosterol/γ-oryzanol oleogels. EJLST 2019, 121, 1800487. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The role of surfactants on the ethylcellulose oleogel structure and mechanical properties. Carbohydr. Polym. 2015, 127, 355–362. [Google Scholar] [CrossRef]
- Zetzl, A.K.; Gravelle, A.J.; Kurylowicz, M.; Dutcher, J.; Barbut, S.; Marangoni, A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Struct. 2014, 2, 27–40. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef]
- Jiang, Q.; Yu, Z.; Meng, Z. Double network oleogels co-stabilised by hydroxypropyl methylcellulose and monoglyceride crystals: Baking application. Int. J. Biol. Macromol. 2022, 209, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and application in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- De Vries, A.; Lopez Gomez, Y.L.; Jansen, B.; Van der Linden, E.; Scholten, E. Controlling agglomeration of protein aggregates for structure formation in liquid oil: A sticky business. ACS Appl. Mater. Interfaces 2017, 9, 10136–10147. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocolloid 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Mezzenga, R. Protein-Templated oil gels and powders Chapter 13. In Edible Oleogels, 2nd ed.; Marangoni, A.G., Garti, N., Eds.; AOCS Press: Champaign, IL, USA, 2018; pp. 307–329. [Google Scholar]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-based structuring of liquid oil into soft solids and oleogels using water-continuous emulsions as templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Valoppi, F.; Calligaris, S.; Barba, L.; Šegatin, N.; Poklar Ulrih, N.; Nicoli, M.C. Influence of oil type on formation, structure, thermal, and physical properties of monoglyceride-based organogel. Eur. J. Lipid Sci. Technol. 2017, 119, 1500549. [Google Scholar] [CrossRef]
- Scharfe, M.; Niksch, J.; Flöter, E. Infuence of Minor Oil Components on sunflower, rice bran, candelilla and beeswax oleogels. EJLST 2022, 124, 2100068. [Google Scholar] [CrossRef]
- Sobolev, R.; Frolova, Y.; Sarkisyan, V.; Makarenko, M.; Kochetkova, A. Effect of beeswax and combinations of its fractions on the oxidative stability of oleogels. Food Biosci. 2022, 48, 101744. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Zbikowska, A. The effect of green tea extract and oleogels on the physico-chemical properties and oxidative stability of short-dough biscuits during storage. LWT 2022, 172, 114197. [Google Scholar] [CrossRef]
- Kupiec, M.; Żbikowska, A.; Marciniak-Lukasiak, K.; Zbikowska, K.; Kowalska, M.; Kowalska, H.; Rutkowska, J. Study on the Introduction of Solid Fat with a High Content of Unsaturated Fatty Acids to Gluten-Free Muffins as a Basis for Designing Food with Higher Health Value. Int. J. Mol. Sci. 2021, 22, 9220. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xia, B.; Ni, Z.J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.L.; Wei, Z.J. Characterization of functional chocolate formulated using oleogels derived from β-sitosterol with γ-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, G. Corn oil-based oleogels with different gelation mechanism as novel cocoa butter alternatives in dark chocolate. J. Food Eng. 2019, 263, 114–122. [Google Scholar] [CrossRef]
- Li, L.; Liu, G.; Lin, Y. Physical and bloom stability of low-saturation chocolates with oleogels based on different gelation mechanism. LWT 2021, 140, 110807. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Cofrades, S.; Espert, M.; Sanz, T.; Salvador, A. Development of Chocolates with Improved Lipid Profile by Replacing Cocoa Butter with an Oleogel. Gels 2021, 7, 220. [Google Scholar] [CrossRef]
- Mert, B.; Demirkesen, I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016, 199, 809–816. [Google Scholar] [CrossRef]
- Oh, I.K.; Lee, S. Utilisation of foam structured hydroxypropyl methylcellulose for oleogels and their application as a solid fat replacer in muffins. Food Hydrocoll. 2018, 77, 796–802. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, K.; Alizadeh, L.; Nayebzadeh, K.; Baranowska, H.M.; Kowalczewski, P.Ł.; Khaneghah, A.M. Potential application of hydrocolloid-based oleogel and beeswax oleogel as partial substitutes of solid fat in margarine. Appl. Sci. 2022, 12, 12136. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Khare, A.; Lal, A.B.; Bebartta, R.P. Utilising oleogel as a frying medium for deep fried Indian traditional product (Mathri) to reduce oil uptake. J. Indian Chem. Soc. 2022, 99, 100378. [Google Scholar] [CrossRef]
- Lim, J.; Jeong, S.; Oh, I.K.; Lee, S. Evaluation of soybean oil-carnauba wax oleogels as an alternative to high saturated fat frying media for instant fried noodles. LWT 2017, 84, 788–794. [Google Scholar] [CrossRef]
- Adrah, K.; Adegoke, S.G.; Tahergorabi, R. Physicochemical and microbial quality of coated raw and oleogel-fried chicken. LWT 2022, 154, 112589. [Google Scholar] [CrossRef]
- Guneser, B.A.; Yılmaz, E.; Uslu, E.K. Sunflower oil-beeswax oleogels are promising frying medium for potato strips. EJLST 2021, 123, 2100063. [Google Scholar] [CrossRef]
- Kim, D.; Oh, I. The characteristic of insect oil for a potential component of oleogel and its application as a solid fat replacer in cookies. Gels 2022, 8, 355. [Google Scholar] [CrossRef]
- Pang, M.; Kang, S.; Liu, L.; Ma, T.; Zheng, Z.; Coa, L. Physicochemical properties and cookie-making performance as fat replacer of wax-based rice bran oil oleogels. Gels 2023, 9, 13. [Google Scholar] [CrossRef]
- Giacomozzi, A.S.; Carrin, M.E.; Palla, C.A. Muffins elaborated with optimised monoglycerides oleogels: From solid fat replacer obtention to product quality evaluation. JFS 2018, 83, 1505–1515. [Google Scholar] [CrossRef]
- Zhao, M.; Rao, J.; Chen, B. Effect of high oleic soybean oil oleogels on the properties of doughs and corresponding bakery products. JAOCS 2022, 99, 1071–1083. [Google Scholar] [CrossRef]
- Bascuas, S.; Morell, P.; Quiles, A.; Salvador, A.; Hernando, I. Use of Oleogels to Replace Margarine in Steamed and Baked Buns. Foods 2021, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Espert, M.; Wang, Q.; Sanz, T.; Salvador, A. Sunflower Oil-based Oleogel as Fat Replacer in Croissants: Textural and Sensory Characterisation. FBT 2023. [Google Scholar] [CrossRef]
- Bascuas, S.; Espert, M.; Llorca, E.; Quiles, A.; Salvador, A.; Hernando, I. Structural and sensory studies on chocolate spreads with hydrocolloid-based oleogels as a fat alternative. LWT 2021, 135, 110228. [Google Scholar] [CrossRef]
- Tirgarian, B.; Yadegari, H.; Bagheri, A.; Neshagaran, E.; Mardani, M.; Farmani, J. Reduced-fat chocolate spreads developed by water-in-oleogel emulsions. JFE 2023, 337, 111233. [Google Scholar] [CrossRef]
- Palla, C.A.; Wasinger, M.F.; Carrin, M.E. Monoglyceride oleogels as fat replacers in filling creams for sandwich cookies. J. Sci. Food Agric. 2021, 101, 2398–2405. [Google Scholar] [CrossRef]
- Airoldi, R.; da Silva, T.L.T.; Ract, J.N.R.; Foguel, A.; Colleran, H.L.; Ibrahim, S.A.; da Silva, R.C. Potential use of carnauba wax oleogel to replace saturated fat in ice cream. JAOCS 2023, 99, 1085–1099. [Google Scholar] [CrossRef]
- Jing, X.; Chen, Z.; Tang, Z.; Tao, Y.; Huang, Q.; Wu, Y.; Zhang, H.; Li, X.; Liang, J.; Liu, Z.; et al. Preparation of camellia oil oleogel and its application in an ice cream system. LWT 2022, 169, 113985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author1(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbikowska, A.; Onacik-Gür, S.; Kowalska, M.; Zbikowska, K.; Feszterová, M. Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application. Gels 2023, 9, 453. https://doi.org/10.3390/gels9060453
Zbikowska A, Onacik-Gür S, Kowalska M, Zbikowska K, Feszterová M. Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application. Gels. 2023; 9(6):453. https://doi.org/10.3390/gels9060453
Chicago/Turabian StyleZbikowska, Anna, Sylwia Onacik-Gür, Małgorzata Kowalska, Katarzyna Zbikowska, and Melánia Feszterová. 2023. "Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application" Gels 9, no. 6: 453. https://doi.org/10.3390/gels9060453
APA StyleZbikowska, A., Onacik-Gür, S., Kowalska, M., Zbikowska, K., & Feszterová, M. (2023). Trends in Fat Modifications Enabling Alternative Partially Hydrogenated Fat Products Proposed for Advanced Application. Gels, 9(6), 453. https://doi.org/10.3390/gels9060453









