Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
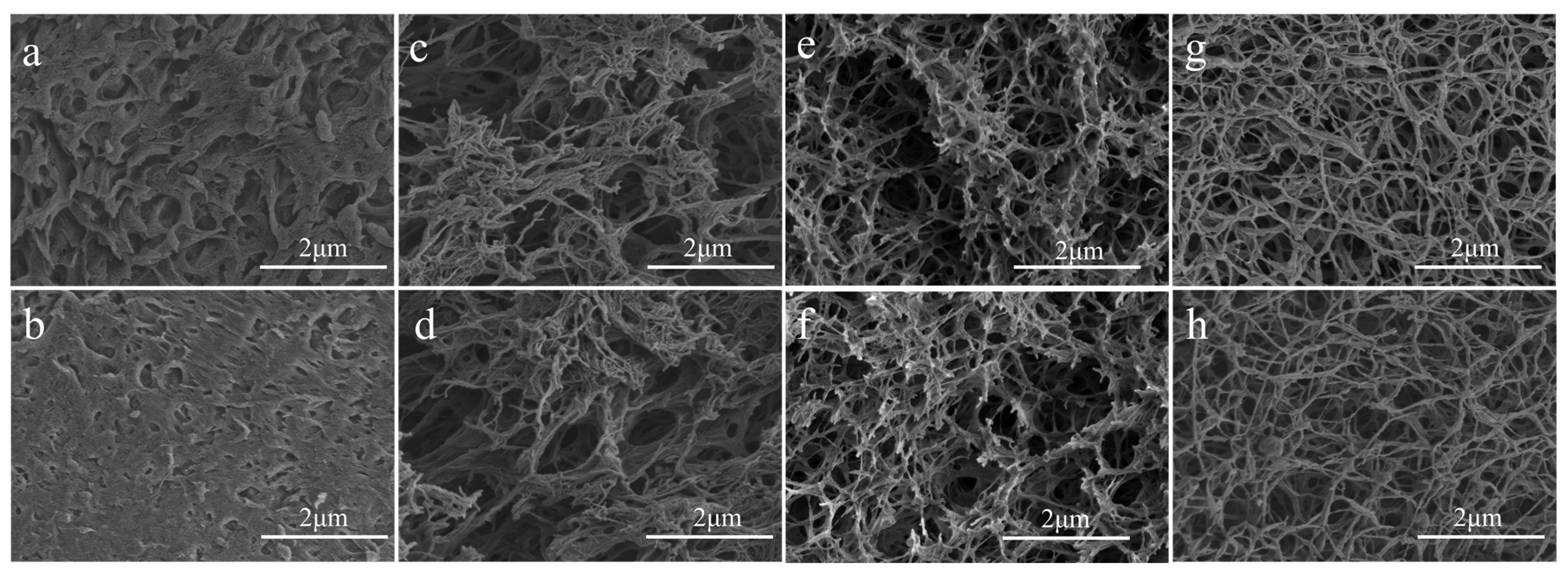
2.1. Scanning Electron Microscopy (SEM)
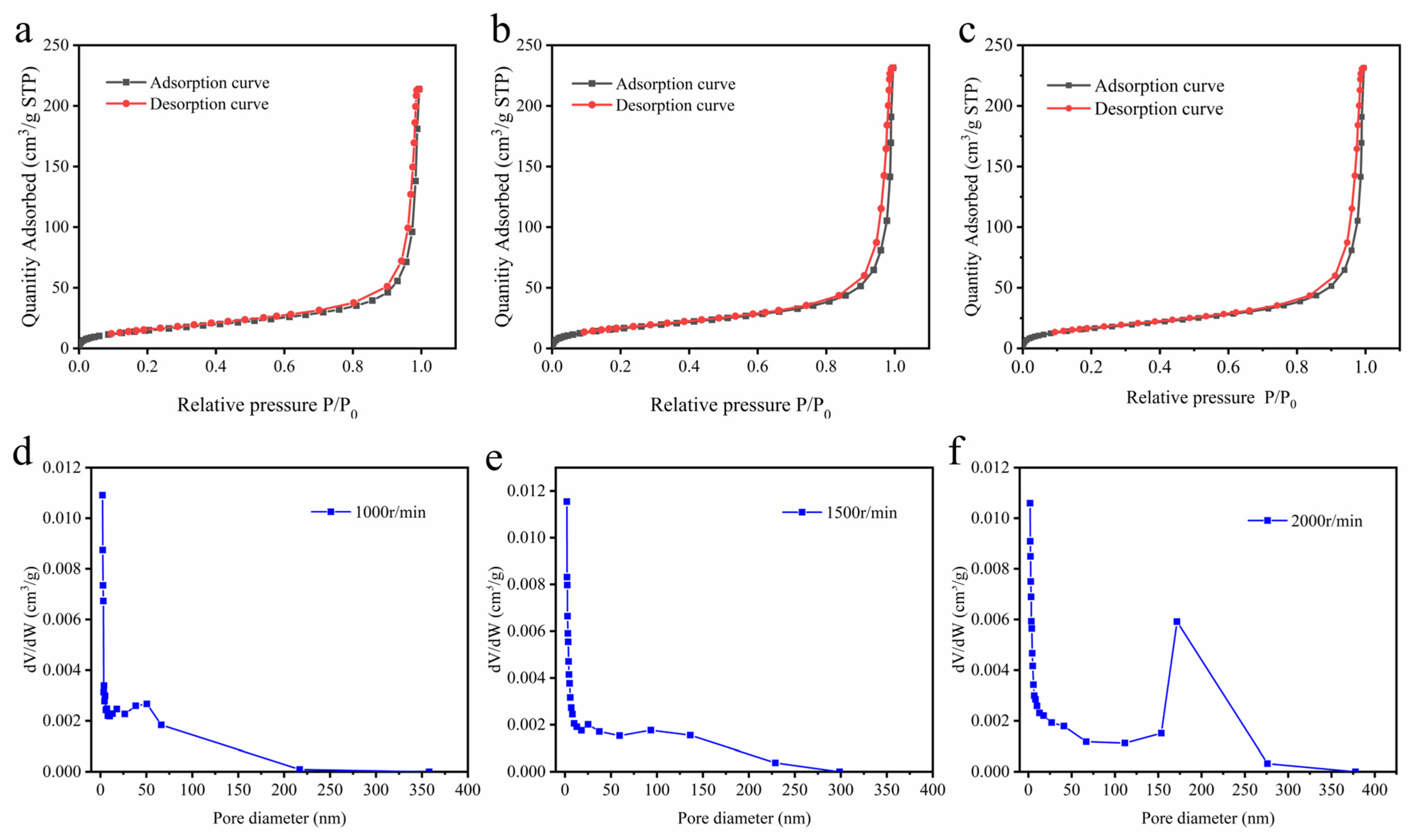
2.2. Surface Area and Porosity Analysis
2.3. X-ray Diffraction (XRD) Analysis
2.4. FTIR Analysis
2.5. Thermal Properties of Cellulose Acetate and Cellulose Acetate Hydrogel
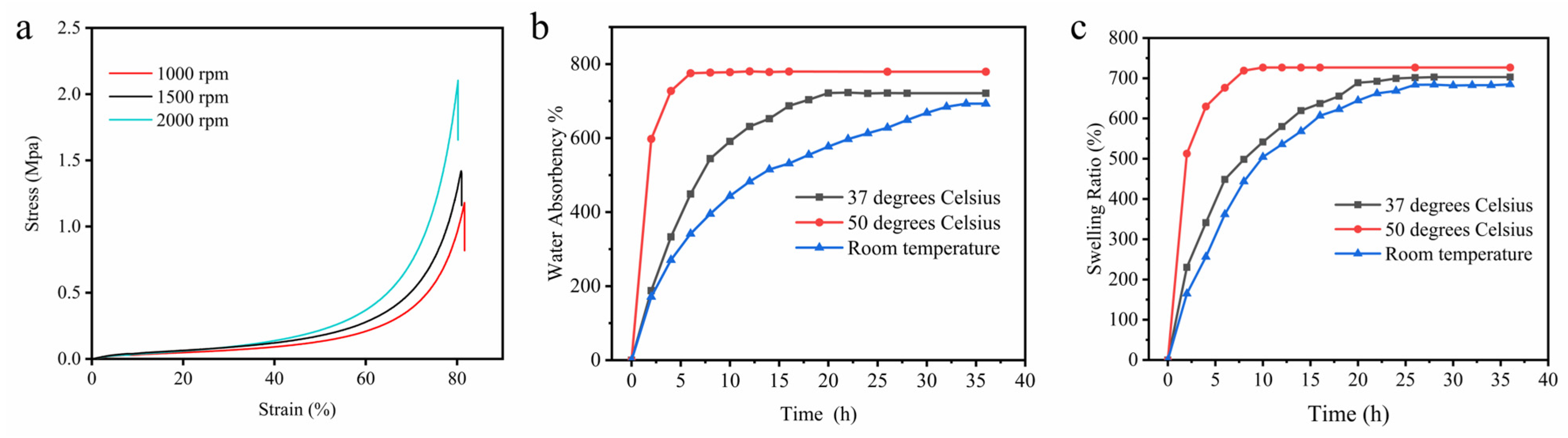
2.6. Compressive Strength of Hydrogel Scaffolds
2.7. Water Absorption and Swelling of Hydrogel Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cellulose Acetate Hydrogel
4.3. Scanning Electron Microscopy (SEM) Analysis
4.4. Specific Surface Area and Pore Size Distribution of Cellulose Acetate Hydrogel
4.5. XRD Analysis
4.6. Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
4.7. Thermogravimetric Analysis (TGA)
4.8. Mechanical Properties
4.9. Water Absorption, Swelling Studies of Hydrogel Scaffolds
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated Cellulose Nanofibers from Cellulose Acetate: Incorporating Hydroxyapatite (HAp) and Silver (Ag) Nanoparticles (NPs), as a Scaffold for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of High-Strength Microcrystalline Cellulose Hydrogel by Viscosity Adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels Based on Cellulose and Chitin: Fabrication, Properties, and Applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Senna, A.M.; Braga do Carmo, J.; Santana da Silva, J.M.; Botaro, V.R. Synthesis, Characterization and Application of Hydrogel Derived from Cellulose Acetate as a Substrate for Slow-Release NPK Fertilizer and Water Retention in Soil. J. Environ. Chem. Eng. 2015, 3, 996–1002. [Google Scholar] [CrossRef]
- Kang, H.-W.; Tabata, Y.; Ikada, Y. Fabrication of Porous Gelatin Sca! Olds for Tissue Engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels Prepared from Unsubstituted Cellulose in NaOH/Urea Aqueous Solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef]
- Nie, H.; Liu, M.; Zhan, F.; Guo, M. Factors on the Preparation of Carboxymethylcellulose Hydrogel and Its Degradation Behavior in Soil. Carbohydr. Polym. 2004, 58, 185–189. [Google Scholar] [CrossRef]
- Qiao, X.; Ni, S.; Lu, H.; Wang, X.; Zhou, X. A Novel Method to Prepare Chemical Fibers by Plasticizing Cotton with 1-Allyl-3-Methylimidazolium Chloride. Int. J. Biol. Macromol. 2021, 166, 1508–1512. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-Based Composite Scaffolds for Bone Tissue Engineering and Localized Drug Delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose Acetate Based 3-Dimensional Electrospun Scaffolds for Skin Tissue Engineering Applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef]
- Huang, H.; Dean, D. 3-D Printed Porous Cellulose Acetate Tissue Scaffolds for Additive Manufacturing. Addit. Manuf. 2020, 31, 100927. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A Review on the Properties of Electrospun Cellulose Acetate and Its Application in Drug Delivery Systems: A New Perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Appaw, C.; Gilbert, R.D.; Khan, S.A.; Kadla, J.F. Viscoelastic Behavior of Cellulose Acetate in a Mixed Solvent System. Biomacromolecules 2007, 8, 1541–1547. [Google Scholar] [CrossRef]
- Senna, A.M.; de Menezes, A.J.; Botaro, V.R. Estudo da densidade de ligações Cruzadas em géis superabsorventes obtidos do acetato de celulose. Polímeros 2012, 23, 59–64. [Google Scholar] [CrossRef]
- Yuan, W.; Wu, K.; Liu, N.; Zhang, Y.; Wang, H. Cellulose Acetate Fibers with Improved Mechanical Strength Prepared with Aqueous NMMO as Solvent. Cellulose 2018, 25, 6395–6404. [Google Scholar] [CrossRef]
- Wan, Y.; Lin, Z.; Gan, D.; Cui, T.; Wan, M.; Yao, F.; Zhang, Q.; Luo, H. Effect of Graphene Oxide Incorporation into Electrospun Cellulose Acetate Scaffolds on Breast Cancer Cell Culture. Fibers Polym. 2019, 20, 1577–1585. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.-G. Electrospun Triaxial Nanofibers with Middle Blank Cellulose Acetate Layers for Accurate Dual-Stage Drug Release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Oliveira, V.A.; Veloso, T.C.; Leão, V.A.; dos Santos, C.G.; Botaro, V.R. Hydrogels of Cellulose Acetate Crosslinked with Pyromellitic Dianhydride: Part I: Synthesis and Swelling Kinetics. Quím. Nova 2013, 36, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Vogrin, N.; Stropnik, Č.; Musil, V.; Brumen, M. The Wet Phase Separation: The Effect of Cast Solution Thickness on the Appearance of Macrovoids in the Membrane Forming Ternary Cellulose Acetate/Acetone/Water System. J. Membr. Sci. 2002, 207, 139–141. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, R.; Modrzejewska, Z.; Nawrotek, K. Drug release from hydrogel matrices. Ecol. Chem. Eng. S 2010, 17, 117–136. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, R.; Malaisamy, R.; Mohan, D.R. Cellulose Acetate and Polyethersulfone Blend Ultrafiltration Membranes. Part I: Preparation and Characterizations: Cellulose Acetate and Polyethersulfone Membranes. Polym. Adv. Technol. 2004, 15, 149–157. [Google Scholar] [CrossRef]
- Frey, M.W. Electrospinning Cellulose and Cellulose Derivatives. Polym. Rev. 2008, 48, 378–391. [Google Scholar] [CrossRef]
- Johnston, H.K.; Sourirajan, S. Viscosity–Temperature Relationships for Cellulose Acetate–Acetone Solutions. J. Appl. Polym. Sci. 1973, 17, 3717–3726. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Qing, Y.; Yan, N.; Wu, Y.; Li, X.; Tian, C. Mechanically Strong and Thermosensitive Hydrogels Reinforced with Cellulose Nanofibrils. Polym. Chem. 2016, 7, 7142–7151. [Google Scholar] [CrossRef]
- Tan, X.; Chen, L.; Li, X.; Xie, F. Effect of Anti-Solvents on the Characteristics of Regenerated Cellulose from 1-Ethyl-3-Methylimidazolium Acetate Ionic Liquid. Int. J. Biol. Macromol. 2019, 124, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K. A Critical Review of Analytical Methods in Pretreatment of Lignocelluloses: Composition, Imaging, and Crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [Green Version]
- Wan Daud, W.R.; Djuned, F.M. Cellulose Acetate from Oil Palm Empty Fruit Bunch via a One Step Heterogeneous Acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef]
- de Freitas, R.R.M.; Senna, A.M.; Botaro, V.R. Influence of Degree of Substitution on Thermal Dynamic Mechanical and Physicochemical Properties of Cellulose Acetate. Ind. Crops Prod. 2017, 109, 452–458. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, J.; Huang, J.; Yang, X.; Zhang, X.; Yuan, S.; Liao, W. Preparation and Characterization of Cellulose/Flaxseed Gum Composite Hydrogel and Its Hemostatic and Wound Healing Functions Evaluation. Cellulose 2020, 27, 3971–3988. [Google Scholar] [CrossRef]
- Ching, T.W.; Haritos, V.; Tanksale, A. Ultrasound-Assisted Conversion of Cellulose into Hydrogel and Functional Carbon Material. Cellulose 2018, 25, 2629–2645. [Google Scholar] [CrossRef]
- Senna, A.M.; Novack, K.M.; Botaro, V.R. Synthesis and Characterization of Hydrogels from Cellulose Acetate by Esterification Crosslinking with EDTA Dianhydride. Carbohydr. Polym. 2014, 114, 260–268. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.-W.; Lu, J.; Liu, Z.-T. Cellulose Triacetate Optical Film Preparation from Ramie Fiber. Ind. Eng. Chem. Res. 2009, 48, 6212–6215. [Google Scholar] [CrossRef]
- Hanna, A.A.; Basta, A.H.; El-Saied, H.; Abadir, I.F. Thermal Properties of Cellulose Acetate and Its Complexes with Some Transition Metals. Polym. Degrad. Stab. 1999, 63, 293–296. [Google Scholar] [CrossRef]
- Maria da Conceicao, C.L.; de Alencar, A.E.V.; Mazzeto, S.E.; de A Soares, S. The Effect of Additives on the Thermal Degradation of Cellulose Acetate. Polym. Degrad. Stab. 2003, 80, 149–155. [Google Scholar]
- Chen, J.; Xu, J.; Wang, K.; Cao, X.; Sun, R. Cellulose Acetate Fibers Prepared from Different Raw Materials with Rapid Synthesis Method. Carbohydr. Polym. 2016, 137, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Jandura, P.; Riedl, B.; Kokta, B.V. Thermal Degradation Behavior of Cellulose fibers Partially Esterified with Some Long Chain Organic Acids. Polym. Degrad. Stab. 2000, 70, 387–394. [Google Scholar] [CrossRef]



| Sample (rpm) | Diameter (nm) | Specific Surface Area (m2/g) |
|---|---|---|
| 1000 | 23.078 ± 1.121 | 56.035 ± 0.753 |
| 1500 | 24.651 ± 1.312 | 58.281 ± 0.647 |
| 2000 | 26.594 ± 1.034 | 62.021 ± 0.712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Huang, X.; Tian, C.; Zhong, Y.; Yan, M.; Miao, C.; Wu, T.; Zhou, X. Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels. Gels 2023, 9, 484. https://doi.org/10.3390/gels9060484
Jiang L, Huang X, Tian C, Zhong Y, Yan M, Miao C, Wu T, Zhou X. Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels. Gels. 2023; 9(6):484. https://doi.org/10.3390/gels9060484
Chicago/Turabian StyleJiang, Lijie, Xingyu Huang, Chaochao Tian, Yidan Zhong, Ming Yan, Chen Miao, Ting Wu, and Xiaofan Zhou. 2023. "Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels" Gels 9, no. 6: 484. https://doi.org/10.3390/gels9060484





