Texturing of Soy Yoghurt Alternatives: Pectin Microgel Particles Serve as Inactive Fillers and Weaken the Soy Protein Gel Structure
Abstract
1. Introduction
2. Results and Discussion
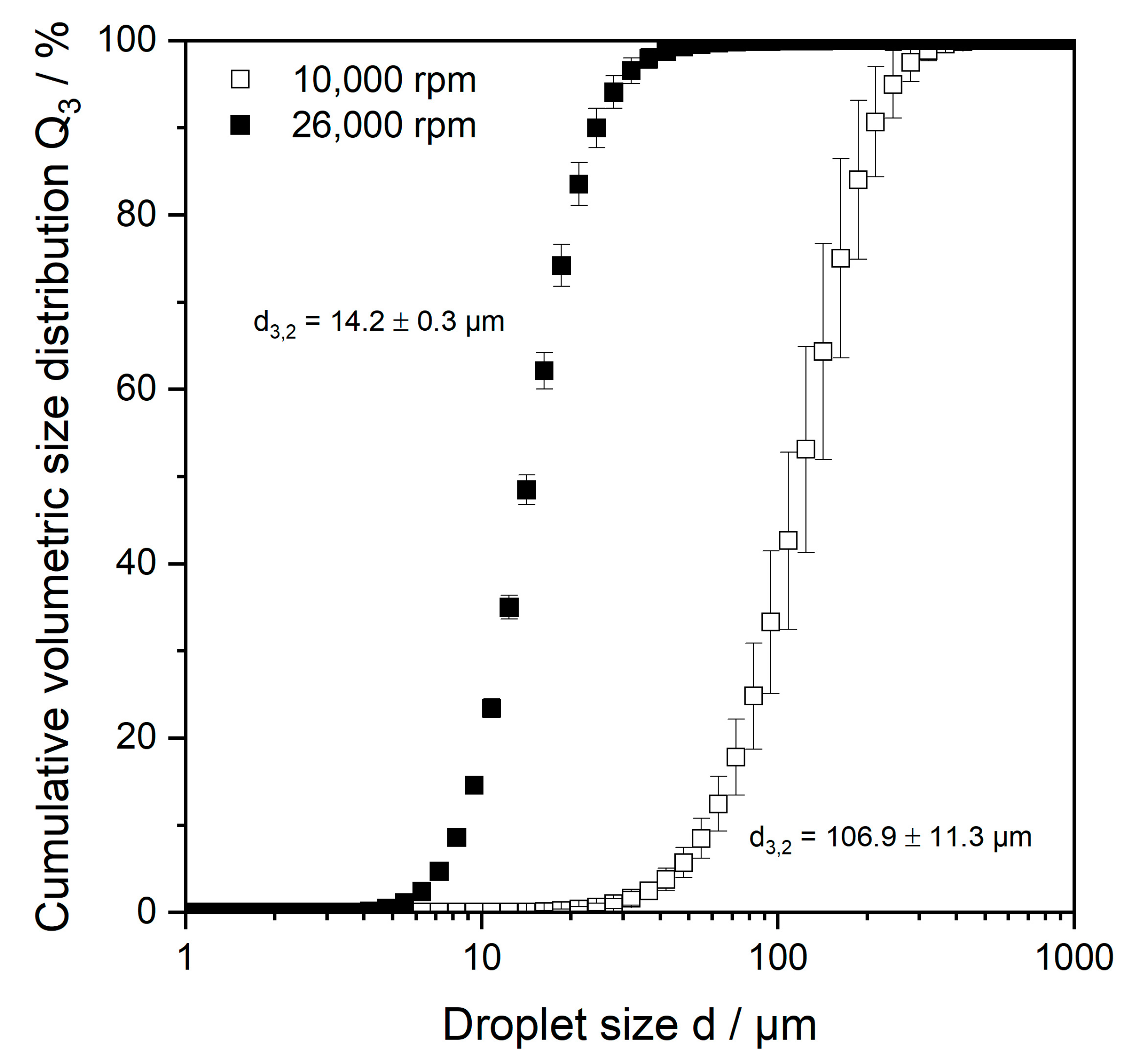
2.1. Microgel Particle Properties
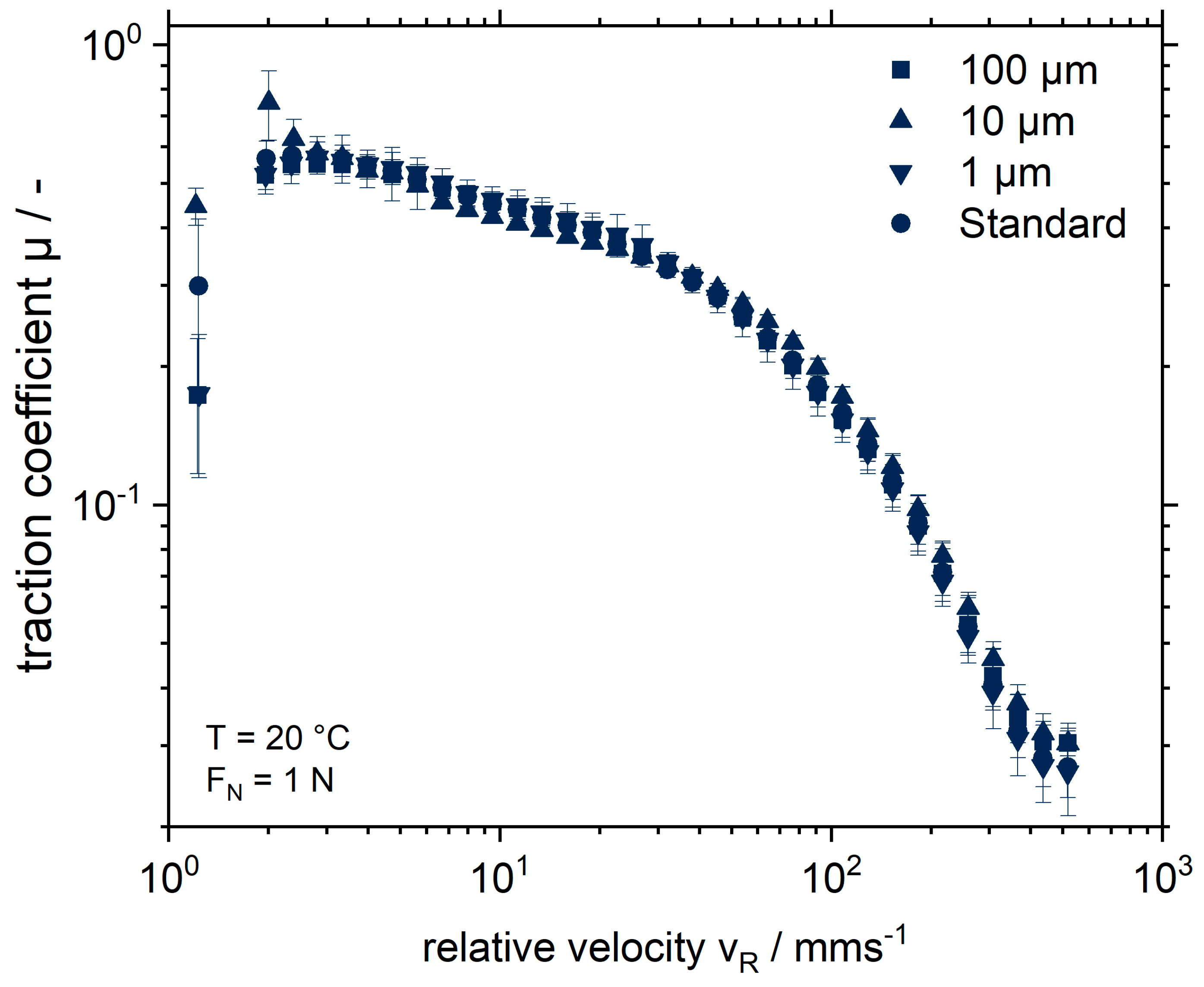
2.2. Influence of the Addition of MGP with Different Particle Sizes on the Rheological and Tribological Properties of an SPI Matrix
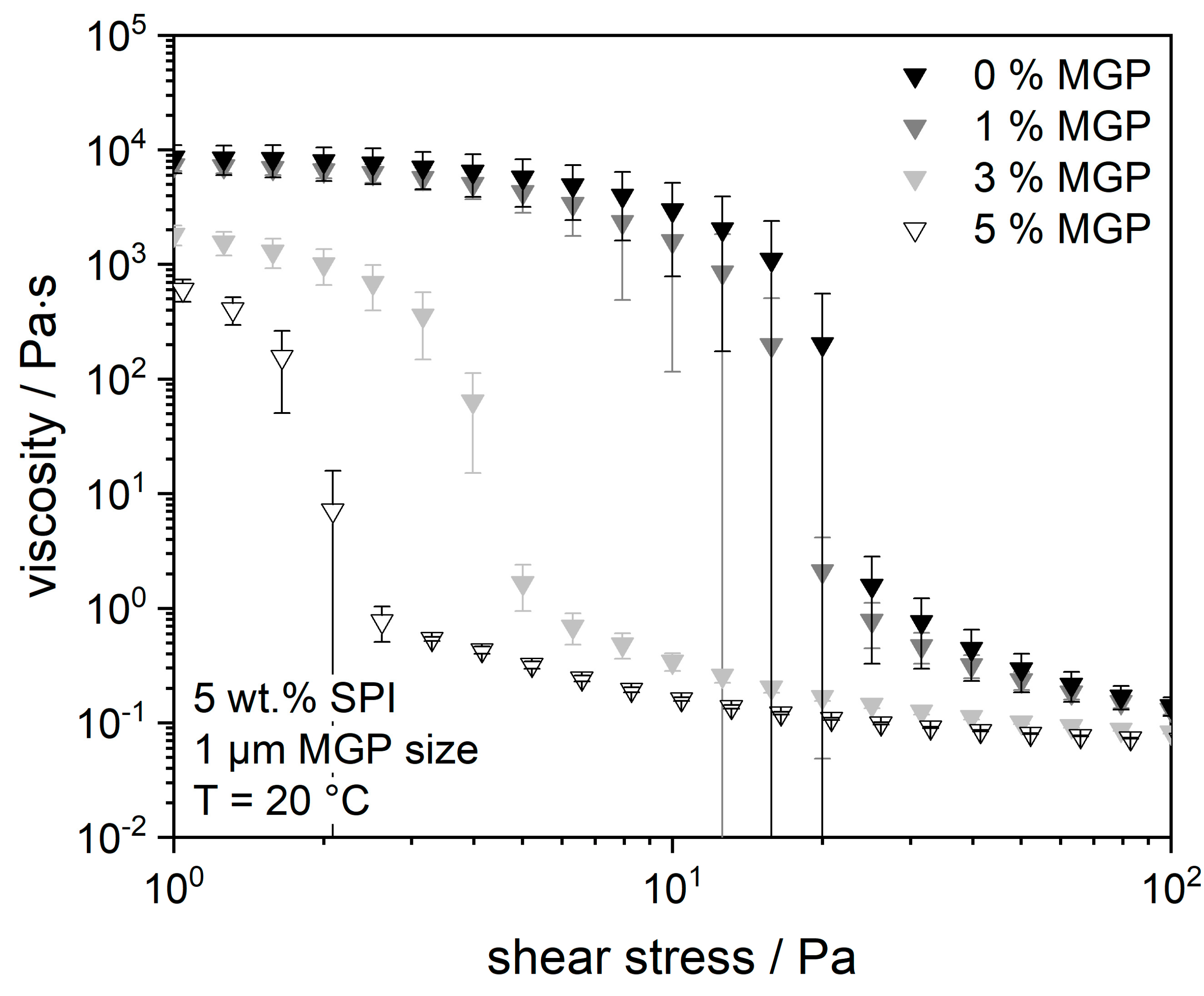
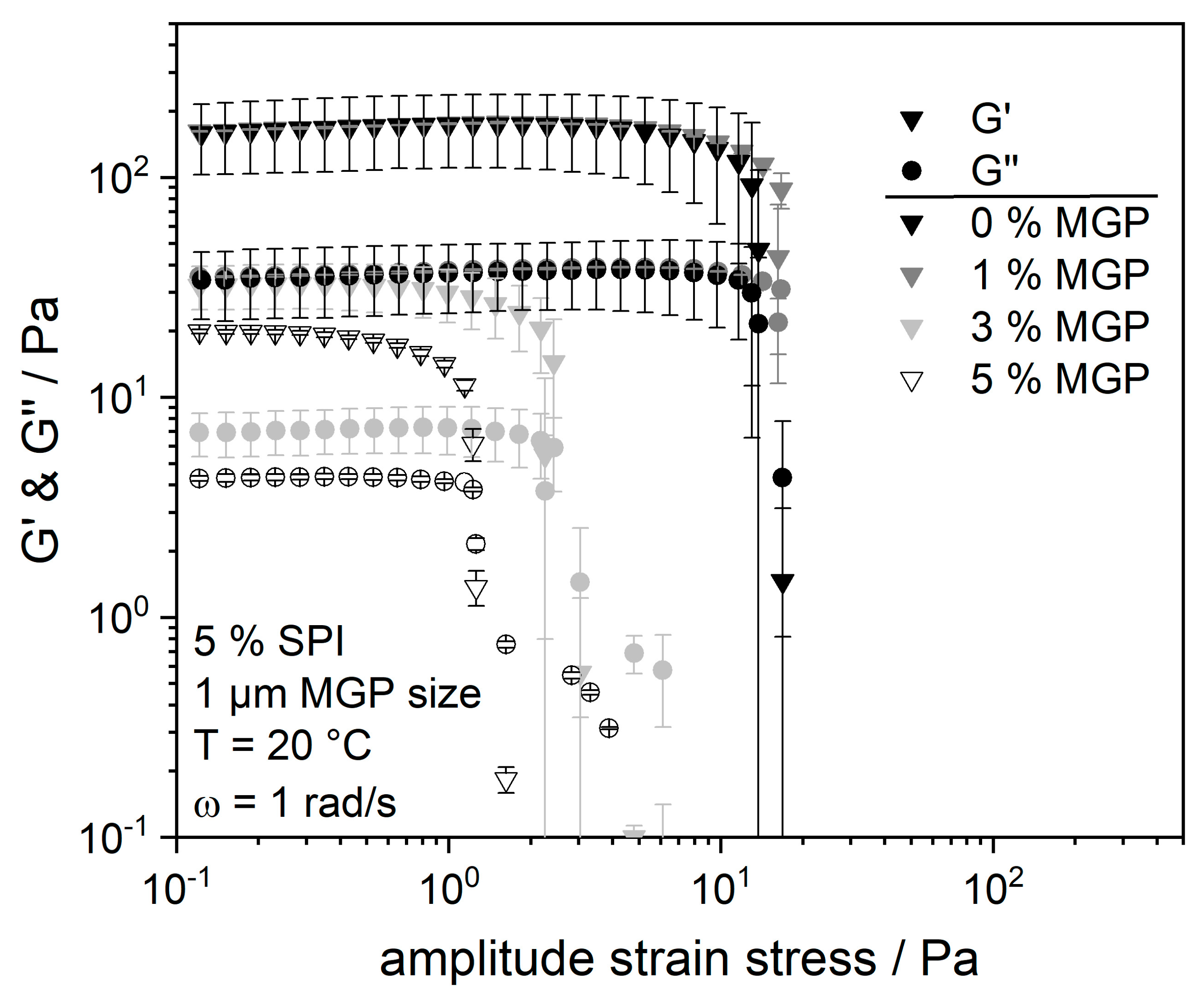
2.3. Influence of MGP Concentration on the Flow Properties and Water-Holding Capacity of the SPI Matrix
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparations of Pectin Solution
4.3. Preparation of Pectin MGP Suspensions
4.4. Determination of MGP Size and Zeta-Potential
4.5. Preparation of Soy Protein Gels
4.6. Tribological Measurements
4.7. Rheological Measurements
4.8. Water-Binding Capacity Measurements
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fu, J.; Li, L. Rheological Characteristics and Microstructure of Probiotic Soy Yogurt Prepared from Germinated Soybeans. Food Technol. Biotechnol. 2012, 50, 73–80. [Google Scholar]
- Buono, M.A.; Setser, C.; Erickson, L.E.; Fung, D.Y.C. Soymilk Yogurt: Sensory Evaluation and Chemical Measurement. J. Food Sci. 1990, 55, 528–531. [Google Scholar] [CrossRef]
- Karagül-Yüceer, Y.; Drake, M.A. Sensory analysis of yogurt. In Manufacturing Yogurt and Fermented Milks; Chandan, R.C., Kilara, A., Eds.; John Wiley & Sons: Oxford, UK, 2013; pp. 354–367. [Google Scholar]
- Ningtyas, D.W.; Tam, B.; Bhandari, B.; Prakash, S. Effect of different types and concentrations of fat on the physico-chemical properties of soy protein isolate gel. Food Hydrocoll. 2021, 111, 106226. [Google Scholar] [CrossRef]
- Batista, A.P.; Portugal CA, M.; Sousa, I.; Crespo, J.G.; Raymundo, A. Accessing gelling ability of vegetable proteins using rheological and fluorescence techniques. Int. J. Biol. Macromol. 2005, 36, 135–143. [Google Scholar] [CrossRef]
- Berghout, J.A.M.; Boom, R.M.; van der Goot, A.J. Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocoll. 2015, 43, 465–472. [Google Scholar] [CrossRef]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure formation and rheological properties of pea protein-based gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef]
- Godoi, F.C.; Bhandari, B.; Prakash, S. Tribo-rheology and sensory analysis of a dairy semi-solid. Food Hydrocoll. 2017, 70, 240–250. [Google Scholar] [CrossRef]
- Kieserling, K.; Meyer, L.; Drusch, S.; Schalow, S. Influence of mechanical and thermal treatment on particle structure, leaching of alcohol insoluble substances and water binding properties of pectin-rich orange fibre. Eur. Food Res. Technol. 2019, 245, 1251–1262. [Google Scholar] [CrossRef]
- Tomic, N.; Dojnov, B.; Miocinovic, J.; Tomasevic, I.; Smigic, N.; Djekic, I.; Vujcic, Z. Enrichment of yoghurt with insoluble dietary fiber from triticale–A sensory perspective. LWT Food Sci. Technol 2017, 80, 59–66. [Google Scholar] [CrossRef]
- Sendra, E.; Kuri, V.; Fernández-López, J.; Sayas-Barberá, E.; Navarro, C.; Pérez-Alvarez, J.A. Viscoelastic properties of orange fiber enriched yogurt as a function of fiber dose, size and thermal treatment. LWT Food Sci. Technol. 2010, 43, 708–714. [Google Scholar] [CrossRef]
- Everett, D.W.; McLeod, R.E. Interactions of polysaccharide stabilisers with casein aggregates in stirred skim-milk yoghurt. Int. Dairy J. 2005, 15, 1175–1183. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Tuinier, R.; Rolin, C.; de Kruif, C.G. Electrosorption of pectin onto casein micelles. Biomacromolecules 2002, 3, 632–638. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Engelen, L.; Wijk, R.; de van der Bilt, A.; Prinz, J.F.; Janssen, A.M.; Bosman, F. Relating particles and texture perception. Physiol. Behav. 2005, 86, 111–117. [Google Scholar] [CrossRef]
- Krzeminski, A.; Prell, K.A.; Busch-Stockfisch, M.; Weiss, J.; Hinrichs, J. Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems. Int. Dairy J. 2014, 36, 118–127. [Google Scholar] [CrossRef]
- Sodini, I.; Lucas, A.; Tissier, J.P.; Corrieu, G. Physical properties and microstructure of yoghurts supplemented with milk protein hydrolysates. Int. Dairy J. 2005, 15, 29–35. [Google Scholar] [CrossRef]
- Sodini, I.; Montella, J.; Tong, P.S. Physical properties of yogurt fortified with various commercial whey protein concentrates. J. Sci. Food Agric. 2005, 85, 853–859. [Google Scholar] [CrossRef]
- Dames, B.; Morrison, B.R.; Willenbacher, N. An empirical model predicting the viscosity of highly concentrated, bimodal dispersions with colloidal interactions. Rheol. Acta 2001, 40, 434–440. [Google Scholar] [CrossRef]
- Hahn, C.; Nöbel, S.; Maisch, R.; Rösingh, W.; Weiss, J.; Hinrichs, J. Adjusting rheological properties of concentrated microgel suspensions by particle size distribution. Food Hydrocoll. 2015, 49, 183–191. [Google Scholar] [CrossRef]
- Wiedenmann, V.; Oehlke, K.; van der Schaaf, U.; Hetzer, B.; Greiner, R.; Karbstein, H.P. Impact of the incorporation of solid lipid nanoparticles on β-lactoglobulin gel matrices. Food Hydrocoll. 2018, 84, 498–507. [Google Scholar] [CrossRef]
- Laguna, L.; Farrell, G.; Bryant, M.; Morina, A.; Sarkar, A. Relating rheology and tribology of commercial dairy colloids to sensory perception. Food Funct. 2017, 8, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Saavedra Isusi, G.I.; Paz Puga, D.; van der Schaaf, U.S. Texturing Fermented Emulsion Gels from Soy Protein: Influence of the Emulsifying Agent—Soy Protein vs. Pectin Microgels—On Gel Microstructure, Rheology and Tribology. Foods 2022, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Frith, W.J.; Stoke, J.R. Influence of particle modulus on the rheological properties of agar microgel suspensions. J. Rheol. 2004, 48, 1195–1213. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid Gel Particles: Formation, Characterization, and Application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Pravinata, L.; Akhtar, M.; Bentley, P.J.; Mahatnirunkul, T.; Murray, B.S. Preparation of alginate microgels in a simple one step process via the Leeds Jet Homogenizer. Food Hydrocoll. 2016, 61, 77–84. [Google Scholar] [CrossRef]
- Hamberg, L.; Wohlwend, M.; Walkenström, P.; Hermansson, A. Shapes and shaping of biopolymer drops in a hyperbolic flow. Food Hydrocoll. 2003, 17, 641–652. [Google Scholar] [CrossRef]
- Wolf, B.; Frith, W.J.; Norton, I.T. Influence of gelation on particle shape in sheared biopolymer blends. J. Rheol. 2001, 45, 1141–1157. [Google Scholar] [CrossRef]
- Wolf, B.; Frith, W.J.; Singleton, S.; Norton, I.T. Shear behaviour of biopolymer suspensions with spheroidal and cylindrical partciles. Rheol. Acta 2001, 40, 238–247. [Google Scholar] [CrossRef]
- Saavedra Isusi, G.I.; Lohner, N.; Karbstein, H.P.; van der Schaaf, U.S. Emulsions stabilised with pectin-based microgels: Investigations into the break-up of droplets in the presence of microgels. J. Food Eng. 2021, 294, 110421. [Google Scholar] [CrossRef]
- Jaramillo, D.P.; Roberts, R.F.; Coupland, J.N. Effect of pH on the properties of soy protein-pectin complexes. Food Res. Int. 2011, 44, 911–916. [Google Scholar] [CrossRef]
- Yuan, Y.; Wan, Z.; Yang, X.; Yin, S. Associative interactions between chitosan and soy protein fractions: Effects of pH, mixing ratio, heat treatment and ionic strength. Food Res Int. 2014, 55, 207–214. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Saavedra Isusi, G.I.; Bindereif, B.; Karbstein, H.P.; van der Schaaf, U.S. Polymer or microgel particle: Differences in emulsifying properties of pectin as microgel or as individual polymer chains. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124793. [Google Scholar] [CrossRef]


| pH | Zeta Potential/mV |
|---|---|
| 3.0 | −17 |
| 4.0 | −33.7 |
| 5.0 | −39.1 |
| MGP Size | Dispersing Device | Rotational Speed/Pressure | Time/Passes |
|---|---|---|---|
| 100 µm | Colloid mill | 10,000 rpm | 1 min |
| 10 µm | Colloid mill | 26,000 rpm | 5 min |
| 1 µm | High-pressure homogenizer | 400 bar | 2 |
| Sample Name | SPI Powder | MGP Size | MGP Suspension Content | Water Content |
|---|---|---|---|---|
| Reference; 5% SPI | 25 g | - | - | 475 g |
| 1% MGP; 5% SPI | 25 g | 1 µm | 100 g | 375.5 g |
| 1% MGP; 5% SPI | 25 g | 10 µm | 100 g | 375.5 g |
| 1% MGP; 5% SPI | 25 g | 100 µm | 100 g | 375.5 g |
| 3% MGP; 5% SPI | 25 g | 1 µm | 300 g | 175.5 g |
| 5% MGP; 5% SPI | 25 g | 1 µm | 500 g | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra Isusi, G.I.; Marburger, J.; Lohner, N.; van der Schaaf, U.S. Texturing of Soy Yoghurt Alternatives: Pectin Microgel Particles Serve as Inactive Fillers and Weaken the Soy Protein Gel Structure. Gels 2023, 9, 473. https://doi.org/10.3390/gels9060473
Saavedra Isusi GI, Marburger J, Lohner N, van der Schaaf US. Texturing of Soy Yoghurt Alternatives: Pectin Microgel Particles Serve as Inactive Fillers and Weaken the Soy Protein Gel Structure. Gels. 2023; 9(6):473. https://doi.org/10.3390/gels9060473
Chicago/Turabian StyleSaavedra Isusi, Gabriela Itziar, Johannes Marburger, Nils Lohner, and Ulrike S. van der Schaaf. 2023. "Texturing of Soy Yoghurt Alternatives: Pectin Microgel Particles Serve as Inactive Fillers and Weaken the Soy Protein Gel Structure" Gels 9, no. 6: 473. https://doi.org/10.3390/gels9060473
APA StyleSaavedra Isusi, G. I., Marburger, J., Lohner, N., & van der Schaaf, U. S. (2023). Texturing of Soy Yoghurt Alternatives: Pectin Microgel Particles Serve as Inactive Fillers and Weaken the Soy Protein Gel Structure. Gels, 9(6), 473. https://doi.org/10.3390/gels9060473








