Lincomycin HCl-Loaded Borneol-Based In Situ Gel for Periodontitis Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. Physical Appearance, Density, and Viscosity
2.1.2. Surface Tension and Contact Angle
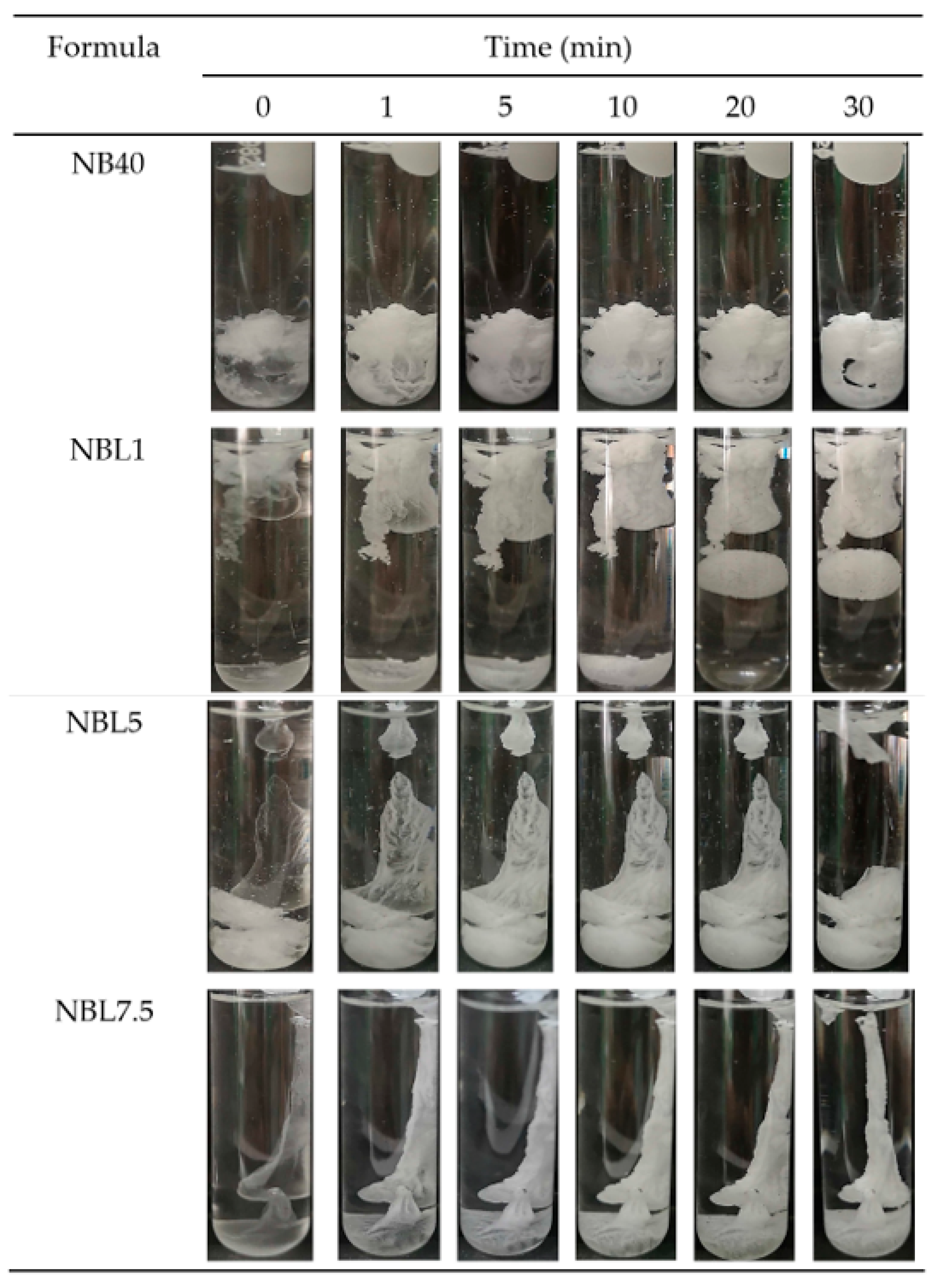
2.1.3. Water Tolerance
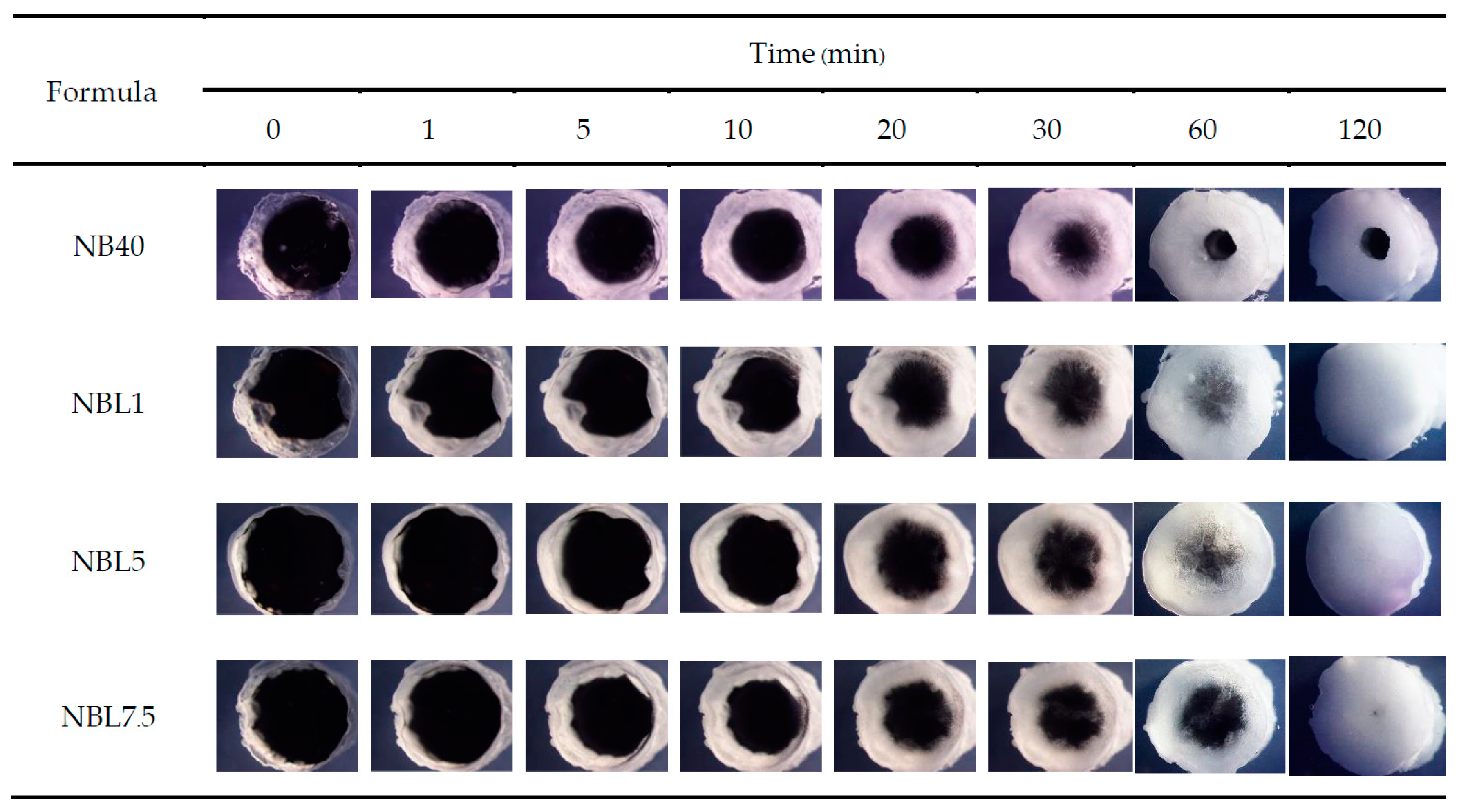
2.1.4. In Situ Gel Formation
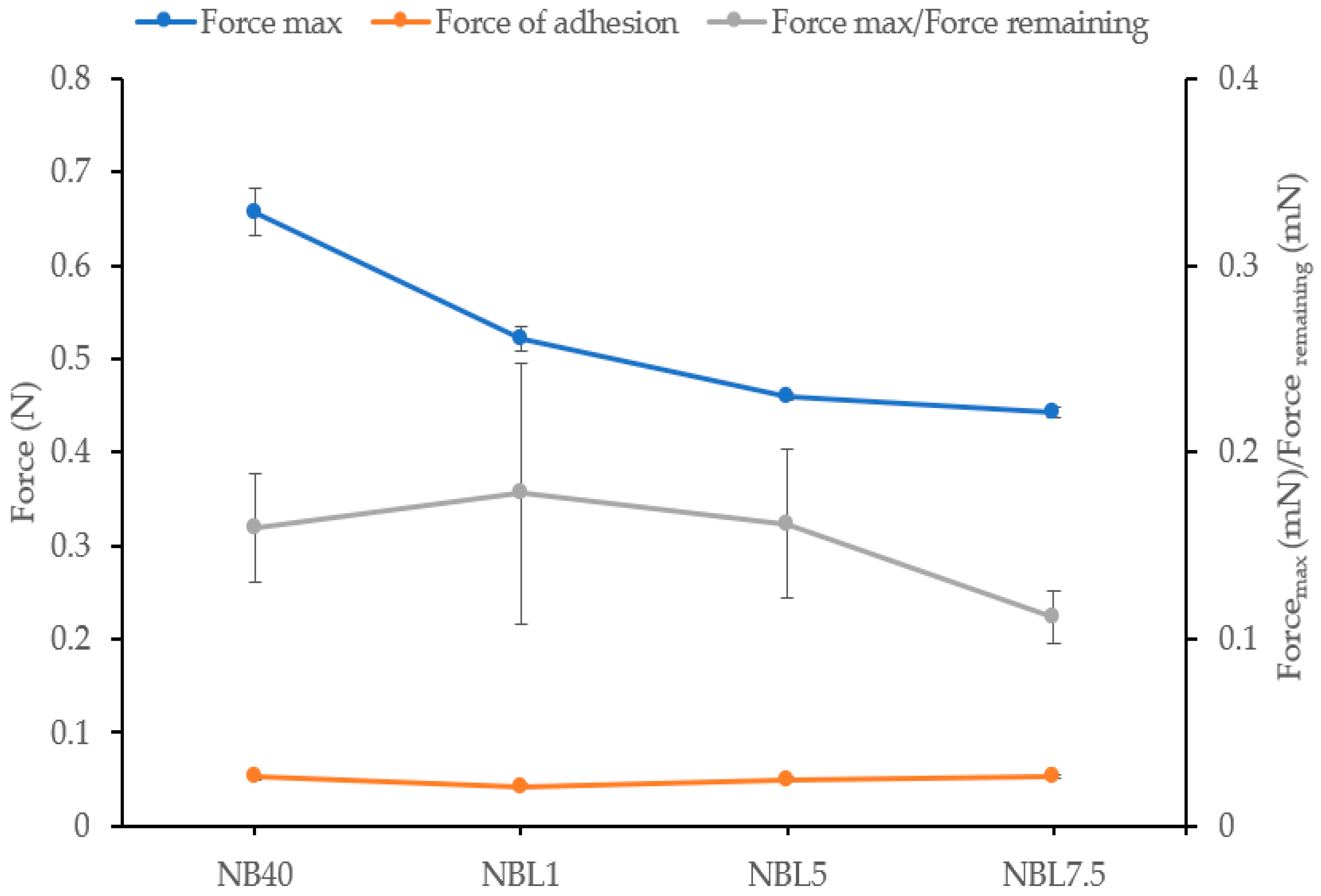
2.1.5. Mechanical Properties
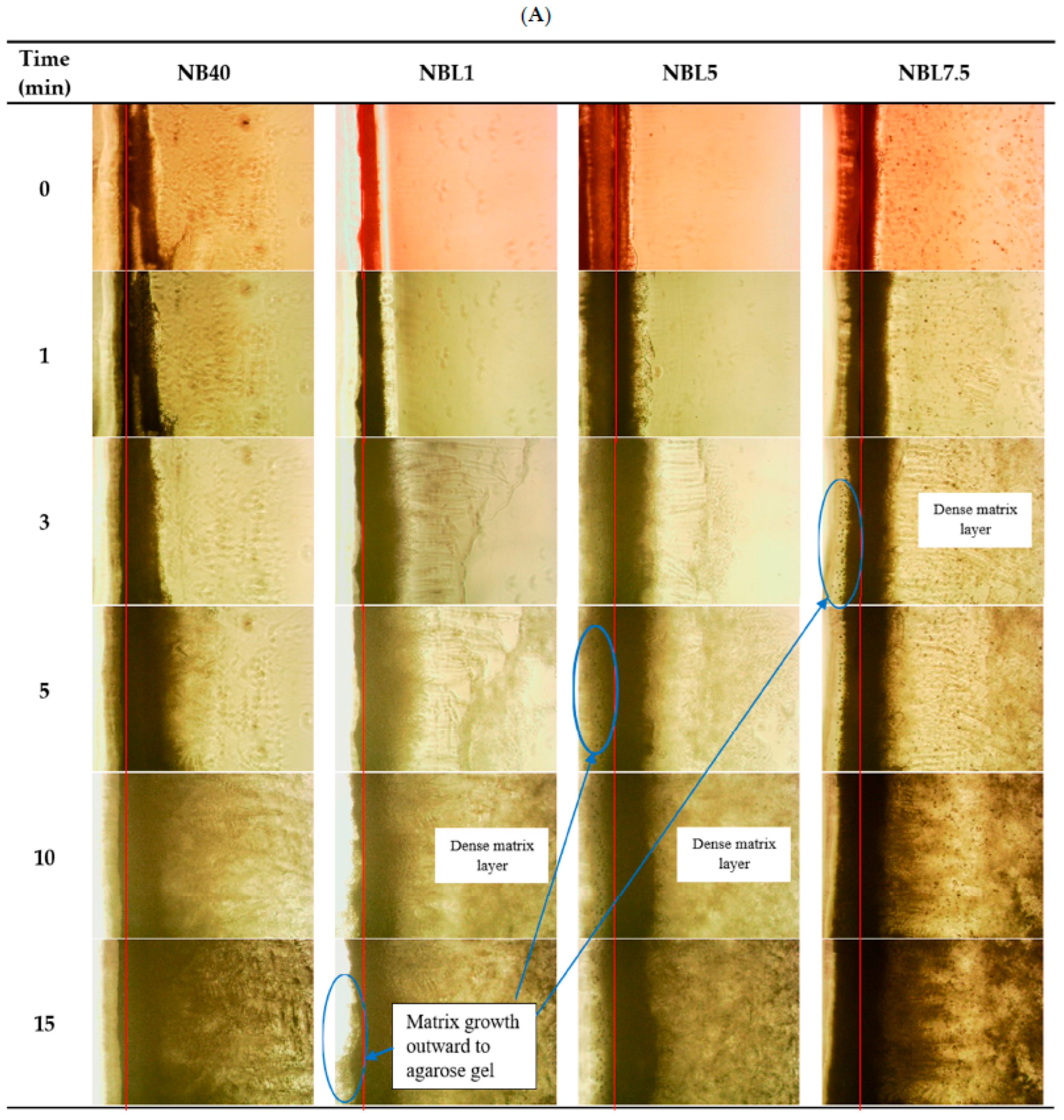
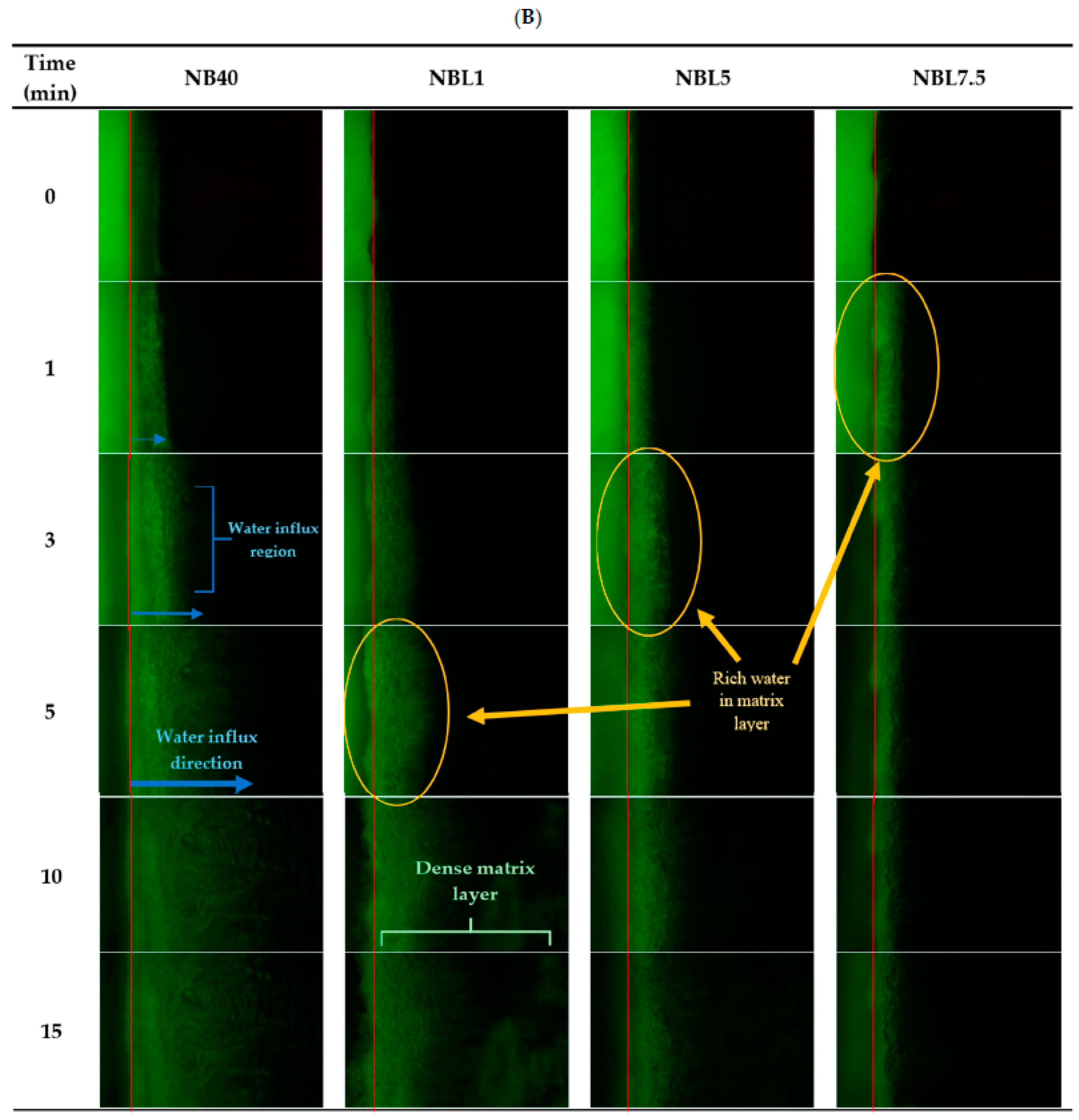
2.1.6. Microscopic Interface Interaction
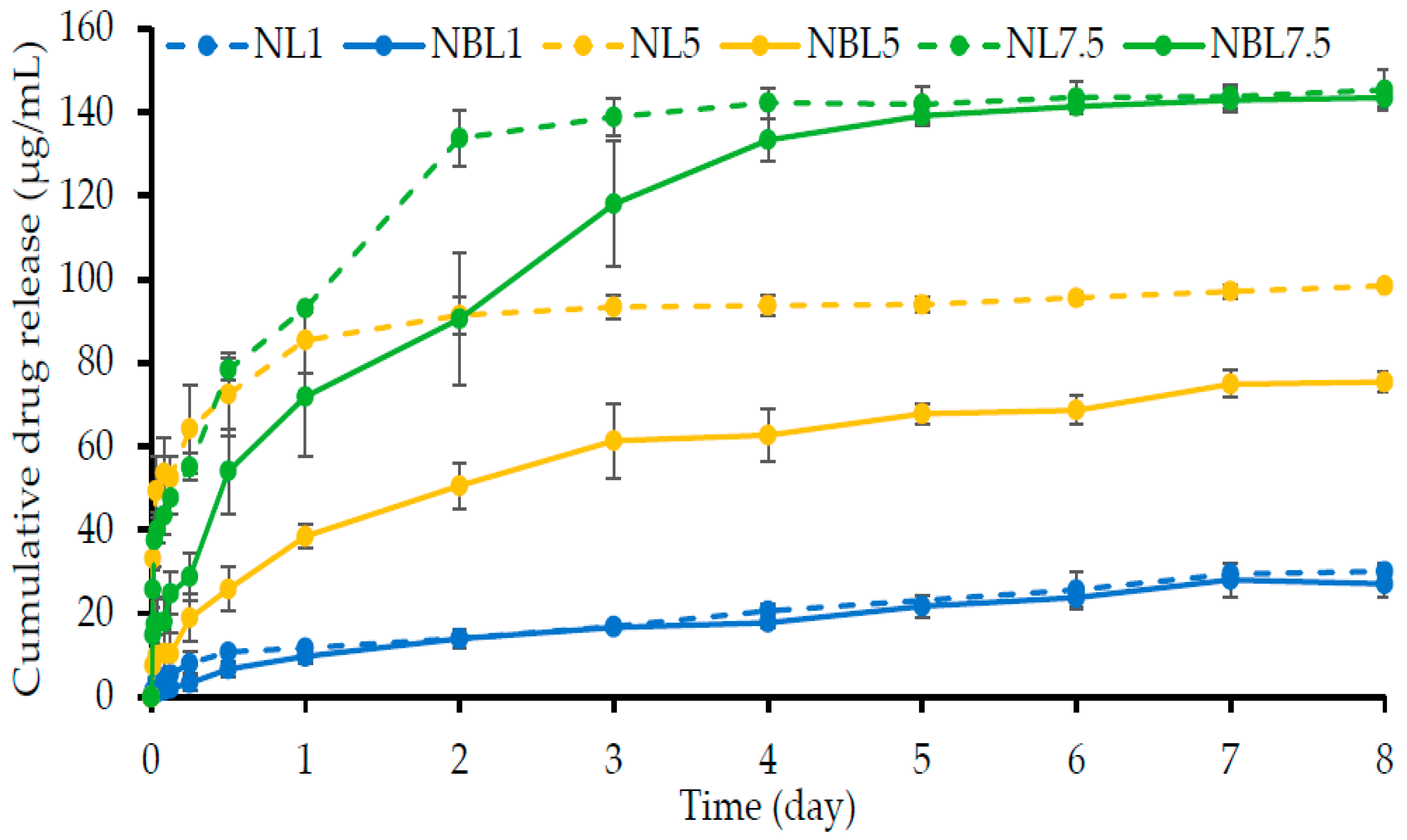
2.1.7. Drug Content and In Vitro Drug Release
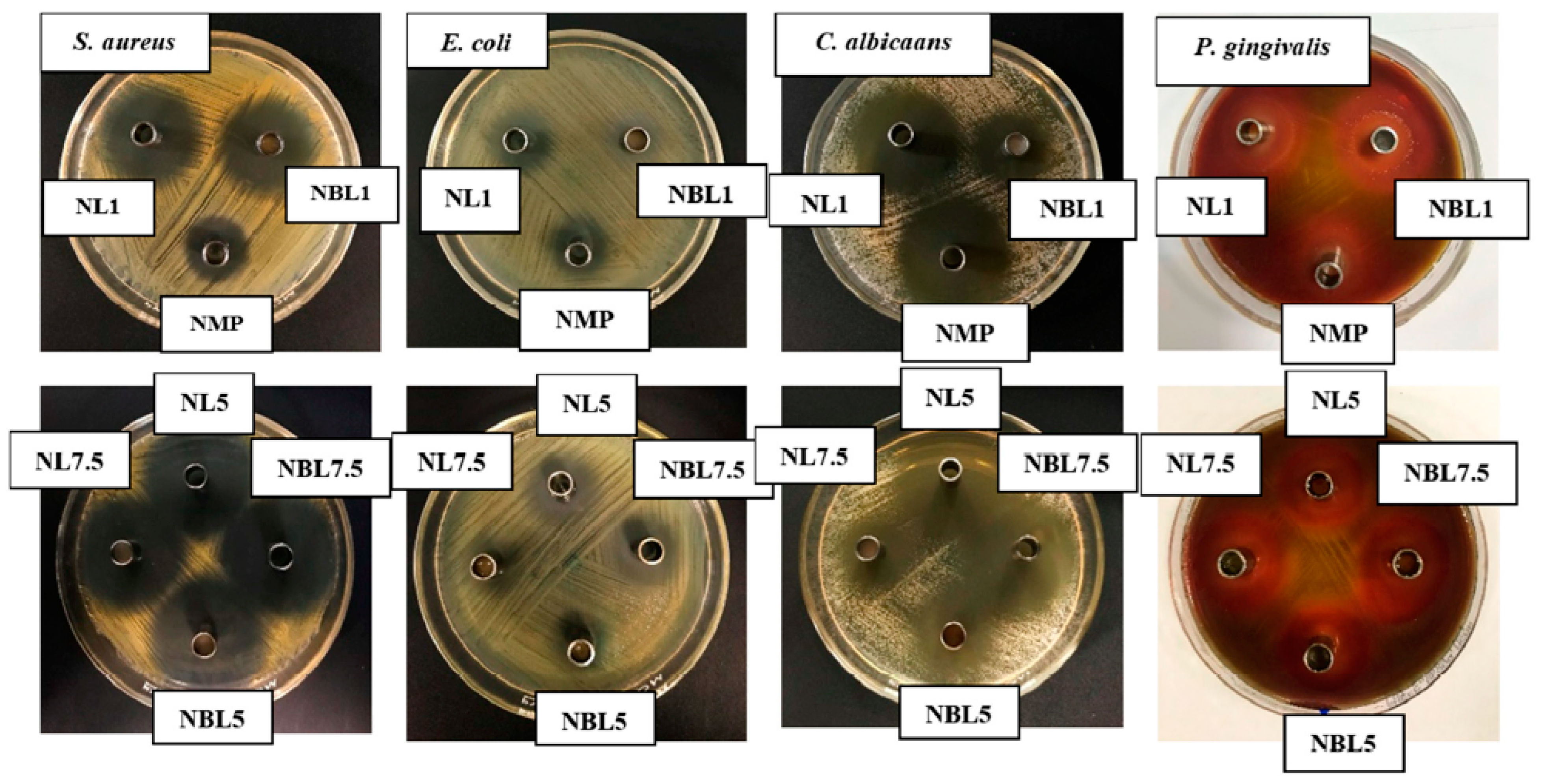
2.2. Antimicrobial Activities
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Drug-Free and Lincomycin HCl-Loaded Borneol-ISG Solutions
4.3. Physicochemical Study
4.3.1. Density and Viscosities
4.3.2. Surface Tension and Contact Angle
4.3.3. Water Tolerance Test
4.3.4. Gel Formation Study
4.3.5. Mechanical Properties
4.3.6. Interfacial Phenomena between Formulation and Aqueous Phase
4.3.7. Drug Content and In Vitro Drug Release Studies
4.3.8. Antimicrobial Activities
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Api, A.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.; Dekant, W.; Fryer, A.; Kromidas, L.; La Cava, S.; et al. RIFM fragrance ingredient safety assessment, borneol, CAS registry number 507-70-0. Food Chem. Toxicol. 2015, 82, S81–S88. [Google Scholar] [CrossRef]
- Zhang, S.; Asghar, S.; Yang, L.; Hu, Z.; Chen, Z.; Shao, F.; Xiao, Y. Borneol and poly (ethylene glycol) dual modified BSA nanoparticles as an itraconazole vehicle for brain targeting. Int. J. Pharm. 2020, 575, 119002. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on borneol. Food Chem. Toxicol. 2008, 46, S77–S80. [Google Scholar] [CrossRef]
- PubChem. Pubchem Compound Summary for CID 64685, Borneol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Borneol (accessed on 14 November 2022).
- Ren, J.; Zou, M.; Gao, P.; Wang, Y.; Cheng, G. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur. J. Pharm. Biopharm. 2013, 83, 141–148. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Fu, B.M.; Zhang, Z.-J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood–brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Agossa, K.; Lizambard, M.; Petit, C.; Bugueno, I.M.; Delcourt-Debruyne, E.; Benkirane-Jessel, N.; Tenenbaum, H.; Siepmann, J.; Siepmann, F.; et al. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treatment: Proof of concept study in vivo. Int. J. Pharm. 2019, 569, 118564. [Google Scholar] [CrossRef]
- Kornman, K.S. Controlled- release local delivery antimicrobials in periodontics: Prospects for the future. J. Periodontol. 1993, 64, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.K.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Ozdogan, A.I.; Ilarslan, Y.D.; Kosemehmetoglu, K.; Akca, G.; Kutlu, H.B.; Comerdov, E.; Iskit, A.B.; Şenel, S. In vivo evaluation of chitosan based local delivery systems for atorvastatin in treatment of periodontitis. Int. J. Pharm. 2018, 550, 470–476. [Google Scholar] [CrossRef]
- Polson, A.M.; Garrett, S.; Stoller, N.H.; Bandt, C.L.; Hanes, P.J.; Killoy, W.J.; Southard, G.L.; Duke, S.P.; Bogle, G.C.; Drisko, C.H.; et al. Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis. II. clinical results. J. Periodontol. 1997, 68, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lizambard, M.; Menu, T.; Fossart, M.; Bassand, C.; Agossa, K.; Huck, O.; Neut, C.; Siepmann, F. In-situ forming implants for the treatment of periodontal diseases: Simultaneous controlled release of an antiseptic and an anti-inflammatory drug. Int. J. Pharm. 2019, 572, 118833. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontal pocket delivery. J. Drug. Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and comparative evaluation of vancomycin HCl-loaded rosin-based in situ forming gel and microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with anti-microbial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Doxycycline hyclate-loaded Eudragit® RS PO in situ-forming microparticles for periodontitis treatment. J. Drug. Deliv. Sci. Technol. 2022, 71, 103294. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Natural-resin in-situ-forming gels: Physicochemical characteristics and bioactivities. Pharm. Sci. Asia 2021, 48, 461–470. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. (Eds.) 32-Antibiotics. In Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006; pp. 425–498. [Google Scholar]
- Spížek, J.; Řezanka, T. Lincosamides: Chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharmacol. 2017, 133, 20–28. [Google Scholar] [CrossRef]
- Tabrizi, R.; Khorshidi, H.; Shahidi, S.; Gholami, M.; Kalbasi, S.; Khayati, A. Use of lincomycin-impregnated demineralized freeze-dried bone allograft in the periodontal defect after third molar surgery. J. Oral. Maxillofac. Surg. 2014, 72, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Czarniak, P.; Boddy, M.; Sunderland, B.; Hughes, J. Stability studies of lincomycin hydrochloride in aqueous solution and intravenous infusion fluids. Drug. Des. Devel. Ther. 2016, 10, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, V.M. Lincomycin in oral surgery. Oral Surg. Oral Med. Oral Pathol. 1970, 29, 485–490. [Google Scholar] [CrossRef]
- Kim, H.-S.; You, H.-K.; Shin, H.-S. Antibiotic susceptibility of Porphyromonas gingivalis and prevotella intennedia from the patients with adult periodontitis. J. Periodontal. Implant. Sci. 1996, 26, 625–639. [Google Scholar]
- Adami, R.; Liparoti, S.; Della Porta, G.; Del Gaudio, P.; Reverchon, E. Lincomycin hydrochloride loaded albumin microspheres for controlled drug release, produced by supercritical assisted atomization. J. Supercrit. Fluids. 2016, 119, 203–210. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- He, G.; Chen, X.; Yin, Y.; Cai, W.; Ke, W.; Kong, Y.; Zheng, H. Preparation and antibacterial properties of O-carboxymethyl chitosan/lincomycin hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-nduced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press L.L.C.: Boca Raton, FL, USA, 2014; pp. 3–58. [Google Scholar]
- Karunarathne, S.S.; Eimer, D.A.; Jens, K.J.; Oi, L.E. Density, viscosity, and excess properties of ternary aqueous mixtures of MDEA + MEA, DMEA + MEA, and DEEA + MEA. Fluids 2020, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Senarat, S.; Lwin, W.W.; Mahadlek, J.; Phaechamud, T. Doxycycline hyclate-loaded in situ forming gels composed from bleached shellac, Ethocel, and Eudragit RS for periodontal pocket delivery. Saudi. Pharm. J. 2021, 29, 252–263. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Sawangsri, P.; Santimaleeworagun, W.; Phaechamud, T. Vancomycin hydrochloride-loaded stearic acid/lauric acid in situ forming matrix for antimicrobial inhibition in patients with joint infection after total knee arthroplasty. Mat. Sci. Eng. C. 2020, 115, 110761. [Google Scholar] [CrossRef]
- Chantadee, T.; Sirirak, J.; Hoshino, T.; Phaechamud, T. Augmentative molecular aspect for phase inversion of vancomycin hydrochloride-loaded fatty acid in situ forming matrices. Mater. Des. 2021, 199, 109429. [Google Scholar] [CrossRef]
- Shardt, N.; Wang, Y.; Jin, Z.; Elliott, J.A.W. Surface tension as a function of temperature and composition for a broad range of mixtures. Chem. Eng. Sci. 2021, 230, 116095. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Tuntarawongsa, S.; Jitrangsriand, K.; Phaechamud, T. Clotrimazole-loaded borneol-based in situ forming gel as oral sprays for oropharyngeal candidiasis therapy. Gels 2023, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; McMillan, H.; Jones, D. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2013, 176, 8–23. [Google Scholar]
- Jouyban, A.; Fakhree, M.A.A.; Shayanfar, A. Review of pharmaceutical applications of n-methyl-2-pyrrolidone. J. Pharm. Sci. 2010, 13, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Sangster, J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Agrawal, A.G.; Kumar, A.; Gide, P.S. Toxicity Study of a Self-nanoemulsifying Drug Delivery System Containing N-methyl pyrrolidone. Drug Res. (Stuttg.) 2015, 65, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Quinn, P.J. Dimethyl sulphoxide: A review of its applications in cell biology. Biosci. Rep. 1994, 14, 259–281. [Google Scholar] [CrossRef]
- Golmaghani-Ebrahimi, E.; Bagheri, A.; Fazli, M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J. Chem. Thermodyn. 2020, 146, 10615. [Google Scholar] [CrossRef]
- Yang, L.-J.; Yang, X.-Q.; Huang, K.-M.; Jia, G.-Z.; Shang, H. Dielectric properties of binary solvent mixtures of dimethyl sulfoxide with water. Int. J. Mol. Sci. 2009, 10, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Garzón, L.C.; Martínez, F. Temperature dependence of solubility for ibuprofen in some organic and aqueous solvents. J. Sol. Chem. 2004, 33, 1379–1395. [Google Scholar] [CrossRef]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In situ forming phase-inversion implants for sustained ocular delivery of triamcinolone acetonide. Drug Deliv. Transl. Res. 2018, 9, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Huang, X.; Xie, Y.; Chen, J.; Huang, Y.; Wang, B.; Wang, H.; Pan, X.; Wu, C. An injectable in situ gel with cubic and hexagonal nanostructures for local treatment of chronic periodontitis. Drug. Deliv. 2017, 24, 1148–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.; Neut, C.; Delcourt, E.; Certo, T.S.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef]
- Do, M.; Neut, C.; Metz, H.; Delcourt, E.; Mäder, K.; Siepmann, J. In-situ forming composite implants for periodontitis treatment: How the formulation determines system performance. Int. J. Pharm. 2015, 486, 38–51. [Google Scholar] [CrossRef]
- Do, M.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mäder, K. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef]
- Belviso, B.D. Crystallization. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 501–502. [Google Scholar] [CrossRef]
- Lipson, B.K.; Yannuzzi, L.A. Complications of intravenous fluorescein injections. Int. Ophthalmol. Clin. 1989, 29, 200–205. [Google Scholar] [CrossRef]
- Poet, T.S.; Kirman, C.R.; Bader, M.; van Thriel, C.; Gargas, M.L.; Hinderliter, P.M. Quantitative risk analysis for N-methyl pyrrolidone using physiologically based pharmacokinetic and benchmark dose modeling. Toxicol. Sci. 2009, 113, 468–482. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Jantadee, T.; Mahadlek, J.; Charoensuksai, P.; Pichayakorn, W. Characterization of antimicrobial agent loaded eudragit RS solvent exchange-induced in situ forming gels for periodontitis treatment. AAPS PharmSciTech. 2017, 18, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Senarat, S.; Rojviriya, C.; Puyathorn, N.; Lertsuphotvanit, N.; Phaechamud, T. Levofloxacin HCl-Incorporated Zein-Based Solvent Removal Phase Inversion In Situ Forming Gel for Periodontitis Treatment. Pharmaceutics 2023, 15, 1199. [Google Scholar] [CrossRef] [PubMed]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C. 2020, 117, 111275. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Sci. 2003, 6, 282–291. [Google Scholar]
- Mathur, S.; Mathur, S. Personalized medicine could transform healthcare (Review). Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar]
- Chaiya, P.; Rojviriya, C.; Pichayakorn, W.; Phaechamud, T. New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques. Pharmaceutics 2022, 14, 2299. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Mahadlek, J.; Okonogi, S.; Pichayakorn, W.; Phaechamud, T. Imatinib Mesylate-Loaded Rosin/Cinnamon Oil-Based In Situ Forming Gel against Colorectal Cancer Cells. Gels 2022, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil–incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18822362 (accessed on 14 February 2020). [CrossRef]
- Kriangkrai, W.; Puttipipatkhachorn, S.; Sriamornsak, P.; Sungthongjeen, S. Borneol as sublimable agent in floating matrix tablet. Adv. Mater. Res. 2014, 1060, 33–36. [Google Scholar] [CrossRef]
- Cai, Z.; Lei, X.; Lin, Z.; Zhao, J.; Wu, F.; Yang, Z.; Pu, J.; Liu, Z. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with borneol as an oral brain-targeting enhancer. Acta Pharm. Sin. B 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.-P.; Gao, X.-C.; Zhang, L.-Q.; Wei, S.-Q.; Bi, S.; Yang, Z.-C.; Cui, H. In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur. J. Pharmacol. 2013, 705, 20–25. [Google Scholar] [CrossRef]
- Raghavan, R.; Devi, M.S.; Varghese, M.; Joseph, A.; Abraham, A.; Sreedevi, P. Evaluation of Different Local Drug Delivery Systems in the Management of Chronic Periodontitis: A Comparative Study. J. Contemp. Dent. Pract. 2020, 21, 280–284. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef]
- Amel, Y.; Bouziane, D.; Leila, M.; Ahmed, B. Microbiological study of periodontitis in the west of Algeria. West. Indian Med. J. 2015, 5, 7–12. [Google Scholar]
- Seymour, R.A.; Heasman, P.A. Pharmacological control of periodontal disease. II. Antimicrobial agents. J. Dent. 1995, 23, 5–14. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Charoenteeraboon, J.; Choopun, S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J. Pharm. Sci. 2012, 74, 498. [Google Scholar] [CrossRef] [Green Version]
- Saw, C.L.L.; Olivo, M.; Wohland, T.; Fu, C.Y.; Kho, K.W.; Soo, K.C.; Heng, P.W.S. Effects of N-methyl pyrrolidone on the uptake of hypericin in human bladder carcinoma and co-staining with DAPI investigated by confocal microscopy. Technol. Cancer Res. Treat. 2007, 6, 383–394. [Google Scholar] [CrossRef]
- Li, J.M.; Hu, S.X.; Li, H.Q. The Commodity Types and Historical Origins of Traditional Chinese Medicine Borneol. Mod. Chin. Med. 2013, 12, 606682. [Google Scholar] [CrossRef]
- Chen, Z.X.; Xu, Q.Q.; Shan, C.S.; Shi, Y.H.; Wang, Y.; Chang, R.C.C.; Zheng, G.-Q. Borneol for Regulating the Permeability of the Blood-Brain Barrier in Experimental Ischemic Stroke: Preclinical Evidence and Possible Mechanism. Oxidative Med. Cell Longevity. 2019, 2019, 2936737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yan, S.; Xu, L.; Zhu, G.; Yu, X.; Tong, X. Use of Angong Niuhuang in Treating Central Nervous System Diseases and Related Research. Evidence-Based Complement. Altern. Med. 2014, 2014, 346918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Qu, X.-Y.; Tao, L.-N.; Zhai, J.-H.; Gao, H.; Song, Y.-Q.; Zhang, S.-Q. XingNaoJing Injection Ameliorates Cerebral Ischaemia/reperfusion Injury via SIRT1-Mediated Inflammatory Response Inhibition. Pharm. Biol. 2020, 58, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.-P.; Chen, J.; Bei, Y.-F.; Han, B.-X.; Wang, S. Influence of borneol on primary mice oral fibroblasts: A penetration enhancer may be used in oral submucous fibrosis J. Oral Pathol. Med. 2009, 38, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Cherneva, E.; Pavlovic, V.; Smelcerovic, A.; Yancheva, D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules 2012, 17, 10258–10266. [Google Scholar] [CrossRef]
- Argoudelis, A.D.; Fox, J.A.; Mason, D.J.; Eble, T.E. New Lincomycin-Related Antibiotics. J. Am. Chem. Soc. 1964, 86, 5044–5045. [Google Scholar] [CrossRef]
- Ganjoo, R.; Soni, S.; Ram, V.; Verma, A. Medium Molecular Weight Chitosan as a Carrier for Delivery of Lincomycin Hydrochloride from Intra-pocket Dental Film: Design, Development, in vitro and ex vivo Characterization. J. Appl. Pharm. Sci. 2016, 6, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Hnatko, S.I. The treatment of acute and chronic staphylococcal osteomyelitis and soft tissue infections with lincomycin. Can. Med. Assoc. J. 1967, 97, 580–584. [Google Scholar]
- Alkabee, H.J. Antimicrobial Activity of Lincomycin and Gentamicin delivered from Chitosan and Chitosan-Gelatin Matrices. J. Kerbala Univ. 2008, 6, 171–180. [Google Scholar]
- Wiśniewska, I.; Slósarczyk, A.; Myśliwiec, L.; Sporniak-Tutak, K. Lincomycin applied to the alveolus on TCP carrier and its effect on wound healing after surgical extraction of a third molar. Ann. Acad. Med. Stetin. 2009, 55, 59–64. [Google Scholar]
- Lertsuphotvanit, N.; Senarat, S.; Tuntarawongsa, S.; Phaechamud, T. Sublimation/Evaporation Behaviors of Borneol In-Situ Forming Matrix. Mater. Today Proceedings, 2023; In Press. [Google Scholar] [CrossRef]

| Formula | Density (g/cm3) | Viscosity (cP) | Surface Tension (mN/m) | Contact Angle (Degree) | % Water Tolerance (% w/w) | |||
|---|---|---|---|---|---|---|---|---|
| Glass Slide | Agarose Gel | Paraffin | 25 °C | 37 °C | ||||
| NMP | 1.027 ± 0.001 a | 2.04 ± 0.13 b | 39.31 ± 0.28 | 31.08 ± 0.40 d | 7.12 ± 1.51 | 44.68 ± 2.15 | - | - |
| NB40 | 1.010 ± 0.000 a | 3.76 ± 0.06 | 45.70 ± 0.25 c | 17.16 ± 1.20 d | 26.98 ± 1.64 e | 46.56 ± 0.53 | 12.76 ± 0.29 | 13.16 ± 0.00 |
| NBL1 | 1.011 ± 0.001 a | 4.01 ± 0.01 b | 40.18 ± 0.05 | 9.15 ± 1.98 | 37.75 ± 1.60 | 45.82 ± 0.39 | 11.52 ± 0.36 | 13.16 ± 0.00 |
| NBL5 | 1.019 ± 0.000 a | 5.51 ± 0.02 b | 40.64 ± 0.20 | 10.37 ± 0.46 | 35.01 ± 1.56 | 46.84 ± 0.93 | 9.39 ± 0.00 | 11.32 ± 0.00 |
| NBL7.5 | 1.025 ± 0.000 | 6.78 ± 0.02 b | 40.12 ± 0.32 | 13.58 ± 0.38 | 32.54 ± 1.28 | 39.16 ± 1.08 | 8.29 ± 0.39 | 9.39 ± 0.65 |
| Formulation | Modeling | Criteria for Model Selection | Kinetic Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | AIC | MSC | |||||
| NL1 | Zero order | 0.7505 | 116.6260 | 1.1271 | k0 = 14.408 | ||
| First order | 0.8812 | 106.1227 | 1.8774 | k1 = 0.363 | |||
| Higuchi’s | 0.9615 | 90.1917 | 3.0153 | kH = 35.490 | |||
| Korsmeyer–Peppas’s | 0.9708 | 87.6678 | 3.1956 | kKP = 39.953 | n = 0.423 | ||
| Peppas–Sahlin’s | 0.9393 | 49.8984 | 2.4443 | k1 = 33.959 | k2 = 6.109 | m = 0.311 | |
| NL5 | Zero order | 1.3967 | 155.4462 | −1.5498 | k0 = 16.864 | ||
| First order | 0.7183 | 123.0316 | 0.6112 | k1 = 6.485 | |||
| Higuchi’s | 0.0977 | 143.5405 | −0.7561 | kH = 44.503 | |||
| Korsmeyer–Peppas’s | 0.9380 | 49.9655 | 2.6167 | kKP = 79.212 | n = 0.173 | ||
| Peppas–Sahlin’s | 0.9488 | 49.4285 | 2.6764 | k1 = 97.941 | k2 = −17.041 | m = 0.216 | |
| NL7.5 | Zero order | 0.1328 | 155.7117 | −0.1901 | k0 = 16.238 | ||
| First order | 0.8631 | 126.0518 | 1.6636 | k1 = 1.397 | |||
| Higuchi’s | 0.7421 | 136.2893 | 1.0237 | kH = 41.861 | |||
| Korsmeyer–Peppas’s | 0.9515 | 48.4314 | 2.8900 | kKP = 64.463 | n = 0.328 | ||
| Peppas–Sahlin’s | 0.9534 | 48.5188 | 2.8803 | k1 = 38.209 | k2 = 26.017 | m = 0.247 | |
| NBL1 | Zero order | 0.8892 | 113.1104 | 1.9081 | k0 = 12.892 | ||
| First order | 0.9700 | 90.2398 | 3.4328 | k1 = 0.261 | |||
| Higuchi’s | 0.9834 | 84.4966 | 3.8157 | kH = 30.920 | |||
| Korsmeyer–Peppas’s | 0.9791 | 36.1348 | 3.6937 | kKP = 29.361 | n = 0.527 | ||
| Peppas-–-Sahlin’s | 0.9777 | 36.8636 | 3.6026 | k1 = 29.806 | k2 = −0.508 | m = 0.585 | |
| NBL5 | Zero order | 0.6336 | 125.7564 | 0.7413 | k0 = 11.854 | ||
| First order | 0.8453 | 111.2304 | 1.7097 | k1 = 0.249 | |||
| Higuchi’s | 0.9395 | 98.3918 | 2.5656 | kH = 29.375 | |||
| Korsmeyer–Peppas’s | 0.9512 | 55.5895 | 2.8777 | kKP = 35.368 | n = 0.389 | ||
| Peppas–Sahlin’s | 0.9819 | 47.7392 | 3.6627 | k1 = 37.226 | k2 = −1.288 | m = 0.547 | |
| NBL7.5 | Zero order | 0.6748 | 141.8234 | 0.9285 | k0 = 15.467 | ||
| First order | 0.9573 | 109.5144 | 2.9478 | k1 = 0.570 | |||
| Higuchi’s | 0.9477 | 111.4238 | 2.8284 | kH = 38.303 | |||
| Korsmeyer–Peppas’s | 0.9697 | 36.5037 | 3.2068 | kKP = 44.108 | n = 0.495 | ||
| Peppas–Sahlin’s | 0.9730 | 35.8043 | 3.3067 | k1 = 47.833 | k2 = −2.585 | m = 0.549 | |
| Formula | Clear Zone Diameter (Mean ± SD) | |||
|---|---|---|---|---|
| S. aureus ATCC 25923 | E. coli ATCC 8739 | C. albicans ATCC 10231 | P. gingivalis ATCC 33277 | |
| NMP | 17.3 ± 0.6 a | 17.0 ± 1.0 | 35.7 ± 0.6 | 18.3 ± 1.2 e |
| NL1 | 30.3 ± 0.6 b | 18.0 ± 0.0 | 36.3 ± 0.6 | 20.3 ± 0.6 |
| NBL1 | 27.7 ± 1.2 b | 12.3 ± 0.6 c | 23.3 ± 0.6 d | 19.3 ± 1.0 |
| NL5 | 35.3 ± 1.5 | 18.3 ± 0.6 | 40.0 ± 0.0 | 25.0 ± 1.0 |
| NBL5 | 33.7 ± 0.6 | 13.0 ± 1.0 | 26.3 ± 1.5 d | 25.3 ± 1.2 |
| NL7.5 | 35.7 ± 0.6 | 19.0 ± 1.0 | 37.7 ± 1.5 | 25.0 ± 1.0 |
| NBL7.5 | 35.0 ± 1.0 | 14.3 ± 0.6 | 23.7 ± 2.5 d | 26.7 ± 0.6 |
| Formulation Code | Lincomycin HCl (% w/w) | Borneol (% w/w) | NMP |
|---|---|---|---|
| NL1 | 1 | - | 99 |
| NL5 | 5 | - | 95 |
| NL7.5 | 7.5 | - | 92.5 |
| NB40 | - | 40 | 60 |
| NBL1 | 1 | 40 | 59 |
| NBL5 | 5 | 40 | 55 |
| NBL7.5 | 7.5 | 40 | 52.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puyathorn, N.; Lertsuphotvanit, N.; Chantadee, T.; Pichayakorn, W.; Phaechamud, T. Lincomycin HCl-Loaded Borneol-Based In Situ Gel for Periodontitis Treatment. Gels 2023, 9, 495. https://doi.org/10.3390/gels9060495
Puyathorn N, Lertsuphotvanit N, Chantadee T, Pichayakorn W, Phaechamud T. Lincomycin HCl-Loaded Borneol-Based In Situ Gel for Periodontitis Treatment. Gels. 2023; 9(6):495. https://doi.org/10.3390/gels9060495
Chicago/Turabian StylePuyathorn, Napaphol, Nutdanai Lertsuphotvanit, Takron Chantadee, Wiwat Pichayakorn, and Thawatchai Phaechamud. 2023. "Lincomycin HCl-Loaded Borneol-Based In Situ Gel for Periodontitis Treatment" Gels 9, no. 6: 495. https://doi.org/10.3390/gels9060495
APA StylePuyathorn, N., Lertsuphotvanit, N., Chantadee, T., Pichayakorn, W., & Phaechamud, T. (2023). Lincomycin HCl-Loaded Borneol-Based In Situ Gel for Periodontitis Treatment. Gels, 9(6), 495. https://doi.org/10.3390/gels9060495










