Carbon Nanofiber—Sodium Alginate Composite Aerogels Loaded with Vitamin D: The Cytotoxic and Apoptotic Effects on Colon Cancer Cells
Abstract
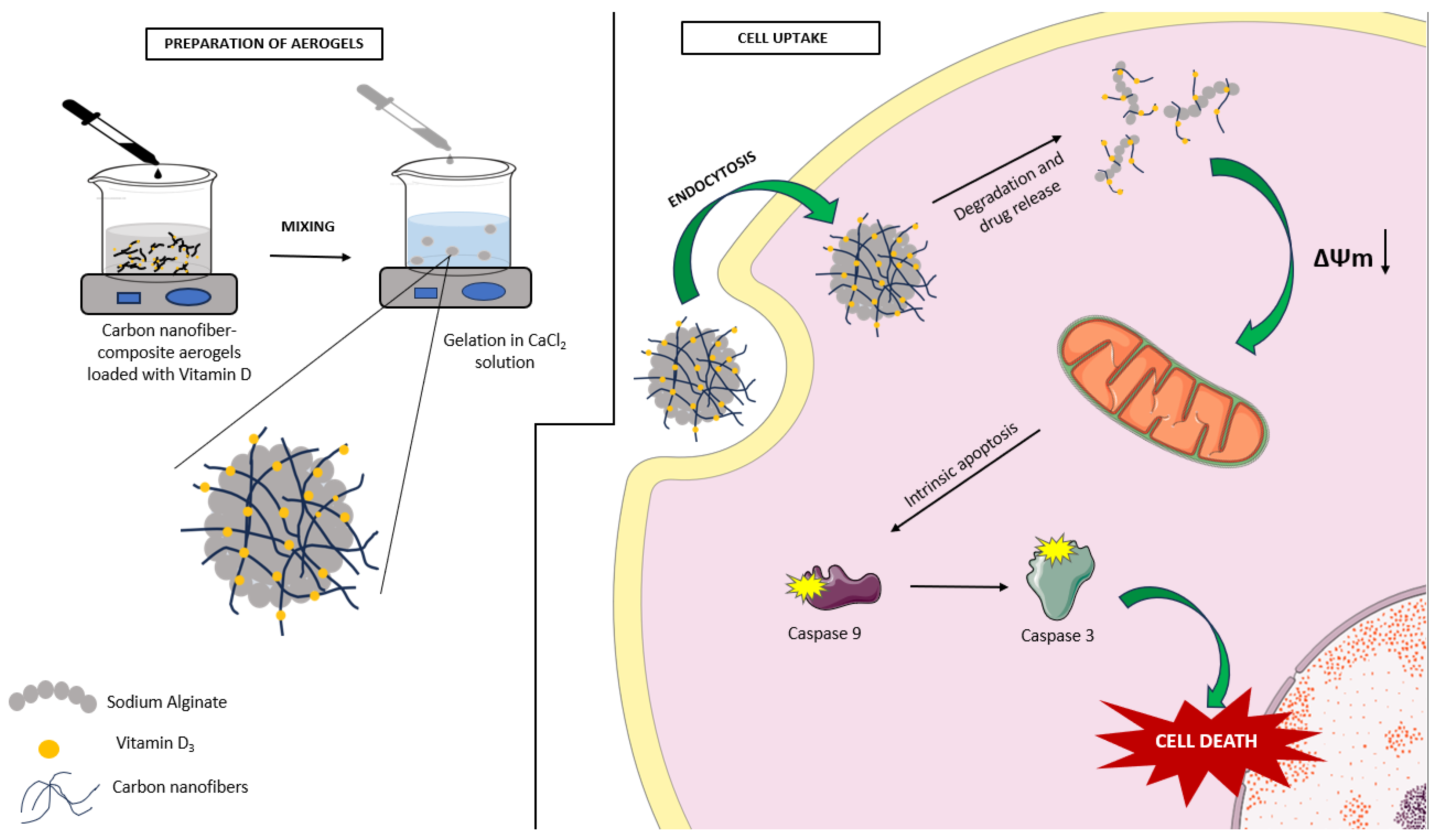
1. Introduction
2. Results and Discussion
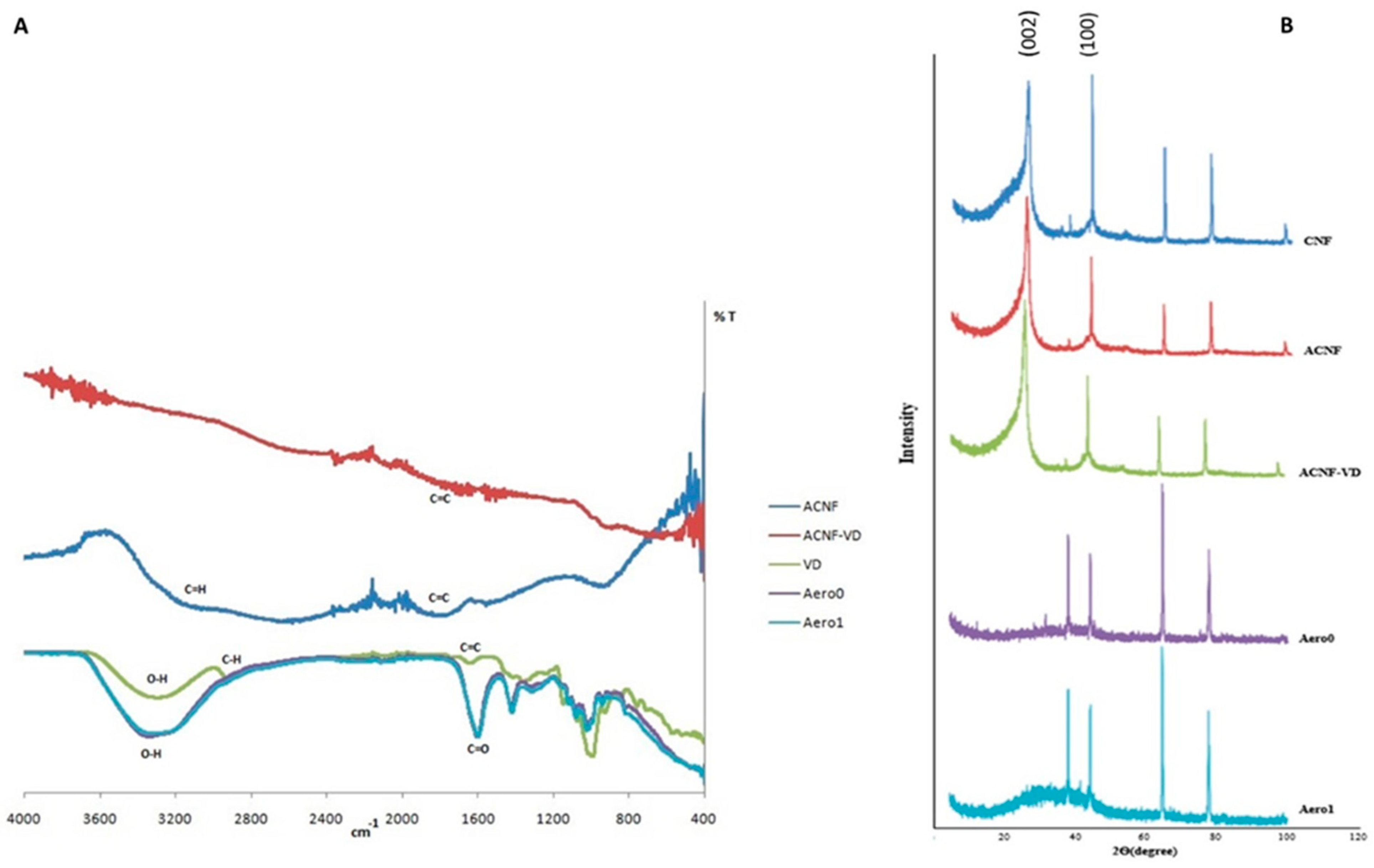
2.1. Characterization of Aerogels
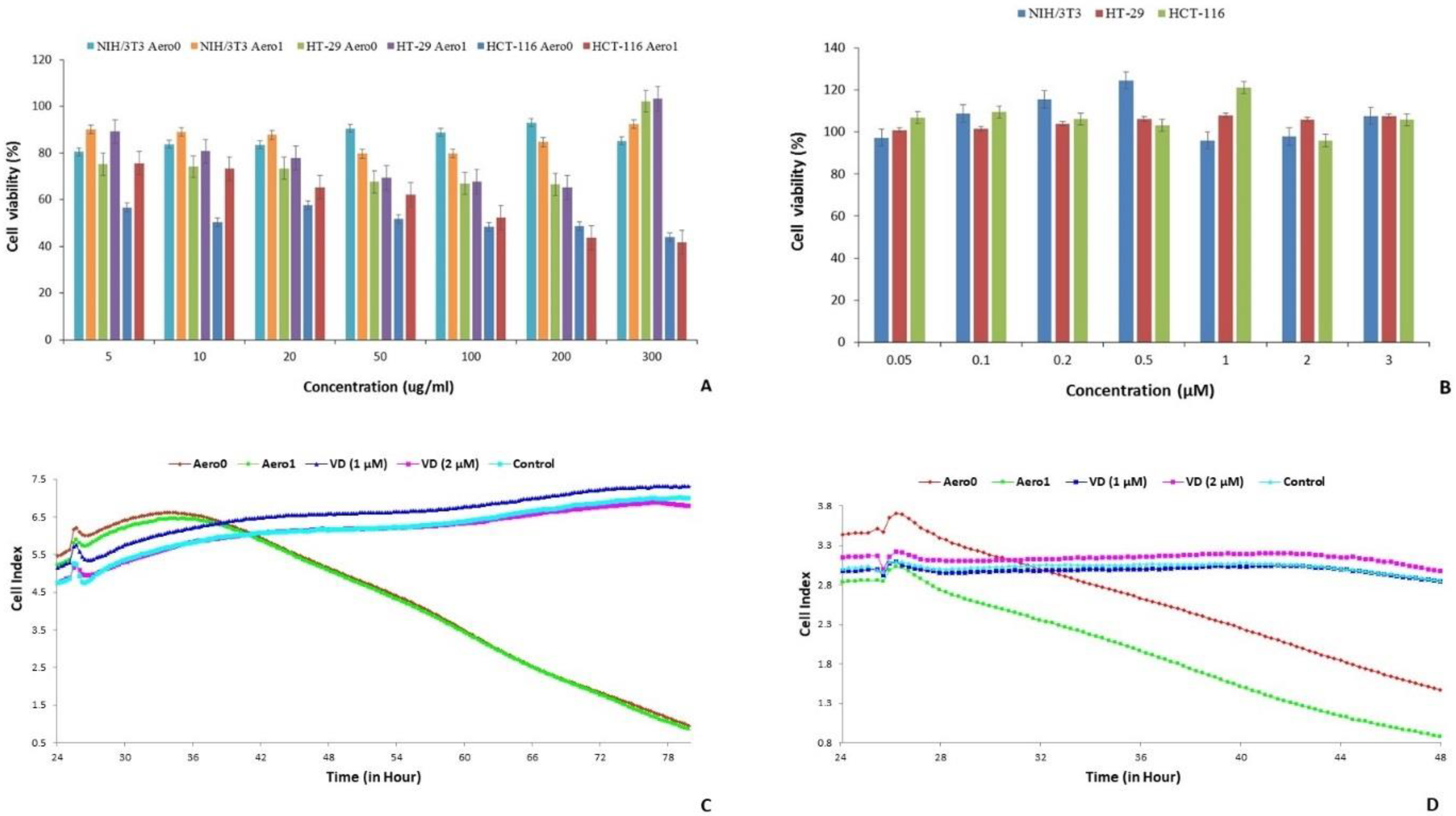
2.2. Assessment of Cell Viability of Aerogels
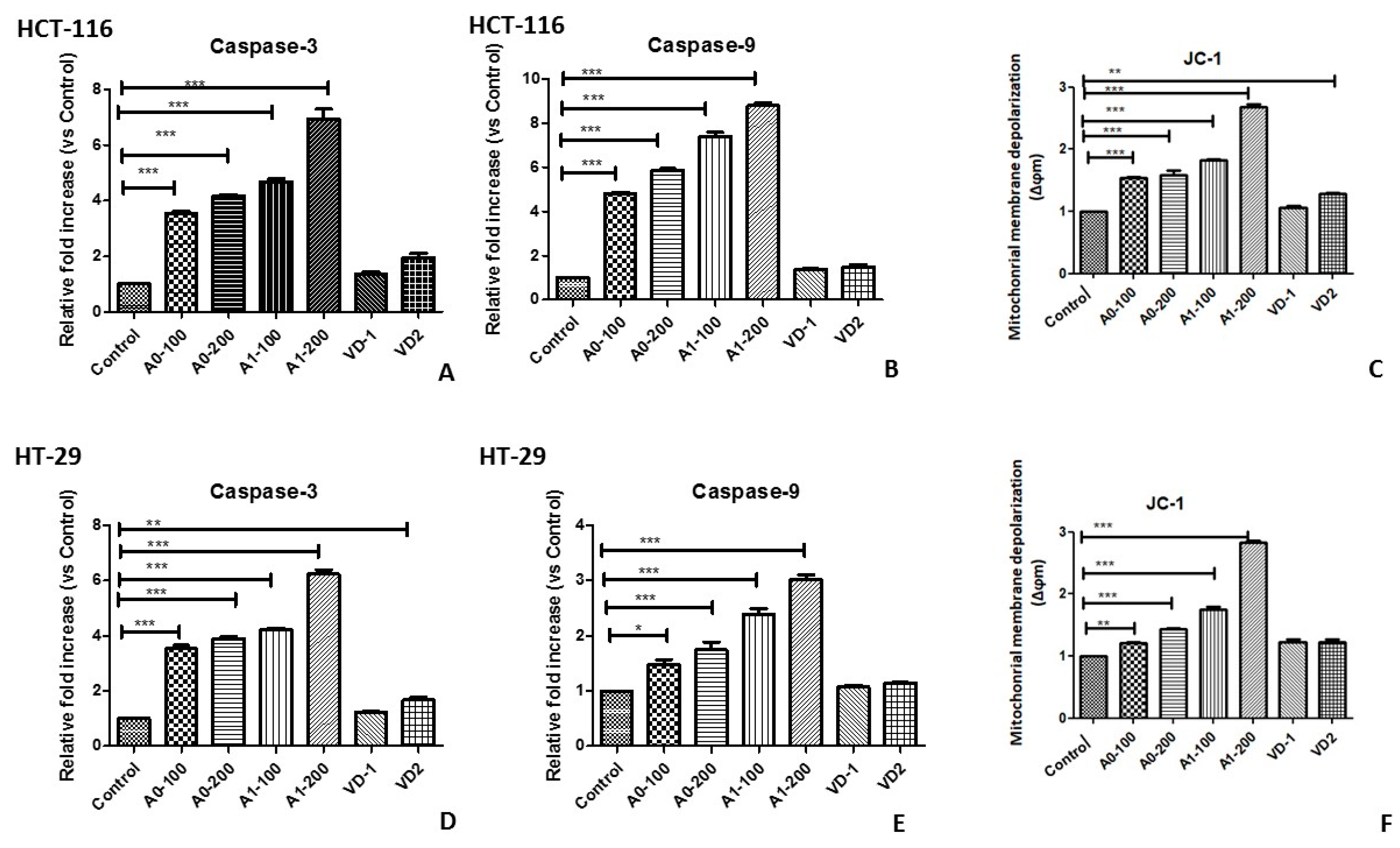
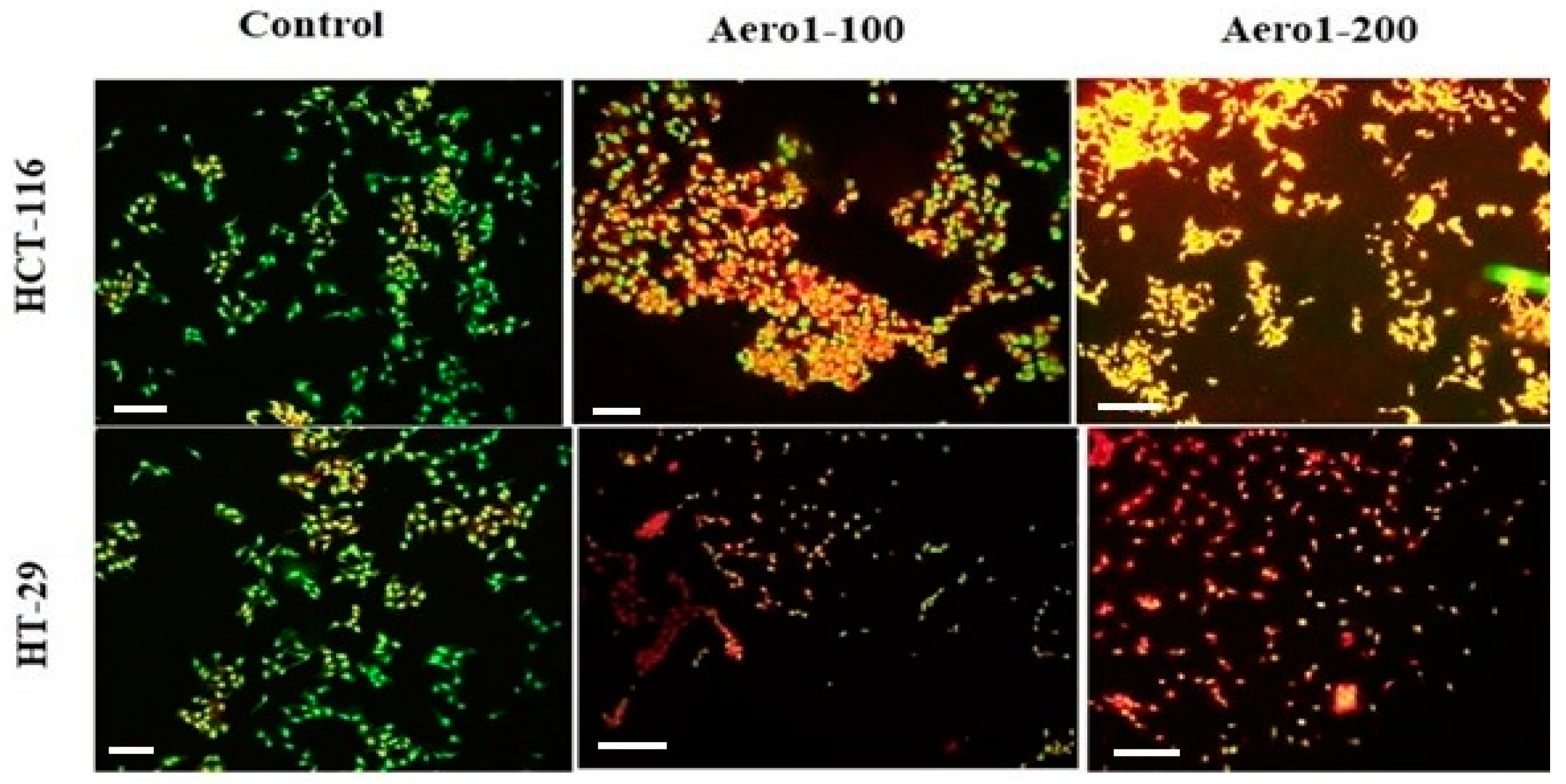
2.3. Apoptotic Potential of Aerogels
2.4. Anti-Migratory Effects of Aerogels
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Vitamin D Solution
4.3. VD Loading and Preparation of Aerogels
- Vitamin D: 1 µM (VD 1);
- Vitamin D: 2 µM (VD 2);
- ACNF (Activated carbon nanofibers) (150 mg) − VD (50 mg) (ACNF − VD);
- Aerogel (sodium alginate) (200 mg) + ACNF (4 mg) (Aero0);
- Aerogel (sodium alginate) (200 mg) + ACNF − VD (4 mg) (Aero1).
4.4. Measurements and Characterization of Aerogels
4.5. Cell Culture Studies
4.6. Assessment of Cell Viability of Aerogels
4.7. Monitoring Cell Proliferation Using the Xcelligence Real-Time Cell Analysis (RTCA)
4.8. Apoptotic Potential of Aerogels
4.8.1. Measurement of Caspase Enzyme Activitiy
4.8.2. Determination of Mitochondrial Membrane Potential (MMP)
4.8.3. Estimation of Apoptosis by Acridine Orange and Ethidium Bromide Staining
4.9. Cell Migration Analysis of Aerogels
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A. Jemal, Global cancer statistics, 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.M.; Tabah-Fisch, I.; Bleiberg, H.; Gramont, A.; Tournigand, C.; Andre, T.; Rothenberg, M.L.; Green, E.; Sargent, D.J. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J. Clin. Oncol. 2006, 24, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Son, H.S.; Lee, W.Y.; Lee, W.S.; Yun, S.H.; Chun, H.K. Compliance and Effective Management of the Hand-Foot Syndrome in Colon Cancer Patients Receiving Capecitabine as Adjuvant Chemotherapy. Yonsei Med. J. 2009, 50, 796–802. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation Therapy-Associated Toxicity: Etiology, Management, and Prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Manz, S.M.; Losa, M.; Fritsch, R.; Scharl, M. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Ther. Adv. Gastroenterol. 2021, 14, 17562848211002018. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Szabados, B.; Leung, C.; Sahdev, A. Adverse effects and radiological manifestations of new immunotherapy agents. Br. J. Radiol. 2018, 92, 20180164. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, M.; Baghbani, E.; Amini, M.; Rezaei, T.; Aghanejad, A.; Mosafer, J.; Mokhtarzadeh, A.; Baradaran, B. MicroRNA-193a and taxol combination: A new strategy for treatment of colorectal cancer. J. Cell. Biochem. 2020, 121, 1388–1399. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Qiu, T.; Tang, M.; Zhang, X.; Dong, W. In vitro and in vivo combinatorial anticancer effects of oxaliplatin- and resveratrol-loaded N,O-carboxymethyl chitosan nanoparticles against colorectal cancer. Eur. J. Pharm. Sci. 2021, 163, 105864. [Google Scholar] [CrossRef]
- Levine, A.J.; Harper, J.M.; Ervin, C.M.; Chen, Y.H.; Harmon, E.; Xue, S.; Lee, E.R.; Frankel, H.D.; Haile, R.W. Serum 25-hydroxyvitamin D, dietary calcium in take, and distal colorectal adenoma risk. Nutr. Cancer 2001, 39, 35–41. [Google Scholar] [CrossRef]
- Freedman, D.M.; Dosemeci, M.; McGlynn, K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: A composite death certificate based case-control study. Occup. Environ. Med. 2002, 59, 257–262. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; Buijs, L.F.; Blackmur, J.P.; Theodoratou, E.; Zgaga, L.; Din, F.V.N.; Farrington, S.M.; Dunlop, M.G. The effect of vitamin D supplementation on survival in patients with colorectal cancer: Systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer 2020, 123, 1705–1712. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, T.; Cao, N.; Ni, J.; Wang, X. Role of Vitamin D Metabolism and Activity on Carcinogenesis. Oncol. Res. 2015, 22, 129. [Google Scholar] [CrossRef]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Peng, X.; Alimirah, F.; Murillo, G.; Mehta, R. Vitamin D and breast cancer: Emerging concepts. Cancer Lett. 2013, 334, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. Vitamin D, Vitamin D Receptor, and Macroautophagy in Inflammation and Infection. Discov. Med. 2011, 11, 325–335. [Google Scholar]
- Nguyen, T.L.U.; Tey, S.W.; Pourgholami, M.H.; Morris, D.L.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Synthesis of semi-biodegradable crosslinked microspheres for the delivery of 1,25 dihydroxyvitamin D3 for the treatment of hepatocellular carcinoma. Eur. Polym. J. 2007, 43, 1754–1767. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.; Pereira, M.C. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J. Nanotechnol. 2015, 12, 1306–1318. [Google Scholar] [CrossRef]
- Nicolas, S.; Bolzinger, M.A.; Jordheim, L.P.; Chevalier, Y.; Fessi, H.; Almouazen, E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 2018, 550, 170–179. [Google Scholar] [CrossRef]
- Xing, R.; Mustapha, O.; Ali, T.; Rehman, M.; Zaidi, S.S.; Baseer, A.; Batool, S.; Mukhtiar, M.; Shafique, S.; Malik, M.; et al. Development, Characterization, and Evaluation of SLN-Loaded Thermoresponsive Hydrogel System of Topotecan as Biological Macromolecule for Colorectal Delivery. BioMed Res. Int. 2021, 3, 9968602. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Jinka, S.; Rachamalla, H.K.; Bhattacharyya, T.; Sridharan, K.; Jaggarapu, M.M.S.; Yakati, V.; Banerjee, R. Glucocorticoid receptor-targeted liposomal delivery system for delivering small molecule ESC8 and anti-miR-Hsp90 gene construct to combat colon cancer. Biomed. Mater. 2021, 18, 024105. [Google Scholar] [CrossRef]
- Mahmood, M.; Karmakar, A.; Fejleh, A.; Mocan, T.; Iancu, C.; Mocan, L.; Iancu, D.T.; Xu, Y.; Dervishi, E.; Li, Z.; et al. Synergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drug. Nanomedicine 2009, 4, 883–893. [Google Scholar] [CrossRef]
- Mahmood, M.; Casciano, D.A.; Mocan, T.; Iancu, C.; Xu, Y.; Mocan, L.; Iancu, D.T.; Dervishi, E.; Li, Z.; Abdalmuhsen, M.; et al. Cytotoxicity and biological effects of functional nanomaterials delivered to various cell lines. J. Appl. Toxicol. 2010, 30, 74–83. [Google Scholar] [CrossRef]
- Arya, N.; Arora, A.; Vasu, K.S.; Sood, A.K.; Katti, D.S. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: A reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 2013, 5, 2818–2829. [Google Scholar] [CrossRef] [PubMed]
- Ringel, J.; Erdmann, K.; Hampel, S.; Kraemer, K.; Maier, D.; Arlt, M.; Kunze, D.; Wirth, M.P.; Fuessel, S. CNFs and carbon nanotubes sensitize prostate and bladder cancer cells to platinum-based chemotherapeutics. J. Biomed. Nanotechnol. 2014, 10, 463–477. [Google Scholar] [CrossRef]
- Vallabani, N.V.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of graphene in normal human lung cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.J.; Gao, X.; Nie, G. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly (lactic-co-glycolic acid): CNF composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Aachmann, F.L.; Serrano-Aroca, A. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of CNFs (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Llorens-Gámez, M.; Serrano-Aroca, Á. Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties. Polymers 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Gámez, M.; Salesa, B.; Serrano-Aroca, A. Physical and biological properties of alginate/carbon nanofibers hydrogel films. Int. J. Biol. Macromol. 2020, 151, 499–507. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.-M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Zhang, H.; Ma, X.; Su, W.; Sun, X.; Li, X. Bio-responsive alginate-keratin composite nanogels with enhanced drug loading efficiency for cancer therapy. Carbohydr. Polym. 2017, 175, 159–169. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; de Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Kiani, A.; Fathi, M.; Ghasemi, S.M. Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int. J. Food Prop. 2017, 20, 2466–2476. [Google Scholar] [CrossRef]
- Helmiyati and Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Malaysia, 26–27 July 2016; IOP Publishing: Bristol, UK, 2017; Volume 188, p. 012019. [Google Scholar]
- Atieh, M.A. Effect of functionalized carbon nanofibers with carboxylic function group on the removal of zinc from water. Int. J. Environ. Sci. Dev. 2011, 2, 142. [Google Scholar] [CrossRef]
- Wang, J.; Salihi, E.C.; Šiller, L. Green reduction of graphene oxide using alanine. Mater. Sci. Eng. 2017, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Demirhan, K.; Ozakpinar, O.B.; Salihi, E.C. Green and one step modification of graphene oxide using natural substances. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 716–723. [Google Scholar] [CrossRef]
- Cong, R.; Choi, J.Y.; Song, J.B.; Jo, M.; Lee, H.; Lee, C.S. Characteristics and electrochemical performances of silicon/carbon nanofiber/graphene composite films as anode materials for binder-free lithium-ion batteries. Sci. Rep. 2021, 11, 1283. [Google Scholar] [CrossRef]
- Salihi, E.C.; Tulay, E.C. Adsorptive removal of antipsychotic drug by carbon nanofibers in a batch and fixed bed column system. Part. Sci. Technol. 2022, 40, 899–910. [Google Scholar] [CrossRef]
- Rajesh, R.; Ravichandran, Y.D. Development of a new carbon nanotube–alginate–hydroxyapatite tricomponent composite scaffold for application in bone tissue engineering. Int. J. Nanomed. 2015, 10 (Suppl. S1), 7. [Google Scholar]
- Shaari, N.; Kamarudin, S.K. Sodium alginate/alumina composite biomembrane preparation and performance in DMFC application. Polym. Test. 2020, 81, 106183. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Utomo, D.P.; Raemas, A.F.A.; Kusworo, T.D.; Jos, B.; Djaeni, M. Alginate/κ-carrageenan-based edible films incorporated with clove essential oil: Physico-chemical characterization and antioxidant-antimicrobial activity. Polymers 2021, 13, 354. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Moukayed, M.; Grant, W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Wierzbicka, J.M.; Binek, A.; Ahrends, T.; Nowacka, J.D.; Szydłowska, A.; Turczyk, L.; Wąsiewicz, T.; Wierzbicki, P.M.; Sądej, R.; Tuckey, R.C.; et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int. J. Oncol. 2015, 47, 1084–1096. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef]
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086. [Google Scholar] [CrossRef]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D (3) on the G (1)-S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar]
- Kisin, E.R.; Murray, A.R.; Sargent, L.; Lowry, D.; Chirila, M.; Siegrist, K.J.; Schwegler-Berry, D.; Leonard, S.; Castranova, V.; Fadeel, B.; et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol. Appl. Pharmacol. 2011, 252, 1–10. [Google Scholar] [CrossRef]
- Grabinski, C.; Hussain, S.; Lafdi, K.; Braydich-Stolle, L.; Schlager, J. Effect of particle dimension on biocompatibility of carbon nanomaterial. Carbon 2007, 45, 2828–2835. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Z.; Novak, Z. Preparation of multi-membrane alginate aerogels used for drug delivery. J. Supercrit. Fluids 2013, 79, 209–218. [Google Scholar] [CrossRef]
- Varghese, J.E.; Shanmugam, V.; Rengarajan, R.L.; Meyyazhagan, A.; Arumugam, V.A.; Al-Misned, F.A.; El-Serehy, H.A. Role of vitamin D3 on apoptosis and inflammatory-associated gene in colorectal cancer: An in vitro approach. J. King Saud Univ. Sci. 2020, 32, 2786–2789. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Almajwal, A.; Alam, I.; Jahan, S. Growth inhibition and apoptosis in colorectal cancer cells induced by Vitamin D-Nanoemulsion (NVD): Involvement of Wnt/β-catenin and other signal transduction pathways. Cell Biosci. 2019, 9, 1–23. [Google Scholar] [CrossRef]
- Sabzichi, M.; Mohammadian, J.; Mohammadi, M.; Jahanfar, F.; MovassaghPour, A.; Hamishehkar, H.; Ostad-Rahimi, A. Vitamin D-Loaded Nanostructured Lipid Carrier (NLC): A New Strategy for Enhancing Efficacy of Doxorubicin in Breast Cancer Treatment. Nutr. Cancer 2017, 69, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Almouazen, E.; Bourgeois, S.; Jordheim, L.P.; Fessi, H.; Briançon, S. Nano-encapsulation of vitamin D3 active metabolites for application in chemotherapy: Formulation study and in vitro evaluation. Pharm. Res. 2013, 30, 1137–1146. [Google Scholar] [CrossRef]
- Li, H.X.; Gao, J.M.; Liang, J.Q.; Xi, J.M.; Fu, M.; Wu, Y.J. Vitamin D3 potentiates the growth inhibitory effects of metformin in DU145 human prostate cancer cells mediated by AMPK/mTOR signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, S.; Lin, G.; Song, C.; He, Z. Vitamin D enhances omega-3 polyunsaturated fatty acids-induced apoptosis in breast cancer cells. Cell Biol. Int. 2017, 41, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Salihi, E.C.; Berber, B.; İsanç, K. Kinetic adsorption of drugs using carbon nanofibers in simulated gastric and intestinal fluids. Int. J. Chem. Kinet. 2022, 55, 102–110. [Google Scholar] [CrossRef]
- Beekman, A.C.; Barentsen, A.R.; Woerdenbag, H.J.; Uden, W.V.; Pras, N. Stereochemistry-dependent cytotoxicity of some artemisinin derivatives. J. Nat. Prod. 1997, 60, 325–330. [Google Scholar] [CrossRef]
- Ozakpinar, O.B.; Ture, A.; Kuçukguzel, I. Molecular modeling and assessment of cytotoxic and apoptotic potential of imatinib analogues featuring (thio)urea motifs in human leukemia and lymphoma. J. Res. Pharm. 2020, 24, 801–811. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingol Ozakpinar, O.; Dastan, H.; Gurboga, M.; Sayin, F.S.; Ozsavci, D.; Caliskan Salihi, E. Carbon Nanofiber—Sodium Alginate Composite Aerogels Loaded with Vitamin D: The Cytotoxic and Apoptotic Effects on Colon Cancer Cells. Gels 2023, 9, 561. https://doi.org/10.3390/gels9070561
Bingol Ozakpinar O, Dastan H, Gurboga M, Sayin FS, Ozsavci D, Caliskan Salihi E. Carbon Nanofiber—Sodium Alginate Composite Aerogels Loaded with Vitamin D: The Cytotoxic and Apoptotic Effects on Colon Cancer Cells. Gels. 2023; 9(7):561. https://doi.org/10.3390/gels9070561
Chicago/Turabian StyleBingol Ozakpinar, Ozlem, Havva Dastan, Merve Gurboga, Fatih Serdar Sayin, Derya Ozsavci, and Elif Caliskan Salihi. 2023. "Carbon Nanofiber—Sodium Alginate Composite Aerogels Loaded with Vitamin D: The Cytotoxic and Apoptotic Effects on Colon Cancer Cells" Gels 9, no. 7: 561. https://doi.org/10.3390/gels9070561
APA StyleBingol Ozakpinar, O., Dastan, H., Gurboga, M., Sayin, F. S., Ozsavci, D., & Caliskan Salihi, E. (2023). Carbon Nanofiber—Sodium Alginate Composite Aerogels Loaded with Vitamin D: The Cytotoxic and Apoptotic Effects on Colon Cancer Cells. Gels, 9(7), 561. https://doi.org/10.3390/gels9070561






