Applying Gel-Supported Liquid Extraction to Tutankhamun’s Textiles for the Identification of Ancient Colorants: A Case Study
Abstract
1. Introduction
2. Results and Discussion
2.1. UV–Vis Spectroscopy
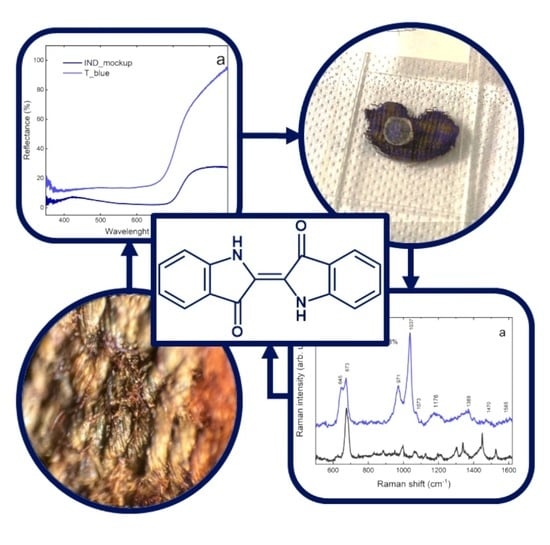
2.2. Case Study: Archaeological Textile Fragment from Tutankhamun’s Tomb
2.2.1. Gel Micro-Extraction In Situ
2.2.2. Fiber Optic Reflectance Spectroscopy (FORS)
2.2.3. Surface Enhanced Raman Scattering (SERS)
3. Conclusions
4. Materials and Methods
4.1. Commercial Products
4.2. Mock-Ups Preparation
4.3. Archaeological Sample: Textile Fragment from Tutankhamun’s Tomb
4.4. Gel Preparation and Micro-Extraction In Situ
4.5. Preparation of Ag-Colloidal Pastes
4.6. Spectroscopic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Oxford Encyclopedia of Ancient Egypt, 1st ed.; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19-510234-5.
- Ahmed, H.E. History of Natural Dyes in North Africa ‘Egypt’. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 27–36. ISBN 978-0-470-74497-0. [Google Scholar]
- Angelini, L.G.; Pistelli, L.; Belloni, P.; Bertoli, A.; Panconesi, S. Rubia Tinctorum a Source of Natural Dyes: Agronomic Evaluation, Quantitative Analysis of Alizarin and Industrial Assays. Ind. Crops Prod. 1997, 6, 303–311. [Google Scholar] [CrossRef]
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype: London, UK, 2007; ISBN 978-1-904982-00-5. [Google Scholar]
- Degano, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical Methods for the Characterization of Organic Dyes in Artworks and in Historical Textiles. Appl. Spectrosc. Rev. 2009, 44, 363–410. [Google Scholar] [CrossRef]
- Sousa, M.M.; Miguel, C.; Rodrigues, I.; Parola, A.J.; Pina, F.; Seixas de Melo, J.S.; Melo, M.J. A Photochemical Study on the Blue Dye Indigo: From Solution to Ancient Andean Textiles. Photochem. Photobiol. Sci. 2008, 7, 1353. [Google Scholar] [CrossRef]
- Cybulska, M. Archaeological Textiles—A Need for New Methods of Analysis and Reconstruction. Fibres Text. East. Eur. 2007, 15, 64–65. [Google Scholar]
- Shahid, M.; Wertz, J.; Degano, I.; Aceto, M.; Khan, M.I.; Quye, A. Analytical Methods for Determination of Anthraquinone Dyes in Historical Textiles: A Review. Anal. Chim. Acta 2019, 1083, 58–87. [Google Scholar] [CrossRef] [PubMed]
- Calà, E.; Gosetti, F.; Gulmini, M.; Serafini, I.; Ciccola, A.; Curini, R.; Salis, A.; Damonte, G.; Kininger, K.; Just, T.; et al. It’s Only a Part of the Story: Analytical Investigation of the Inks and Dyes Used in the Privilegium Maius. Molecules 2019, 24, 2197. [Google Scholar] [CrossRef] [PubMed]
- Calà, E.; Benzi, M.; Gosetti, F.; Zanin, A.; Gulmini, M.; Idone, A.; Serafini, I.; Ciccola, A.; Curini, R.; Whitworth, I.; et al. Towards the Identification of the Lichen Species in Historical Orchil Dyes by HPLC-MS/MS. Microchem. J. 2019, 150, 104140. [Google Scholar] [CrossRef]
- Han, J.; Wanrooij, J.; Van Bommel, M.; Quye, A. Characterisation of Chemical Components for Identifying Historical Chinese Textile Dyes by Ultra High Performance Liquid Chromatography—Photodiode Array—Electrospray Ionisation Mass Spectrometer. J. Chromatogr. A 2017, 1479, 87–96. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M.; Picollo, M.; Ricciardi, P.; Delaney, J.K. Characterisation of Colourants on Illuminated Manuscripts by Portable Fibre Optic UV-Visible-NIR Reflectance Spectrophotometry. Anal. Methods 2014, 6, 1488. [Google Scholar] [CrossRef]
- Picollo, M.; Cucci, C.; Casini, A.; Stefani, L. Hyper-Spectral Imaging Technique in the Cultural Heritage Field: New Possible Scenarios. Sensors 2020, 20, 2843. [Google Scholar] [CrossRef] [PubMed]
- Cazenobe, I.; Bacci, M.; Picollo, M.; Radicati, B.; Bacci, G.; Conti, S.; Lanterna, G.; Porcinai, S. Non-Destructive Spectroscopic Investigation of Dyed Textiles: An Application to Yellow Dyed Wool Samples. In Proceedings of the Preprints of the 13th Triennial ICOM Meeting, Rio de Janeiro, Brazil, 22–27 September 2002; Volume 1, pp. 238–244. [Google Scholar]
- Peruzzi, G.; Cucci, C.; Picollo, M.; Quercioli, F.; Stefani, L. Non-Invasive Identification of Dyed Textiles by Using Vis-NIR FORS and Hyperspectral Imaging Techniques. Cult. Sci.Color.-Color Cult. Sci. J. 2021, 13, 61. [Google Scholar] [CrossRef]
- Ciccola, A.; Tozzi, L.; Romani, M.; Serafini, I.; Ripanti, F.; Curini, R.; Vitucci, F.; Cestelli Guidi, M.; Postorino, P. Lucio Fontana and the Light: Spectroscopic Analysis of the Artist’s Collection at the National Gallery of Modern and Contemporary Art. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118319. [Google Scholar] [CrossRef]
- Ciccola, A.; Serafini, I.; D’Agostino, G.; Giambra, B.; Bosi, A.; Ripanti, F.; Nucara, A.; Postorino, P.; Curini, R.; Bruno, M. Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage 2021, 4, 1807–1820. [Google Scholar] [CrossRef]
- Alp, Z.; Ciccola, A.; Serafini, I.; Nucara, A.; Postorino, P.; Gentili, A.; Curini, R.; Favero, G. Photons for Photography: A First Diagnostic Approach to Polaroid Emulsion Transfer on Paper in Paolo Gioli’s Artworks. Molecules 2022, 27, 7023. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Leona, M. Surface-Enhanced Raman Spectroscopy in Art and Archaeology: SERS in Art and Archaeology. J. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Whitney, A.V.; Van Duyne, R.P.; Casadio, F. An Innovative Surface-Enhanced Raman Spectroscopy (SERS) Method for the Identification of Six Historical Red Lakes and Dyestuffs. J. Raman Spectrosc. 2006, 37, 993–1002. [Google Scholar] [CrossRef]
- Leona, M. Sub-Nanogram Level Identification of Alizarin by Surface Enhanced Raman Scattering. In Proceedings of the IRUG6, Florence, Italy, 29 March–1 April 2004; pp. 105–112. [Google Scholar]
- Chang, J.; Cañamares, M.V.; Aydin, M.; Vetter, W.; Schreiner, M.; Xu, W.; Lombardi, J.R. Surface-Enhanced Raman Spectroscopy of Indanthrone and Flavanthrone: SERS from Indanthrone and Flavanthrone. J. Raman Spectrosc. 2009, 40, 1557–1563. [Google Scholar] [CrossRef]
- Colantonio, C.; Lanteri, L.; Ciccola, A.; Serafini, I.; Postorino, P.; Censorii, E.; Rotari, D.; Pelosi, C. Imaging Diagnostics Coupled with Non-Invasive and Micro-Invasive Analyses for the Restoration of Ethnographic Artifacts from French Polynesia. Heritage 2022, 5, 215–232. [Google Scholar] [CrossRef]
- Serafini, I.; Ciccola, A. Nanotechnologies and Nanomaterials. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–380. ISBN 978-0-12-813910-3. [Google Scholar]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Kneipp, K.; Moskovits, M.; Kneipp, H. (Eds.) Surface-Enhanced Raman Scattering: Physics and Applications; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006; ISBN 978-3-540-33566-5. [Google Scholar]
- Leona, M.; Decuzzi, P.; Kubic, T.A.; Gates, G.; Lombardi, J.R. Nondestructive Identification of Natural and Synthetic Organic Colorants in Works of Art by Surface Enhanced Raman Scattering. Anal. Chem. 2011, 83, 3990–3993. [Google Scholar] [CrossRef]
- Lofrumento, C.; Ricci, M.; Platania, E.; Becucci, M.; Castellucci, E. SERS Detection of Red Organic Dyes in Ag-Agar Gel: Detection of Red Organic Dyes in Ag-Agar Gel. J. Raman Spectrosc. 2013, 44, 47–54. [Google Scholar] [CrossRef]
- Becucci, M.; Ricci, M.; Lofrumento, C.; Castellucci, E. Identification of Organic Dyes by Surface-Enhanced Raman Scattering in Nano-Composite Agar-Gel Matrices: Evaluation of the Enhancement Factor. Opt. Quant. Electron. 2016, 48, 449. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Peruzzi, G.; Nigro, V.; Postorino, P.; Curini, R.; Favero, G. Gel Microextraction from Hydrophilic Paint Layers: A Comparison between Agar-Gel and Nanorestore Gel® HWR for Spectroscopic Identification of Madder. Microchem. J. 2023, 187, 108447. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Lombardi, L.; Ciccola, A.; Serafini, I.; Bianco, A.; Postorino, P.; Pellegrino, L.; Bruno, M. Deepening Inside the Pictorial Layers of Etruscan Sarcophagus of Hasti Afunei: An Innovative Micro-Sampling Technique for Raman/SERS Analyses. Molecules 2019, 24, 3403. [Google Scholar] [CrossRef]
- Platania, E.; Lofrumento, C.; Lottini, E.; Azzaro, E.; Ricci, M.; Becucci, M. Tailored Micro-Extraction Method for Raman/SERS Detection of Indigoids in Ancient Textiles. Anal. Bioanal. Chem. 2015, 407, 6505–6514. [Google Scholar] [CrossRef]
- Germinario, G.; Ciccola, A.; Serafini, I.; Ruggiero, L.; Sbroscia, M.; Vincenti, F.; Fasolato, C.; Curini, R.; Ioele, M.; Postorino, P.; et al. Gel Substrates and Ammonia-EDTA Extraction Solution: A New Non-Destructive Combined Approach for the Identification of Anthraquinone Dyes from Wool Textiles. Microchem. J. 2020, 155, 104780. [Google Scholar] [CrossRef]
- Lombardi, L.; Serafini, I.; Guiso, M.; Sciubba, F.; Bianco, A. A New Approach to the Mild Extraction of Madder Dyes from Lake and Textile. Microchem. J. 2016, 126, 373–380. [Google Scholar] [CrossRef]
- Platania, E.; Lombardi, J.R.; Leona, M.; Shibayama, N.; Lofrumento, C.; Ricci, M.; Becucci, M.; Castellucci, E. Suitability of Ag-Agar Gel for the Micro-Extraction of Organic Dyes on Different Substrates: The Case Study of Wool, Silk, Printed Cotton and a Panel Painting Mock-up: Suitability of Ag-Agar Gel. J. Raman Spectrosc. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Fonseca, B.; Schmidt Patterson, C.; Ganio, M.; MacLennan, D.; Trentelman, K. Seeing Red: Towards an Improved Protocol for the Identification of Madder- and Cochineal-Based Pigments by Fiber Optics Reflectance Spectroscopy (FORS). Herit. Sci. 2019, 7, 92. [Google Scholar] [CrossRef]
- Gulmini, M.; Idone, A.; Diana, E.; Gastaldi, D.; Vaudan, D.; Aceto, M. Identification of Dyestuffs in Historical Textiles: Strong and Weak Points of a Non-Invasive Approach. Dye. Pigment. 2013, 98, 136–145. [Google Scholar] [CrossRef]
- Bloxam, W.P.; Perkin, A.G. CXXXV—Indirubin. Part I. J. Chem. Soc., Trans. 1910, 97, 1460–1475. [Google Scholar] [CrossRef]
- Ju, Z.; Sun, J.; Liu, Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules 2019, 24, 3831. [Google Scholar] [CrossRef] [PubMed]
- Poulin, J. Identification of Indigo and Its Degradation Products on a Silk Textile Fragment Using Gas Chromatography-Mass Spectrometry. J. Can. Assoc. Conserv. 2007, 32, 48–56. [Google Scholar]
- Chadha, R.; Maiti, N.; Kapoor, S. Interaction of Anthranilic Acid with Silver Nanoparticles: A Raman, Surface-Enhanced Raman Scattering and Density Functional Theoretical Study. J. Mol. Struct. 2014, 1076, 35–41. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F. Historical Organic Dyes: A Surface-Enhanced Raman Scattering (SERS) Spectral Database on Ag Lee-Meisel Colloids Aggregated by NaClO 4: SERS Database on Historical Organic Dyes. J. Raman Spectrosc. 2011, 42, 1267–1281. [Google Scholar] [CrossRef]
- Nanotechnologies in the Conservation of Cultural Heritage; Springer: New York, NY, USA, 2014; ISBN 978-94-017-9302-5.
- Royal Society of Chemistry. Nanoscience for the Conservation of Works of Art; Baglioni, P., Chelazzi, D., Eds.; The Royal Society of Chemistry: London, UK, 2013; ISBN 978-1-84973-566-7. [Google Scholar]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Pensabene Buemi, L.; Petruzzellis, M.L.; Baglioni, P. Twin-Chain Polymer Hydrogels Based on Poly(Vinyl Alcohol) as New Advanced Tool for the Cleaning of Modern and Contemporary Art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Baglioni, M.; Guaragnone, T.; Mastrangelo, R.; Sekine, F.H.; Ogura, T.; Baglioni, P. Nonionic Surfactants for the Cleaning of Works of Art: Insights on Acrylic Polymer Films Dewetting and Artificial Soil Removal. ACS Appl. Mater. Interfaces 2020, 12, 26704–26716. [Google Scholar] [CrossRef]
- Daniels, V.; Devièse, T.; Hacke, M.; Higgitt, C. Technological Insights into Madder Pigment Production in Antiquity; Archetype Books: London, UK, 2014. [Google Scholar]
- Hofenk de Graaff, J.H.; Roelofs, W.G.T.; van Bommel, M.R. The Colourful Past: Origins, Chemistry and Identification of Natural Dyestuffs; Archetype Publ: London, UK, 2004; ISBN 978-1-873132-13-5. [Google Scholar]
- Deutsches Archäologisches Institute (Ed.) Mitteilungen des Deutschen Archäologischen Instituts; Harrassowitz: Berlin, Germany, 2019; ISBN 978-3-447-11194-2. [Google Scholar]
- Idone, A.; Gulmini, M.; Henry, A.-I.; Casadio, F.; Chang, L.; Appolonia, L.; Van Duyne, R.P.; Shah, N.C. Silver Colloidal Pastes for Dye Analysis of Reference and Historical Textile Fibers Using Direct, Extractionless, Non-Hydrolysis Surface-Enhanced Raman Spectroscopy. Analyst 2013, 138, 5895. [Google Scholar] [CrossRef]
- Saviello, D.; Alyami, A.; Trabace, M.; Giorgi, R.; Baglioni, P.; Mirabile, A.; Iacopino, D. Plasmonic Colloidal Pastes for Surface-Enhanced Raman Spectroscopy (SERS) of Historical Felt-Tip Pens. RSC Adv. 2018, 8, 8365–8371. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruzzi, G.; Ciccola, A.; Bosi, A.; Serafini, I.; Negozio, M.; Hamza, N.M.; Moricca, C.; Sadori, L.; Favero, G.; Nigro, V.; et al. Applying Gel-Supported Liquid Extraction to Tutankhamun’s Textiles for the Identification of Ancient Colorants: A Case Study. Gels 2023, 9, 514. https://doi.org/10.3390/gels9070514
Peruzzi G, Ciccola A, Bosi A, Serafini I, Negozio M, Hamza NM, Moricca C, Sadori L, Favero G, Nigro V, et al. Applying Gel-Supported Liquid Extraction to Tutankhamun’s Textiles for the Identification of Ancient Colorants: A Case Study. Gels. 2023; 9(7):514. https://doi.org/10.3390/gels9070514
Chicago/Turabian StylePeruzzi, Greta, Alessandro Ciccola, Adele Bosi, Ilaria Serafini, Martina Negozio, Nagmeldeen Morshed Hamza, Claudia Moricca, Laura Sadori, Gabriele Favero, Valentina Nigro, and et al. 2023. "Applying Gel-Supported Liquid Extraction to Tutankhamun’s Textiles for the Identification of Ancient Colorants: A Case Study" Gels 9, no. 7: 514. https://doi.org/10.3390/gels9070514
APA StylePeruzzi, G., Ciccola, A., Bosi, A., Serafini, I., Negozio, M., Hamza, N. M., Moricca, C., Sadori, L., Favero, G., Nigro, V., Postorino, P., & Curini, R. (2023). Applying Gel-Supported Liquid Extraction to Tutankhamun’s Textiles for the Identification of Ancient Colorants: A Case Study. Gels, 9(7), 514. https://doi.org/10.3390/gels9070514