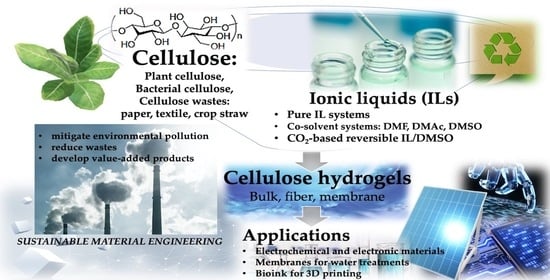
Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes
Abstract
1. Introduction
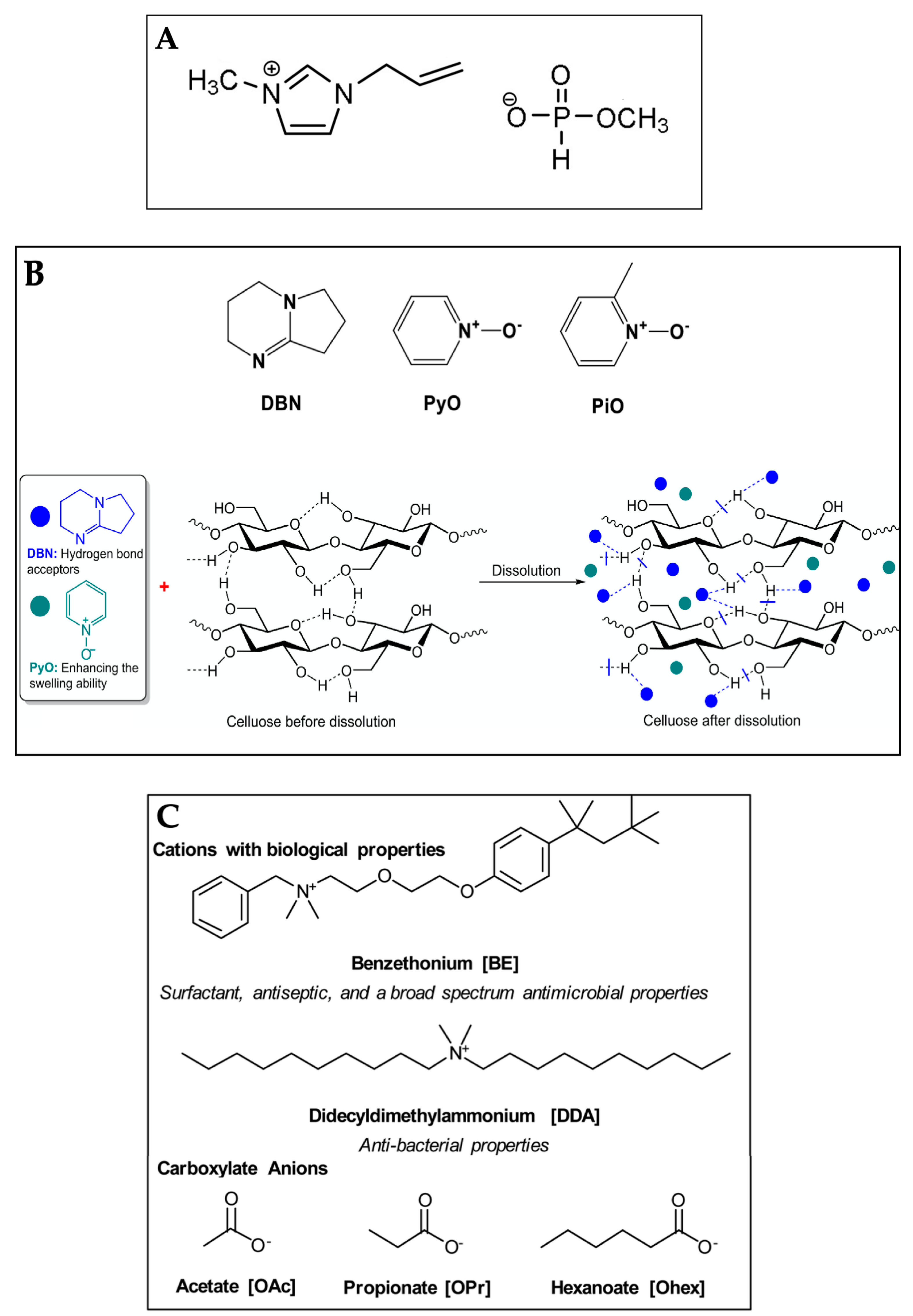
2. Ionic Liquids Used in Dissolution of Cellulose
2.1. Pure Ionic Liquid Systems
2.2. Ionic Liquid–Cosolvent Systems
3. Regeneration of Cellulose in Antisolvents
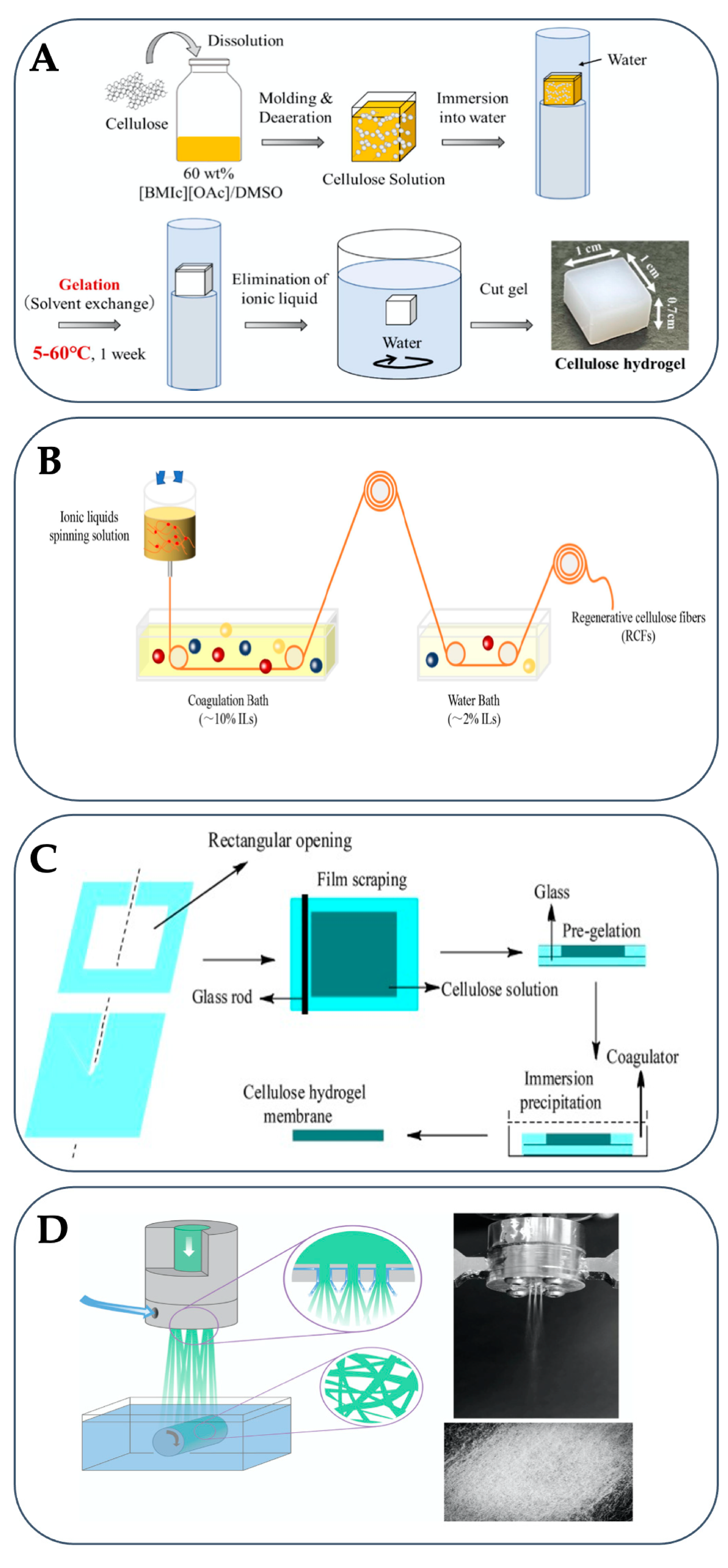
4. Applications
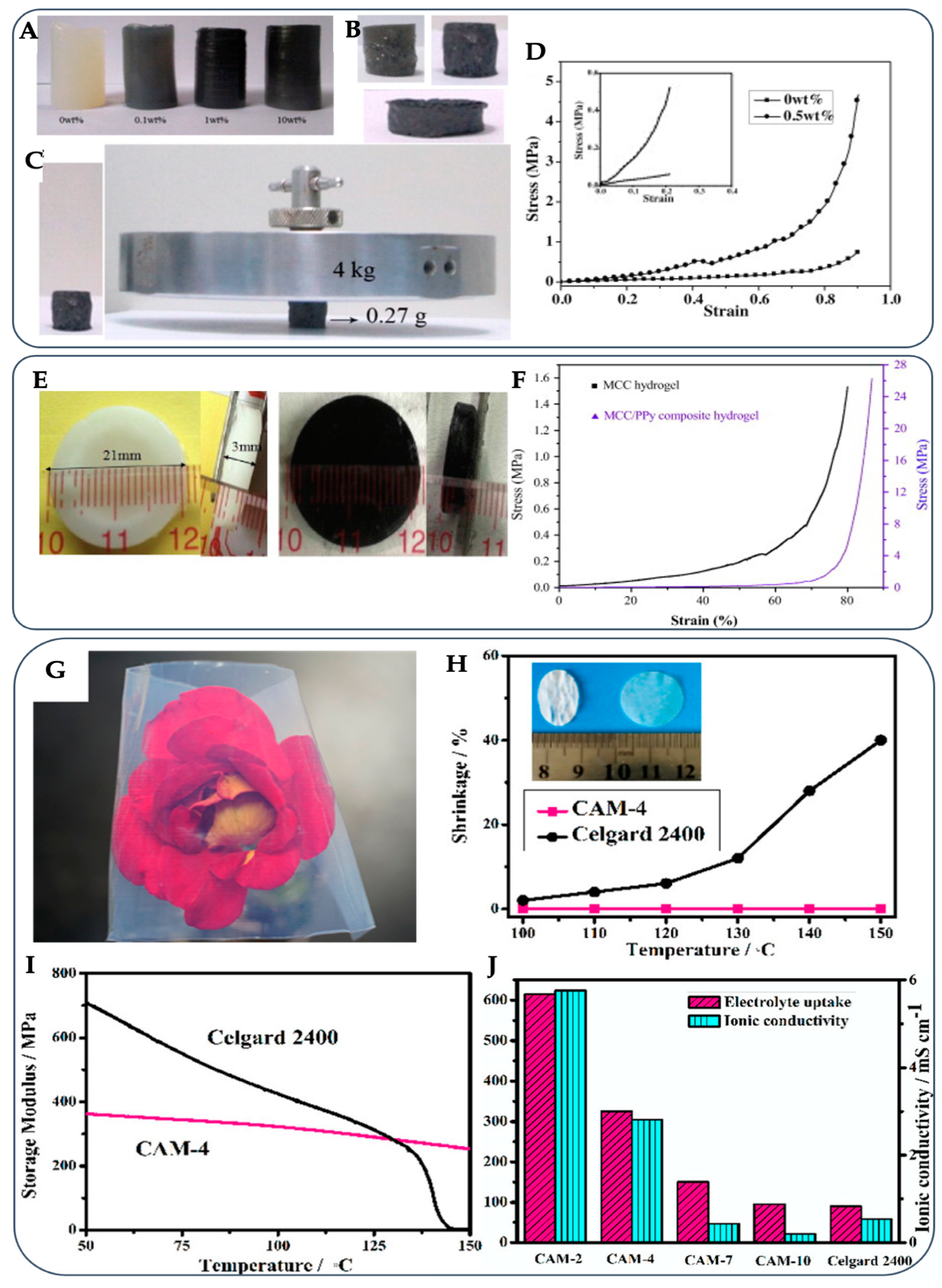
4.1. Electrochemical and Electronic Materials
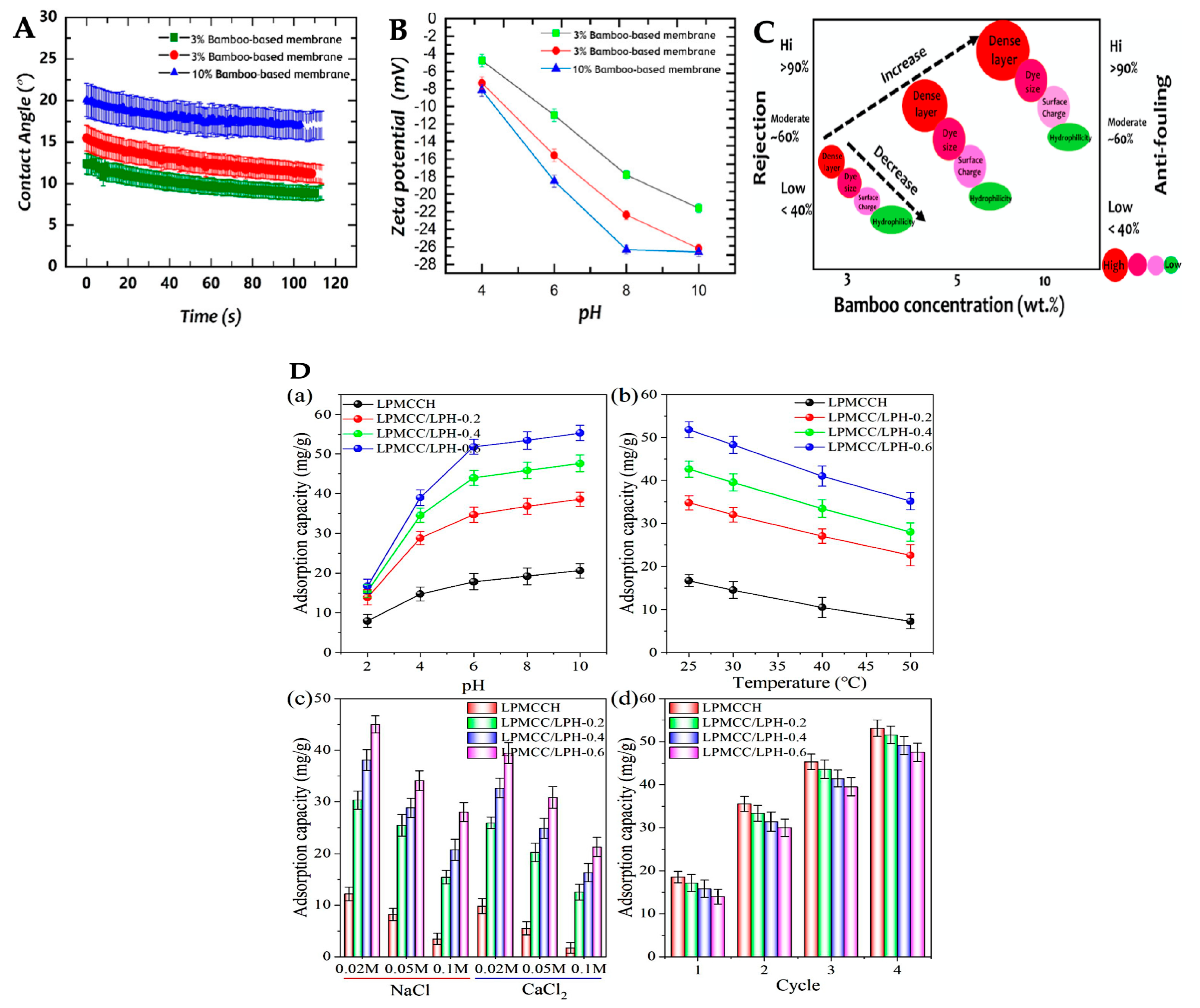
4.2. Membranes for Water Treatments
4.3. Bioink for 3D Printing
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Yeboah, W.O.; Kwofie, E.M.; Wang, D. Circular bioeconomy potential of rice husk as a bioplastic resource: Techno-environmental assessment. Bioresour. Technol. Rep. 2022, 20, 101248. [Google Scholar] [CrossRef]
- Ahrens, T.; Drescher-Hartung, S.; Anne, O. Sustainability of future bioenergy production. Waste Manag. 2017, 67, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Wang, Y.; Xiao, S.; Gao, R.; Shang, Y.; Xie, Y.; Liu, J.; Li, J. Magnetically Driven 3D Cellulose Film for Improved Energy Efficiency in Solar Evaporation. ACS Appl. Mater. Interfaces 2021, 13, 7756–7765. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Hao, N.; Zhang, K.; Xing, C.; Wang, S. Cellulose nanofibrils-based thermally conductive composites for flexible electronics: A mini review. Cellulose 2020, 27, 4173–4187. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crops Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Taokaew, S.; Nakson, N.; Zhang, X.; Kongklieng, P.; Kobayashi, T. Biotransformation of okara extracted protein to nanocellulose and chitin by Gluconacetobacter xylinus and Bacillus pumilus. Bioresour. Technol. Rep. 2022, 17, 100904. [Google Scholar] [CrossRef]
- Taokaew, S.; Nakson, N.; Thienchaimongkol, J.; Kobayashi, T. Enhanced production of fibrous bacterial cellulose in Gluconacetobacter xylinus culture medium containing modified protein of okara waste. J. Biosci. Bioeng. 2023, 135, 71–78. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Wang, L.; Huang, S.; Wang, Y. Recycling of Waste Cotton Textile Containing Elastane Fibers through Dissolution and Regeneration. Membranes 2022, 12, 355. [Google Scholar] [CrossRef]
- Zhong, X.; Li, R.; Wang, Z.; Wang, Y.; Wang, W.; Yu, D. Highly flexible, transparent film prepared by upcycle of wasted jute fabrics with functional properties. Process Saf. Environ. Prot. 2021, 146, 718–725. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, B.; Wang, X.; Byrne, N. Circular Textiles: Closed Loop Fiber to Fiber Wet Spun Process for Recycling Cotton from Denim. ACS Sustain. Chem. Eng. 2019, 7, 11937–11943. [Google Scholar] [CrossRef]
- Selvakumaran, N.; Lazim, A. Superabsorbent hydrogel from extracted oil palm frond waste cellulose using microwave irradiation for cadmium ion removal from aqueous solution. Chem. Chem. Technol. 2019, 13, 518–525. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Bian, F. Utilization of discarded crop straw to produce cellulose nanofibrils and their assemblies. J. Bioresour. Bioprod. 2020, 5, 26–36. [Google Scholar] [CrossRef]
- Su, R.; Wang, F.; Ding, J.; Li, Q.; Zhou, W.; Liu, Y.; Gao, B.; Yue, Q. Magnetic hydrogel derived from wheat straw cellulose/feather protein in ionic liquids as copper nanoparticles carrier for catalytic reduction. Carbohydr. Polym. 2019, 220, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef]
- Wang, H.; Qian, H.; Luo, Z.; Zhang, K.; Shen, X.; Zhang, Y.; Zhang, M.; Liebner, F. ZCIS/ZnS QDs fluorescent aerogels with tunable emission prepared from porous 3D nanofibrillar bacterial cellulose. Carbohydr. Polym. 2019, 224, 115173. [Google Scholar] [CrossRef]
- Afsahi, G.; Dimic-Misic, K.; Gane, P.; Budtova, T.; Maloney, T.; Vuorinen, T. The investigation of rheological and strength properties of NFC hydrogels and aerogels from hardwood pulp by short catalytic bleaching (Hcat). Cellulose 2018, 25, 1637–1655. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; Li, L.; He, M.; Li, Y.; Teng, F. Fabrication of composite hydrogels by assembly of okara cellulose nanofibers and gum Arabic in ionic liquids: Structure and properties. J. Mol. Liq. 2022, 349, 118132. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Tian, W.; Gao, X.; Zhang, J.; Yu, J.; Zhang, J. Cellulose nanosphere: Preparation and applications of the novel nanocellulose. Carbohydr. Polym. 2022, 277, 118863. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-H.; Pham, T.P.T.; Yun, Y.-S. Ionic liquid-assisted cellulose coating of chitosan hydrogel beads and their application as drug carriers. Sci. Rep. 2020, 10, 13905. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.M.; Kafle, K. Characterization of crystalline cellulose in biomass: Basic principles, applications, and limitations of XRD, NMR, IR, Raman, and SFG. Korean J. Chem. Eng. 2013, 30, 2127–2141. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Wu, F.; Cao, R. Deep oxidative desulfurization of fuels based on [C4mimCl]CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Han, Q.; Gao, X.; Zhang, H.; Chen, K.; Peng, L.; Jia, Q. Preparation and comparative assessment of regenerated cellulose films from corn (Zea mays) stalk pulp fines in DMAc/LiCl solution. Carbohydr. Polym. 2019, 218, 315–323. [Google Scholar] [CrossRef]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef]
- From, M.; Larsson, P.T.; Andreasson, B.; Medronho, B.; Svanedal, I.; Edlund, H.; Norgren, M. Tuning the properties of regenerated cellulose: Effects of polarity and water solubility of the coagulation medium. Carbohydr. Polym. 2020, 236, 116068. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, K.; Zhou, X.; Luo, L.; Zeng, J.; Huang, R.; Lu, A.; Liu, X.; Chen, F.; Zhang, L.; et al. Influences of Coagulation Conditions on the Structure and Properties of Regenerated Cellulose Filaments via Wet-Spinning in LiOH/Urea Solvent. ACS Sustain. Chem. Eng. 2018, 6, 4056–4067. [Google Scholar] [CrossRef]
- Peng, J.; Li, Y.; Liu, X.; Ke, G.; Song, D.; Wu, S.; Xu, W.; Zhu, K. Cellulose film with air barrier and moisture-conducting character fabricated by NMMO. J. Mater. Sci. 2021, 56, 18313–18326. [Google Scholar] [CrossRef]
- Zhang, J.; Tominaga, K.; Yamagishi, N.; Gotoh, Y. Comparison of Regenerated Cellulose Fibers Spun from Ionic Liquid Solutions with Lyocell Fiber. J. Fiber Sci. Technol. 2020, 76, 257–266. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Yadykova, A.Y.; Ilyin, S.O. A novel method for producing cellulose nanoparticles and their possible application as thickeners for biodegradable low-temperature greases. Cellulose 2021, 28, 10203–10219. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, M.; Li, S.; Guan, Z.; Xia, Y.; Yang, J.; Lin, X. Room temperature preparation of cellulose nanocrystals with high yield via a new ZnCl2 solvent system. Carbohydr. Polym. 2022, 278, 118946. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Meng, J.; Liu, S.; Liu, Y.; Zeng, S.; Wang, L.; Xia, Q.; Yu, H. Room temperature dissolving cellulose with a metal salt hydrate-based deep eutectic solvent. Carbohydr. Polym. 2021, 272, 118473. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.-F.; Wang, Z.; Yao, J. Structure reorganization of cellulose hydrogel by green solvent exchange for potential plastic replacement. Carbohydr. Polym. 2022, 275, 118695. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic Salts Induce Thermally Reversible and Anti-Freezing Cellulose Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Shen, C.; Hu, C.; Zhang, W.; Lin, X.; Qi, W.; Zhang, Z.; Gu, J. Acidified ZnCl2 molten salt hydrate systems as hydrolytic media for cellulose I and II nanocrystal production: From rods to spheres. Cellulose 2022, 29, 7629–7647. [Google Scholar] [CrossRef]
- Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. Inorganic Molten Salts as Solvents for Cellulose. Cellulose 2003, 10, 227–236. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Wu, J.; Huang, W.; Li, Y.; Mao, Y.; Wang, L.; Wang, Y. Cellulose pretreatment with inorganic salt hydrate: Dissolution, regeneration, structure and morphology. Ind. Crops Prod. 2022, 180, 114722. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.; Feng, X.; Wu, M.; Tang, Y.; Li, B. Effect of regeneration solvent on the characteristics of regenerated cellulose from lithium bromide trihydrate molten salt. Cellulose 2020, 27, 9243–9256. [Google Scholar] [CrossRef]
- Hattori, K.; Arai, A. Preparation and Hydrolysis of Water-Stable Amorphous Cellulose. ACS Sustain. Chem. Eng. 2016, 4, 1180–1186. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Zhang, J.; Cheng, Y.; Wu, J.; Yu, J.; Zhang, J. Molecular weight characterization of cellulose using ionic liquids. Polym. Test. 2021, 93, 106985. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of Cellulose Non-Derivatizing Solvent Interactions with Emphasis on Activity in Inorganic Molten Salt Hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Wang, C.; Lu, Y.; Dong, K.; Huo, F.; Zhang, S. Insights into Ionic Liquids: From Z-Bonds to Quasi-Liquids. JACS Au 2022, 2, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Hopson, C.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodríguez, F. A new approach for the use of cellulose-rich solids from biorefinery in the formulation of gel-like materials. Ind. Crops Prod. 2022, 186, 115230. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Yu, J.; Wu, J.; Zhang, X.; He, J.; Zhang, J. Understanding cellulose dissolution: Effect of the cation and anion structure of ionic liquids on the solubility of cellulose. Sci. China Chem. 2016, 59, 1421–1429. [Google Scholar] [CrossRef]
- Paiva, T.; Echeverria, C.; Godinho, M.H.; Almeida, P.L.; Corvo, M.C. On the influence of imidazolium ionic liquids on cellulose derived polymers. Eur. Polym. J. 2019, 114, 353–360. [Google Scholar] [CrossRef]
- Jadhav, S.; Ganvir, V.; Singh, M.K.; Shanmuganathan, K. Synthesis of N-oxyethylene substituted imidazolium-based zwitterions as a recyclable solvent for cellulose dissolution. Cellulose 2023, 30, 87–109. [Google Scholar] [CrossRef]
- Morita, T.; Okada, H.; Yamada, T.; Hidaka, R.; Ueki, T.; Niitsuma, K.; Kitazawa, Y.; Watanabe, M.; Nishikawa, K.; Higashi, K. A study combining magic-angle spinning NMR and small-angle X-ray scattering on the interaction in the mixture of poly(benzyl methacrylate) and ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide. Phys. Chem. Chem. Phys. 2022, 24, 26575–26582. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Thomsen, K.; Nie, Y.; Zhang, S.-J.; Meyer, A.S. Predictive screening of ionic liquids for dissolving cellulose and experimental verification. Green Chem. 2016, 18, 6246–6254. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E. Effect of Oil on Cellulose Dissolution in the Ionic Liquid 1-Butyl-3-methyl Imidazolium Acetate. ACS Omega 2022, 7, 37532–37545. [Google Scholar] [CrossRef]
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef]
- Haq, M.A.; Habu, Y.; Yamamoto, K.; Takada, A.; Kadokawa, J.-I. Ionic liquid induces flexibility and thermoplasticity in cellulose film. Carbohydr. Polym. 2019, 223, 115058. [Google Scholar] [CrossRef] [PubMed]
- Idenoue, S.; Oga, Y.; Hashimoto, D.; Yamamoto, K.; Kadokawa, J.I. Preparation of Reswellable Amorphous Porous Celluloses through Hydrogelation from Ionic Liquid Solutions. Materials 2019, 12, 3249. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Zhang, J.; Dang, B.; Li, Y. Green fabrication of an ionic liquid-activated lignocellulose flame-retardant composite. Ind. Crops Prod. 2022, 178, 114602. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, Q.; Liu, P.; Zhu, R.; Lu, F.; Ramaswamy, S.; Wu, Y.; Xu, F.; Zhang, X. Fabrication of Novel Cellulose-Based Antibacterial Film Loaded with Poacic Acid against Staphylococcus Aureus. J. Polym. Environ. 2021, 29, 745–754. [Google Scholar] [CrossRef]
- Spörl, J.M.; Batti, F.; Vocht, M.-P.; Raab, R.; Müller, A.; Hermanutz, F.; Buchmeiser, M.R. Ionic Liquid Approach Toward Manufacture and Full Recycling of All-Cellulose Composites. Macromol. Mater. Eng. 2018, 303, 1700335. [Google Scholar] [CrossRef]
- Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid. Polymers 2020, 12, 1010. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Mathew, A.P.; Oksman, K. All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride. Compos. Part A Appl. Sci. Manuf. 2009, 40, 2031–2037. [Google Scholar] [CrossRef]
- Khakalo, A.; Tanaka, A.; Korpela, A.; Hauru, L.K.J.; Orelma, H. All-Wood Composite Material by Partial Fiber Surface Dissolution with an Ionic Liquid. ACS Sustain. Chem. Eng. 2019, 7, 3195–3202. [Google Scholar] [CrossRef]
- Vinogradova, Y.S.; Chen, J.Y. Micron- and nano-cellulose fiber regenerated from ionic liquids. J. Text. Inst. 2016, 107, 472–476. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, Y.; Huang, W.; Gong, P.; Peng, S.; He, J.; Fan, M.; Wang, K. An insight into the influence of hydrogen bond acceptors on cellulose/1-allyl-3-methyl imidazolium chloride solution. Carbohydr. Polym. 2017, 178, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pan, F.; Liu, Y.; Kang, Z.; Zeng, S.; Nie, Y. Study on the regularity of cellulose degradation in ionic liquids. J. Mol. Liq. 2020, 308, 113153. [Google Scholar] [CrossRef]
- Xu, K.; Xiao, Y.; Cao, Y.; Peng, S.; Fan, M.; Wang, K. Dissolution of cellulose in 1-allyl-3-methylimidazolium methyl phosphonate ionic liquid and its composite system with Na2PHO3. Carbohydr. Polym. 2019, 209, 382–388. [Google Scholar] [CrossRef]
- Fumino, K.; Peppel, T.; Geppert-Rybczyńska, M.; Zaitsau, D.H.; Lehmann, J.K.; Verevkin, S.P.; Köckerling, M.; Ludwig, R. The influence of hydrogen bonding on the physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 14064–14075. [Google Scholar] [CrossRef] [PubMed]
- Zweckmair, T.; Hettegger, H.; Abushammala, H.; Bacher, M.; Potthast, A.; Laborie, M.-P.; Rosenau, T. On the mechanism of the unwanted acetylation of polysaccharides by 1,3-dialkylimidazolium acetate ionic liquids: Part 1—Analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 2015, 22, 3583–3596. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ling, Z.; Xu, D.; You, T.; Wu, Y.-Y.; Xu, F. Room-Temperature Superbase-Derived Ionic Liquids with Facile Synthesis and Low Viscosity: Powerful Solvents for Cellulose Dissolution by Destroying the Cellulose Aggregate Structure. Macromolecules 2020, 53, 3284–3295. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W.; Guo, Z.; Nawaz, H.; You, T.; Li, X.; Xu, F. Rheological characteristics of novel cellulose/superbase-derived ionic liquid solutions and the coagulation process towards regenerated cellulose films. Green Chem. 2023, 25, 1597–1610. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Nawaz, H.; Zhang, J.; Chen, X.; Cheng, P.; You, T.; Ramaswamy, S.; Xu, F. The choice of ionic liquid ions to mitigate corrosion impacts: The influence of superbase cations and electron-donating carboxylate anions. Green Chem. 2022, 24, 2114–2128. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Mu, T.; Xue, Z.; Xu, F. Robust superbase-based emerging solvents for highly efficient dissolution of cellulose. Carbohydr. Polym. 2021, 272, 118454. [Google Scholar] [CrossRef] [PubMed]
- Galamba, J.; Alves, V.D.; Jordão, N.; Neves, L.A. Development of cellulose-based polymeric structures using dual functional ionic liquids. RSC Adv. 2021, 11, 39278–39286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Insight into the Cosolvent Effect of Cellulose Dissolution in Imidazolium-Based Ionic Liquid Systems. J. Phys. Chem. B 2013, 117, 9042–9049. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhang, Y.; Zhao, Y.; Wang, J. Cellulose dissolution at ambient temperature: Role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr. Polym. 2013, 92, 540–544. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Yin, D.; Zhang, J.; Mi, Q.; Lu, H.; Liang, D.; Zhang, J. The solution state and dissolution process of cellulose in ionic-liquid-based solvents with different hydrogen-bonding basicity and microstructures. Green Chem. 2022, 24, 3824–3833. [Google Scholar] [CrossRef]
- Sánchez, P.B.; Tsubaki, S.; Pádua, A.A.H.; Wada, Y. Kinetic analysis of microwave-enhanced cellulose dissolution in ionic solvents. Phys. Chem. Chem. Phys. 2020, 22, 1003–1010. [Google Scholar] [CrossRef]
- Hawkins, J.E.; Liang, Y.; Ries, M.E.; Hine, P.J. Time temperature superposition of the dissolution of cellulose fibres by the ionic liquid 1-ethyl-3-methylimidazolium acetate with cosolvent dimethyl sulfoxide. Carbohydr. Polym. Technol. Appl. 2021, 2, 100021. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Jamil, S.N.A.M. Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review. e-Polymers 2021, 21, 869–880. [Google Scholar] [CrossRef]
- Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Swelling and dissolution kinetics of natural and man-made cellulose fibers in solvent power tuned ionic liquid. Cellulose 2020, 27, 7399–7415. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Oliveira, M.L.; Bioni, T.A.; Nawaz, H.; King, A.W.T.; Kilpeläinen, I.; Hummel, M.; Sixta, H.; El Seoud, O.A. Binary mixtures of ionic liquids-DMSO as solvents for the dissolution and derivatization of cellulose: Effects of alkyl and alkoxy side chains. Carbohydr. Polym. 2019, 212, 206–214. [Google Scholar] [CrossRef]
- Wittmar, A.S.M.; Koch, D.; Prymak, O.; Ulbricht, M. Factors Affecting the Nonsolvent-Induced Phase Separation of Cellulose from Ionic Liquid-Based Solutions. ACS Omega 2020, 5, 27314–27322. [Google Scholar] [CrossRef]
- Satani, H.; Kuwata, M.; Shimizu, A. Simple and environmentally friendly preparation of cellulose hydrogels using an ionic liquid. Carbohydr. Res. 2020, 494, 108054. [Google Scholar] [CrossRef]
- Kikuchi, K.; Kaneko, K.; Seonju, J.; Fukaya, R.; Yamada, M.; Ishii, H.; Inoue, T.; Shimizu, A. Influence of gelation temperature on physicochemical properties of cellulose hydrogels prepared from ionic liquid/DMSO solution. J. Mol. Liq. 2023, 376, 121465. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kostyuk, A.V.; Anokhina, T.S.; Melekhina, V.Y.; Bakhtin, D.S.; Antonov, S.V.; Volkov, A.V. The Effect of Non-Solvent Nature on the Rheological Properties of Cellulose Solution in Diluted Ionic Liquid and Performance of Nanofiltration Membranes. Int. J. Mol. Sci. 2023, 24, 8057. [Google Scholar] [CrossRef]
- Layek, S.; Banerjee, P.; Sarkar, N. An insight into the dissolution of cellulose in 1-butyl-3-methylimidazolium chloride-DMSO binary Mixture: Exploring the dynamics of rhodamine 6G and fluorescein. J. Mol. Liq. 2021, 339, 116817. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, S.; Carvalho, D.; Fu, J.; Martins, M.; Cavaco-Paulo, A. Cellulose Dissolved in Ionic Liquids for Modification of the Shape of Keratin Fibers. ACS Sustain. Chem. Eng. 2021, 9, 4102–4110. [Google Scholar] [CrossRef]
- Müller, K.; Zollfrank, C. Ionic liquid aided solution-precipitation method to prepare polymer blends from cellulose with polyesters or polyamide. Eur. Polym. J. 2020, 133, 109743. [Google Scholar] [CrossRef]
- Lei, L.; Lindbråthen, A.; Sandru, M.; Gutierrez, M.T.; Zhang, X.; Hillestad, M.; He, X. Spinning Cellulose Hollow Fibers Using 1-Ethyl-3-methylimidazolium Acetate–Dimethylsulfoxide Co-Solvent. Polymers 2018, 10, 972. [Google Scholar] [CrossRef]
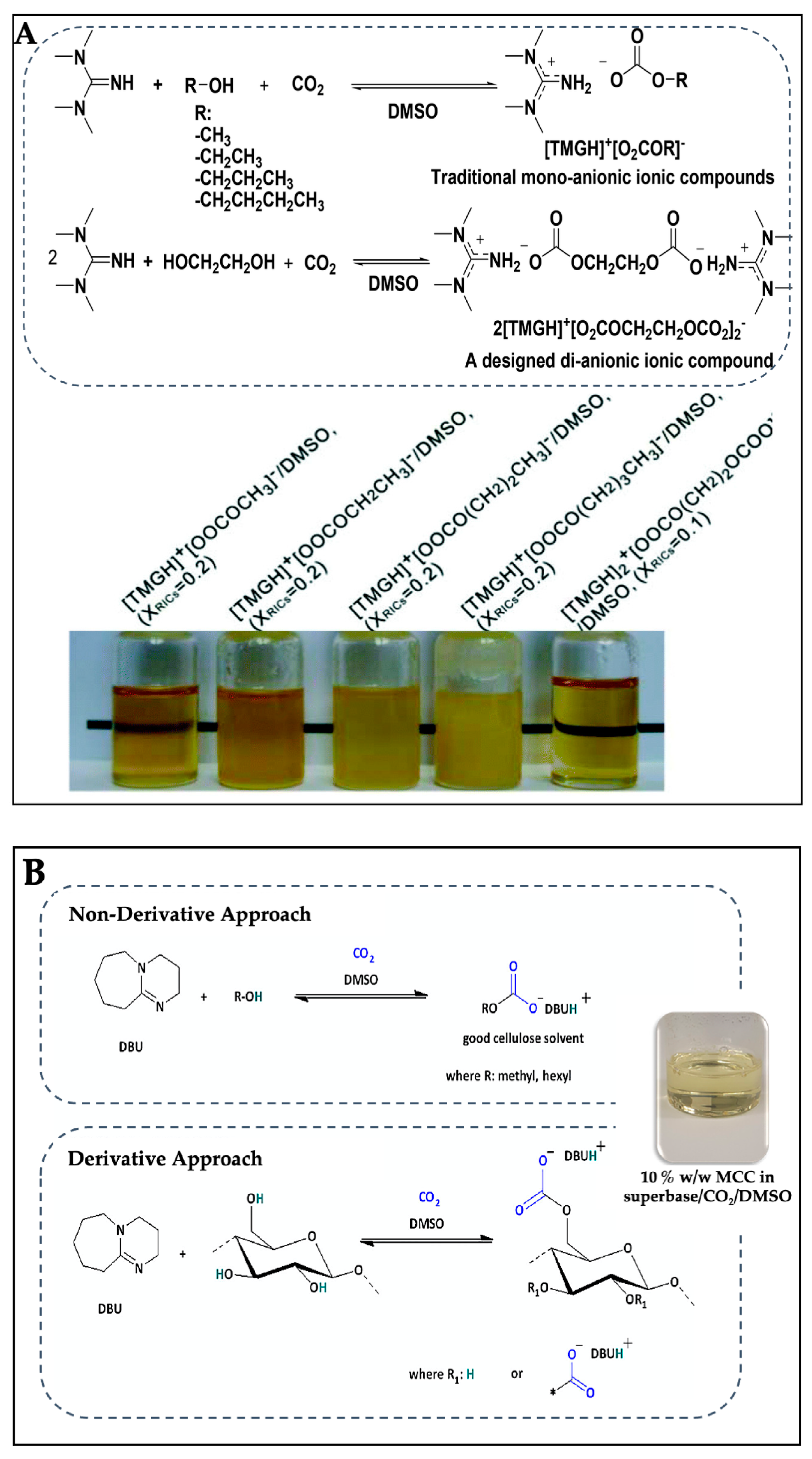
- Xie, H.; Yu, X.; Yang, Y.; Zhao, Z.K. Capturing CO2 for cellulose dissolution. Green Chem. 2014, 16, 2422–2427. [Google Scholar] [CrossRef]
- Jin, L.; Gan, J.; Hu, G.; Cai, L.; Li, Z.; Zhang, L.; Zheng, Q.; Xie, H. Preparation of Cellulose Films from Sustainable CO2/DBU/DMSO System. Polymers 2019, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, W.; Sheng, H.; Feng, S.; Yao, M.; Chen, P.; Zheng, Q.; Xie, H. Unique CO2-switched cellulose solution properties in the CO2/DBU/DMSO solvent system and the preparation of regenerated materials. Green Chem. 2021, 23, 5856–5865. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Peng, C.; Liu, E.; Xie, H. Activating cellulose via its reversible reaction with CO2 in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene for the efficient synthesis of cellulose acetate. Green Chem. 2015, 17, 2758–2763. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Lapuyade, L.; Maillé, L.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Sustainable Approach for Cellulose Aerogel Preparation from the DBU–CO2 Switchable Solvent. ACS Sustain. Chem. Eng. 2019, 7, 3329–3338. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, J.; Jin, L.; Hu, G.; Zheng, Q.; Xie, H.; Chen, P. Unique gelation and rheological properties of the cellulose/CO2-based reversible ionic liquid/DMSO solutions. Carbohydr. Polym. 2019, 222, 115024. [Google Scholar] [CrossRef]
- Zhang, Q.; Oztekin, N.S.; Barrault, J.; De Oliveira Vigier, K.; Jérôme, F. Activation of Microcrystalline Cellulose in a CO2-Based Switchable System. ChemSusChem 2013, 6, 593–596. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Tassaing, T.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Detailed Understanding of the DBU/CO2 Switchable Solvent System for Cellulose Solubilization and Derivatization. ACS Sustain. Chem. Eng. 2018, 6, 1496–1503. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Z.; Yan, C.; Li, Z.; Mu, T. Fine regulation of cellulose dissolution and regeneration by low pressure CO2 in DMSO/organic base: Dissolution behavior and mechanism. Phys. Chem. Chem. Phys. 2016, 18, 32772–32779. [Google Scholar] [CrossRef]
- Nanta, P.; Skolpap, W.; Kasemwong, K.; Shimoyama, Y. Dissolution and modification of cellulose using high-pressure carbon dioxide switchable solution. J. Supercrit. Fluids 2017, 130, 84–90. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Liu, F.; Qiao, Q.; Na, H.; Zhu, J. Structure and Properties of Regenerated Cellulose Fibers Based on Dissolution of Cellulose in a CO2 Switchable Solvent. ACS Sustain. Chem. Eng. 2021, 9, 4744–4754. [Google Scholar] [CrossRef]
- Xu, Q.; Song, L.; Zhang, L.; Hu, G.; Du, J.; Liu, E.; Zheng, Q.; Liu, Y.; Li, N.; Xie, H. Organocatalytic Cellulose Dissolution and In Situ Grafting of ϵ-Caprolactone via ROP in a Reversible DBU/DMSO/CO2 System. ChemistrySelect 2017, 2, 7128–7134. [Google Scholar] [CrossRef]
- Wang, C.-G.; Li, N.; Wu, G.; Lin, T.T.; Lee, A.M.X.; Yang, S.-W.; Li, Z.; Luo, D.H.-K. Carbon Dioxide Mediated Cellulose Dissolution and Derivatization to Cellulose Carbonates in a Low-pressure System. Carbohydr. Polym. Technol. Appl. 2022, 3, 100186. [Google Scholar] [CrossRef]
- Zhang, J.; Mi, S.; Liu, F.; Qiao, Q.; Na, H.; Zhu, J. Enhanced dissolution of cellulose in CO2 switchable system by solvent with low Henry’s constants as CO2 absorbent. Cellulose 2022, 29, 6745–6758. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Molecular insight into cellulose regeneration from a cellulose/ionic liquid mixture: Effects of water concentration and temperature. RSC Adv. 2013, 3, 4425–4433. [Google Scholar] [CrossRef]
- Olsson, C.; Idström, A.; Nordstierna, L.; Westman, G. Influence of water on swelling and dissolution of cellulose in 1-ethyl-3-methylimidazolium acetate. Carbohydr. Polym. 2014, 99, 438–446. [Google Scholar] [CrossRef]
- Berton, P.; Shen, X.; Rogers, R.D.; Shamshina, J.L. 110th Anniversary: High-Molecular-Weight Chitin and Cellulose Hydrogels from Biomass in Ionic Liquids without Chemical Crosslinking. Ind. Eng. Chem. Res. 2019, 58, 19862–19876. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Anti-solvents tuning cellulose nanoparticles through two competitive regeneration routes. Cellulose 2018, 25, 4513–4523. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Crystallinity of regenerated cellulose from [Bmim]Cl dependent on the hydrogen bond acidity/basicity of anti-solvents. RSC Adv. 2017, 7, 41004–41010. [Google Scholar] [CrossRef]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 9593–9603. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Xu, Y.; Zhang, H.; Cai, R.; Guo, Z.; Zhou, L.; Zhang, J.; Yuan, Y. Mechanism of cellulose regeneration from its ionic liquid solution as revealed by infrared spectroscopy. Polymer 2022, 257, 125280. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Shen, F.; Wang, D.; Zhang, S. Efficient removal of metal ions from the ionic liquid aqueous solution by selective electrodialysis. Sep. Purif. Technol. 2022, 295, 121322. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.; Xu, H.; Dai, G. Preparations, properties, and formation mechanism of novel cellulose hydrogel membrane based on ionic liquid. J. Appl. Polym. Sci. 2018, 135, 45488. [Google Scholar] [CrossRef]
- Jedvert, K.; Idström, A.; Köhnke, T.; Alkhagen, M. Cellulosic nonwovens produced via efficient solution blowing technique. J. Appl. Polym. Sci. 2020, 137, 48339. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Sun, S.; Zhou, Q. Surfactant-assisted fabrication of ultra-permeable cellulose gels with macro channels and insights on regeneration of cellulose from ionic liquids. J. Mol. Liq. 2019, 280, 64–70. [Google Scholar] [CrossRef]
- Heasman, P.; Mehandzhiyski, A.Y.; Ghosh, S.; Zozoulenko, I. A computational study of cellulose regeneration: All-atom molecular dynamics simulations. Carbohydr. Polym. 2023, 311, 120768. [Google Scholar] [CrossRef]
- Pang, J.; Mehandzhiyski, A.Y.; Zozoulenko, I. A computational study of cellulose regeneration: Coarse-grained molecular dynamics simulations. Carbohydr. Polym. 2023, 313, 120853. [Google Scholar] [CrossRef]
- Ding, Z.-D.; Chi, Z.; Gu, W.-X.; Gu, S.-M.; Liu, J.-H.; Wang, H.-J. Theoretical and experimental investigation on dissolution and regeneration of cellulose in ionic liquid. Carbohydr. Polym. 2012, 89, 7–16. [Google Scholar] [CrossRef]
- Fu, L.; Ju, Z.; Yu, M.; Luo, H.; Zhang, C.; Zhang, X.; Cheng, H.; Zheng, M.; Jin, L.; Ge, C. Cellulose Regeneration in Imidazolium-Based Ionic Liquids and Antisolvent Mixtures: A Density Functional Theory Study. ACS Omega 2022, 7, 42170–42180. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.Y.; Jiang, W.; Lynch, V. Crystalline characteristics of cellulose fiber and film regenerated from ionic liquid solution. Carbohydr. Polym. 2015, 118, 150–155. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Pan, F.; Zhou, L.; Ji, X.; Kang, Z.; Zhang, S. Study on ionic liquid/cellulose/coagulator phase diagram and its application in green spinning process. J. Mol. Liq. 2019, 289, 111127. [Google Scholar] [CrossRef]
- Sangtarashani, S.M.H.; Rahmaninia, M.; Behrooz, R.; Khosravani, A. Lignocellulosic hydrogel from recycled old corrugated container resources using ionic liquid as a green solvent. J. Environ. Manag. 2020, 270, 110853. [Google Scholar] [CrossRef] [PubMed]
- Ingildeev, D.; Effenberger, F.; Bredereck, K.; Hermanutz, F. Comparison of direct solvents for regenerated cellulosic fibers via the lyocell process and by means of ionic liquids. J. Appl. Polym. Sci. 2013, 128, 4141–4150. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shi, Z. Influences of diffusion coefficient of 1-allyl-3-methylimidazolium chloride on structure and properties of regenerated cellulose fiber obtained via dry-jet-wet spinning. J. Appl. Polym. Sci. 2019, 136, 47609. [Google Scholar] [CrossRef]
- Tan, X.; Chen, L.; Li, X.; Xie, F. Effect of anti-solvents on the characteristics of regenerated cellulose from 1-ethyl-3-methylimidazolium acetate ionic liquid. Int. J. Biol. Macromol. 2019, 124, 314–320. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Role of Solvent Parameters in the Regeneration of Cellulose from Ionic Liquid Solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, L.; Guo, G.; Xie, H.; Zhang, L.; Jin, L.; Liang, S.; Hu, L.; Xu, Q.; Zheng, Q. Cellulose Membranes from Cellulose CO2-Based Reversible Ionic Liquid Solutions. ACS Sustain. Chem. Eng. 2021, 9, 11847–11854. [Google Scholar] [CrossRef]
- Wang, B.; Nie, Y.; Kang, Z.; Liu, X. Effects of coagulating conditions on the crystallinity, orientation and mechanical properties of regenerated cellulose fibers. Int. J. Biol. Macromol. 2023, 225, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Larkiala, S.; Moriam, K.; Sawada, D.; Elsayed, S.; Rantasalo, S.; Hummel, M.; Sixta, H. Air gap spinning of a cellulose solution in [DBNH][OAc] ionic liquid with a novel vertically arranged spinning bath to simulate a closed loop operation in the Ioncell® process. J. Appl. Polym. Sci. 2021, 138, 49787. [Google Scholar] [CrossRef]
- To, T.Q.; Kenny, C.; Cheong, S.; Aldous, L. Carbon dioxide as a pH-switch anti-solvent for biomass fractionation and pre-treatment with aqueous hydroxide solutions. Green Chem. 2017, 19, 2129–2134. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Qin, Y.; Belmore, K.; Daly, D.T.; Rogers, R.D. Switchable carbamate coagulants to improve recycling ionic liquid from biomass solutions. Green Chem. Eng. 2021, 2, 384–391. [Google Scholar] [CrossRef]
- Barber, P.S.; Griggs, C.S.; Gurau, G.; Liu, Z.; Li, S.; Li, Z.; Lu, X.; Zhang, S.; Rogers, R.D. Coagulation of chitin and cellulose from 1-ethyl-3-methylimidazolium acetate ionic-liquid solutions using carbon dioxide. Angew. Chem. Int. Ed. 2013, 52, 12350–12353. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chi, Y.; Mu, T. Studies on staged precipitation of cellulose from an ionic liquid by compressed carbon dioxide. Green Chem. 2014, 16, 2736–2744. [Google Scholar] [CrossRef]
- Xu, A.-R.; Guo, X.; Ma, J. Properties of Cellulose Regenerated from Powerful 1-Butyl-3-methylimidazolium Acetate/Dimethyl Sulfoxide Solvent. J. Macromol. Sci. Part B 2016, 55, 559–565. [Google Scholar] [CrossRef]
- Ebrahiminejadhasanabadi, M.; Nelson, W.M.; Naidoo, P.; Mohammadi, A.H.; Ramjugernath, D. Investigation of Mixed MEA-Based Solvents Featuring Ionic Liquids and NMP for CO2 Capture: Experimental Measurement of CO2 Solubility and Thermophysical Properties. J. Chem. Eng. Data 2021, 66, 899–914. [Google Scholar] [CrossRef]
- Gelles, T.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Recent advances in development of amine functionalized adsorbents for CO2 capture. Adsorption 2020, 26, 5–50. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 capture adsorbents functionalized by amine–bearing polymers: A review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Li, J.; Yun, X.; Hu, Z.; Xi, L.; Li, N.; Tang, H.; Lu, P.; Zhu, Y. Three-dimensional nitrogen and phosphorus co-doped carbon quantum dots/reduced graphene oxide composite aerogels with a hierarchical porous structure as superior electrode materials for supercapacitors. J. Mater. Chem. A 2019, 7, 26311–26325. [Google Scholar] [CrossRef]
- Yun, X.; Wu, S.; Li, J.; Li, L.; Zhou, J.; Lu, P.; Tang, H.; Zhu, Y. Facile synthesis of crystalline RuSe2 nanoparticles as a novel pseudocapacitive electrode material for supercapacitors. Chem. Commun. 2019, 55, 12320–12323. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Chitin and chitin-cellulose composite hydrogels prepared by ionic liquid-based process as the novel electrolytes for electrochemical capacitors. J. Solid State Electrochem. 2021, 25, 2549–2563. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Isogai, A. Ionic Liquid Mediated Dispersion and Support of Functional Molecules on Cellulose Fibers for Stimuli-Responsive Chromic Paper Devices. ACS Appl. Mater. Interfaces 2017, 9, 40914–40920. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Seelam, S.; Love, S.A.; O’Malley, S.M.; Hu, X.; Salas-de la Cruz, D. Reduced graphene oxide influences morphology and thermal properties of silk/cellulose biocomposites. Int. J. Biol. Macromol. 2023, 236, 123971. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Sotenko, M.; Cruz-Izquierdo, A.; Rymansaib, Z.; Iravani, P.; Kirwan, K.; Scott, J.L. Preparation of Printable and Biodegradable Cellulose-Laponite Composite for Electronic Device Application. J. Polym. Environ. 2021, 29, 17–27. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Chen, R.; Wang, X. High performance fully paper-based all-solid-state supercapacitor fabricated by a papermaking process with silver nanoparticles and reduced graphene oxide-modified pulp fibers. EcoMat 2021, 3, e12076. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; Ruan, K.; Kong, J.; Dong, M.; Zhang, J.; Gu, J.; Guo, Z. Reduced Graphene Oxide Heterostructured Silver Nanoparticles Significantly Enhanced Thermal Conductivities in Hot-Pressed Electrospun Polyimide Nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 25465–25473. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Kang, R.; Liang, Y.; Guo, L.; Dai, W.; Nishimura, K.; Lin, C.-T.; Jiang, N.; Yu, J. Highly flexible biodegradable cellulose nanofiber/graphene heat-spreader films with improved mechanical properties and enhanced thermal conductivity. J. Mater. Chem. C 2018, 6, 12739–12745. [Google Scholar] [CrossRef]
- Ahmed, A.; Adak, B.; Bansala, T.; Mukhopadhyay, S. Green Solvent Processed Cellulose/Graphene Oxide Nanocomposite Films with Superior Mechanical, Thermal, and Ultraviolet Shielding Properties. ACS Appl. Mater. Interfaces 2020, 12, 1687–1697. [Google Scholar] [CrossRef]
- Zhang, K.; Ketterle, L.; Järvinen, T.; Lorite, G.S.; Hong, S.; Liimatainen, H. Self-assembly of graphene oxide and cellulose nanocrystals into continuous filament via interfacial nanoparticle complexation. Mater. Des. 2020, 193, 108791. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Q.; Wang, X.; Sun, R. Highly tough cellulose/graphene composite hydrogels prepared from ionic liquids. Ind. Crops Prod. 2015, 70, 56–63. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, L.; Shi, N.; Yu, Y.; Min, Y.; Epstein, A.J. Self-assembled high-performance graphene oxide fibers using ionic liquid as coagulating agent. J. Mater. Sci. 2017, 52, 7698–7708. [Google Scholar] [CrossRef]
- Murugesan, B.; Pandiyan, N.; Arumugam, M.; Veerasingam, M.; Sonamuthu, J.; Jeyaraman, A.R.; Samayanan, S.; Mahalingam, S. Two dimensional graphene oxides converted to three dimensional P, N, F and B, N, F tri-doped graphene by ionic liquid for efficient catalytic performance. Carbon 2019, 151, 53–67. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, C.; Lei, M.; Gao, Q.; Nie, S.; Liu, X.; Wang, S. Reduced graphene oxide-based highly sensitive pressure sensor for wearable electronics via an ordered structure and enhanced interlayer interaction mechanism. RSC Adv. 2020, 10, 2150–2159. [Google Scholar] [CrossRef]
- Liang, X.; Qu, B.; Li, J.; Xiao, H.; He, B.; Qian, L. Preparation of cellulose-based conductive hydrogels with ionic liquid. React. Funct. Polym. 2015, 86, 1–6. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yu, J.; Zhang, J. Cellulose Aerogel Membranes with a Tunable Nanoporous Network as a Matrix of Gel Polymer Electrolytes for Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 24591–24599. [Google Scholar] [CrossRef] [PubMed]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef]
- Chereddy, S.; Aguirre, J.; Dikin, D.; Wunder, S.L.; Chinnam, P.R. Gel Electrolyte Comprising Solvate Ionic Liquid and Methyl Cellulose. ACS Appl. Energy Mater. 2020, 3, 279–289. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Hydroxypropyl Cellulose Based Non-Volatile Gel Polymer Electrolytes for Dye-Sensitized Solar Cell Applications using 1-methyl-3-propylimidazolium iodide ionic liquid. Sci. Rep. 2015, 5, 18056. [Google Scholar] [CrossRef]
- Dai, C.; Ye, C.; Ren, J.; Yang, S.; Cao, L.; Yu, H.; Liu, S.; Shao, Z.; Li, J.; Chen, W.; et al. Humanoid Ionotronic Skin for Smart Object Recognition and Sorting. ACS Mater. Lett. 2023, 5, 189–201. [Google Scholar] [CrossRef]
- Ge, D.; Yu, H.-Y.; Miao, Z.; He, X.; Abdalkarim, S.Y.H. Intrinsically Conductive Bifunctional Nanocellulose-Reinforced Robust and Self-Healable Electronic Skin: Deep Insights into Multiple Bonding Network, Property Reinforcement, and Sensing Mechanism. ACS Sustain. Chem. Eng. 2023, 11, 1157–1167. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Qin, C.; Qian, X.; Zhang, L.; Zhou, J.; Lu, A. Transparent, conductive cellulose hydrogel for flexible sensor and triboelectric nanogenerator at subzero temperature. Carbohydr. Polym. 2021, 265, 118078. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Li, S.; Liu, X.; Sun, J. Mechanically Robust, Elastic, and Healable Ionogels for Highly Sensitive Ultra-Durable Ionic Skins. Adv. Mater. 2020, 32, 2002706. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, S.; Guo, Y.; Song, J.; Zhang, L.; Xiao, L.; Guan, Q.; You, Z. Ionogel-based, highly stretchable, transparent, durable triboelectric nanogenerators for energy harvesting and motion sensing over a wide temperature range. Nano Energy 2019, 63, 103847. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Hu, K.; He, P.; Zhao, Z.; Huang, L.; Liu, K.; Lin, S.; Zhang, M.; Wu, H.; Chen, L.; Ni, Y. Nature-inspired self-powered cellulose nanofibrils hydrogels with high sensitivity and mechanical adaptability. Carbohydr. Polym. 2021, 264, 117995. [Google Scholar] [CrossRef]
- Geng, L.; Hu, S.; Cui, M.; Wu, J.; Huang, A.; Shi, S.; Peng, X. Muscle-inspired double-network hydrogels with robust mechanical property, biocompatibility and ionic conductivity. Carbohydr. Polym. 2021, 262, 117936. [Google Scholar] [CrossRef]
- Park, S.; Parida, K.; Lee, P.S. Deformable and Transparent Ionic and Electronic Conductors for Soft Energy Devices. Adv. Energy Mater. 2017, 7, 1701369. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Huang, J.; Liu, M. Low Temperature Tolerant Organohydrogel Electrolytes for Flexible Solid-State Supercapacitors. Adv. Energy Mater. 2018, 8, 1801967. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Dou, S.; Sun, J.; Cong, Z.; Jiang, C.; Du, C.; Pu, X.; Hu, W.; Wang, Z.L. Triboelectric-Nanogenerator-Based Soft Energy-Harvesting Skin Enabled by Toughly Bonded Elastomer/Hydrogel Hybrids. ACS Nano 2018, 12, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Hopson, C.; Villar-Chavero, M.M.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Cellulose ionogels, a perspective of the last decade: A review. Carbohydr. Polym. 2021, 274, 118663. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, L.; He, S.; Hong, H.; Liu, J.; Gan, L.; Long, M. Highly Efficient and Stable Cellulose-Based Ion Gel Polymer Electrolyte for Solid-State Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 5992–6001. [Google Scholar] [CrossRef]
- Uto, T.; Yamamoto, K.; Kadokawa, J.-i. Cellulose Crystal Dissolution in Imidazolium-Based Ionic Liquids: A Theoretical Study. J. Phys. Chem. B 2018, 122, 258–266. [Google Scholar] [CrossRef]
- Kowalczuk, J.; Bielejewski, M.; Tritt-Goc, J. Ionic liquid dynamics and electrical conductivity under confinement within micro and nanocellulose ionogels. Cellulose 2023, 30, 3551–3567. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J.; Chen, C.; Yu, H.; Hu, L. A Dynamic Gel with Reversible and Tunable Topological Networks and Performances. Matter 2020, 2, 390–403. [Google Scholar] [CrossRef]
- Livazovic, S.; Li, Z.; Behzad, A.R.; Peinemann, K.V.; Nunes, S.P. Cellulose multilayer membranes manufacture with ionic liquid. J. Membr. Sci. 2015, 490, 282–293. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Pleshivtseva, T.S.; Ignatenko, V.Y.; Antonov, S.V.; Volkov, A.V. Fabrication of composite nanofiltration membranes from cellulose solutions in an [Emim]OAc–DMSO mixture. Pet. Chem. 2017, 57, 477–482. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Taylor, A.; Serwinowski, N.; Parkerson, Z.J.; Confer, M.P.; Kammakakam, I.; Bara, J.E.; Esfahani, A.R.; Mahmoodi, S.N.; Koutahzadeh, N.; et al. Sustainable Novel Bamboo-Based Membranes for Water Treatment Fabricated by Regeneration of Bamboo Waste Fibers. ACS Sustain. Chem. Eng. 2020, 8, 4225–4235. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Ma, L.; Zhang, Y.; Cui, B. Direct regeneration of hydrogels based on lemon peel and its isolated microcrystalline cellulose: Characterization and application for methylene blue adsorption. Int. J. Biol. Macromol. 2021, 191, 129–138. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, Y.; Zhang, H.; Huang, H. Green and facile fabrication of pineapple peel cellulose/magnetic diatomite hydrogels in ionic liquid for methylene blue adsorption. Cellulose 2019, 26, 3825–3844. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, S.; Chu, Y.; Yuan, B.; Tao, X.; Hu, X.; Wang, Y. An injectable bioink with rapid prototyping in the air and in-situ mild polymerization for 3D bioprinting. Biofabrication 2021, 13, 045026. [Google Scholar] [CrossRef] [PubMed]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Sears, N.A.; Wilems, T.S.; Gold, K.A.; Lan, Z.; Cereceres, S.N.; Dhavalikar, P.S.; Foudazi, R.; Cosgriff-Hernandez, E.M. Hydrocolloid Inks for 3D Printing of Porous Hydrogels. Adv. Mater. Technol. 2019, 4, 1800343. [Google Scholar] [CrossRef]
- He, X.; Lu, Q. Design and fabrication strategies of cellulose nanocrystal-based hydrogel and its highlighted application using 3D printing: A review. Carbohydr. Polym. 2023, 301, 120351. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Mohan, D.; Teong, Z.K.; Sajab, M.S.; Kamarudin, N.H.; Kaco, H. Intact Fibrillated 3D-Printed Cellulose Macrofibrils/CaCO3 for Controlled Drug Delivery. Polymers 2021, 13, 1912. [Google Scholar] [CrossRef]
- AbouHashem, Y.; Dayal, M.; Savanah, S.; Štrkalj, G. The application of 3D printing in anatomy education. Med. Educ. Online 2015, 20, 29847. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, A.; Lee, S.-E.; Mamp, M. The application of 3D printing technology in the fashion industry. Int. J. Fash. Des. Technol. Educ. 2017, 10, 170–179. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Fang, Z. 3D printing of food: Pretreatment and post-treatment of materials. Crit. Rev. Food Sci. Nutr. 2020, 60, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef]
- Xu, W.; Molino, B.Z.; Cheng, F.; Molino, P.J.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On Low-Concentration Inks Formulated by Nanocellulose Assisted with Gelatin Methacrylate (GelMA) for 3D Printing toward Wound Healing Application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio Mater. 2021, 4, 325–369. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiao, D.; Zhao, S.; Lin, Q.; Zhang, B.; Xie, F. 3D printing to innovate biopolymer materials for demanding applications: A review. Mater. Today Chem. 2021, 20, 100459. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Yang, J. 3D bioprinting of cellulose with controlled porous structures from NMMO. Mater. Lett. 2018, 210, 136–138. [Google Scholar] [CrossRef]
- Hausmann, M.K.; Siqueira, G.; Libanori, R.; Kokkinis, D.; Neels, A.; Zimmermann, T.; Studart, A.R. Complex-Shaped Cellulose Composites Made by Wet Densification of 3D Printed Scaffolds. Adv. Funct. Mater. 2020, 30, 1904127. [Google Scholar] [CrossRef]
- Hausmann, M.K.; Rühs, P.A.; Siqueira, G.; Läuger, J.; Libanori, R.; Zimmermann, T.; Studart, A.R. Dynamics of Cellulose Nanocrystal Alignment during 3D Printing. ACS Nano 2018, 12, 6926–6937. [Google Scholar] [CrossRef]
- Mulakkal, M.C.; Trask, R.S.; Ting, V.P.; Seddon, A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018, 160, 108–118. [Google Scholar] [CrossRef]
- Studart, A.R. Additive manufacturing of biologically-inspired materials. Chem. Soc. Rev. 2016, 45, 359–376. [Google Scholar] [CrossRef]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Gauss, C.; Pickering, K.L.; Muthe, L.P. The use of cellulose in bio-derived formulations for 3D/4D printing: A review. Compos. Part C 2021, 4, 100113. [Google Scholar] [CrossRef]
- Roman, M.; Navarro, F. Deposition of Cellulose Nanocrystals by Inkjet Printing. In Model Cellulosic Surfaces; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1019, pp. 157–171. [Google Scholar]
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Li, V.C.-F.; Kuang, X.; Mulyadi, A.; Hamel, C.M.; Deng, Y.; Qi, H.J. 3D printed cellulose nanocrystal composites through digital light processing. Cellulose 2019, 26, 3973–3985. [Google Scholar] [CrossRef]
- Amini, M.; Kamkar, M.; Ahmadijokani, F.; Ghaderi, S.; Rojas, O.J.; Hosseini, H.; Arjmand, M. Mapping 3D Printability of Ionically Cross-Linked Cellulose Nanocrystal Inks: Architecting from Nano- to Macroscale Structures. Biomacromolecules 2023, 24, 775–788. [Google Scholar] [CrossRef]
- Shin, S.; Hyun, J. Rheological properties of cellulose nanofiber hydrogel for high-fidelity 3D printing. Carbohydr. Polym. 2021, 263, 117976. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef]
- Markstedt, K.; Sundberg, J.; Gatenholm, P. 3D Bioprinting of Cellulose Structures from an Ionic Liquid. 3D Print. Addit. Manuf. 2014, 1, 115–121. [Google Scholar] [CrossRef]
- Hopson, C.; Rigual, V.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Eucalyptus bleached kraft pulp-ionic liquid inks for 3D printing of ionogels and hydrogels. Carbohydr. Polym. 2023, 313, 120897. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, D.H.A.T.; Kuek, S.; Hasanaj, D.; He, Y.; Tuck, C.; Croft, A.K.; Wildman, R.D. Three dimensional ink-jet printing of biomaterials using ionic liquids and co-solvents. Faraday Discuss. 2016, 190, 509–523. [Google Scholar] [CrossRef]
- Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Lukasheva, N.V.; Smirnov, M.A. Water Influence on the Physico-Chemical Properties and 3D Printability of Choline Acrylate—Bacterial Cellulose Inks. Polymers 2023, 15, 2156. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Ribeiro, M.C.C.; Smirnov, M.A. Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers 2022, 14, 5148. [Google Scholar] [CrossRef] [PubMed]

| IL | Concentration of DMSO in IL and Temperature to Dissolve Cellulose | Concentration of Cellulose | Ref. |
|---|---|---|---|
| C3OMeImAc | 60% mole, 60 °C | 12% w/w MCC a | [91] |
| C4MeImAc | 60% mole, 60 °C | 16% w/w MCC a | |
| (BmimAc) | |||
| C3OMe2ImAc | 60% mole, 60 °C | 19% w/w MCC a | |
| C4Me2ImAc | 60% mole, 60 °C | 22% w/w MCC a | |
| BmimAc | 20% w/w, 70 °C | 8% w/w MCC, Avicel, and α-cellulose b | [93] |
| 40% w/w, R.T. c | 20% w/w Avicel b | [94] | |
| 40% w/w, R.T. c | ~10% w/w MCC b | [95] | |
| 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] | |
| BmimCl | 50% w/w, 15–75 °C | 3% w/w cellulose powder b | [92] |
| 30% w/w, 120 °C | - | [97] | |
| EmimAc | 50% w/w, 60 °C | 2.5% w/w cotton b | [98] |
| 25% w/w, 50 °C | 10% w/w MCC b | [99] | |
| 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] | |
| EmimCl | 75% w/w, 80 °C | 14% w/w cellulose powder b | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taokaew, S. Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes. Gels 2023, 9, 546. https://doi.org/10.3390/gels9070546
Taokaew S. Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes. Gels. 2023; 9(7):546. https://doi.org/10.3390/gels9070546
Chicago/Turabian StyleTaokaew, Siriporn. 2023. "Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes" Gels 9, no. 7: 546. https://doi.org/10.3390/gels9070546
APA StyleTaokaew, S. (2023). Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes. Gels, 9(7), 546. https://doi.org/10.3390/gels9070546