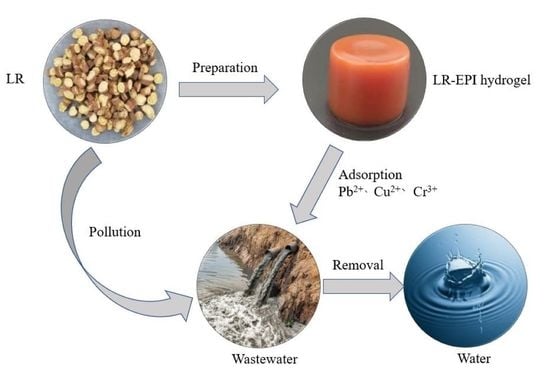
Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Chemistry Composition Analysis
2.2. Morphology and Structure of Hydrogels
2.3. Adsorption Performance of LR-EPI Hydrogels
2.3.1. Effect of Solution pH on Adsorption
2.3.2. Effect of Contact Time on Adsorption
2.3.3. Adsorption Kinetics
2.3.4. Effect of Initial Ion Concentration on Adsorption
2.3.5. Adsorption Isotherm
2.3.6. The Effects of Salt Ions on the Adsorption Capacity of LR-EPI-8
2.3.7. Regeneration
2.4. Adsorption Mechanism of LR-EPI Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.2.1. Pretreatment of LR
4.2.2. Dissolution of LR-NaOH
4.2.3. Preparation of LR-EPI Hydrogels
4.3. Characterization
4.4. Equilibrium Degree of Swelling and Gel Fraction Yield of Hydrogels
4.5. Zeta Potential Measurement
4.6. Adsorption Experiment
4.7. The Reusability of Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Komijani, M.; Shamabadi, N.S.; Shahin, K.; Eghbalpour, F.; Tahsili, M.R.; Bahram, M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Environ. Pollut. 2021, 274, 116569. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, P.; Yang, K.; Wang, Q.; Feng, W.; Tang, Y. PAA/TiO2@ C composite hydrogels with hierarchical pore structures as high efficiency adsorbents for heavy metal ions and organic dyes removal. Desalination 2023, 558, 116620. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.N.; Jang, A.; Jang, M.; Park, C.M.; Son, A.; Her, N.; Heo, J.; Yoon, Y. Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 2022, 286, 131916. [Google Scholar] [CrossRef]
- Yin, X.C.; Liu, X.; Fan, J.C.; Wu, J.J.; Men, J.L.; Zheng, G.S. Preparation of gel resins and removal of copper and lead from water. J. Appl. Polym. Sci. 2016, 134, 44466–44474. [Google Scholar] [CrossRef]
- Chandrabose, G.; Dey, A.; Gaur, S.S.; Pitchaimuthu, S.; Jagadeesan, H.; Braithwaite, N.S.J.; Selvaraj, V.; Kumar, V.; Krishnamurthy, S. Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 2021, 279, 130467–130478. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Xie, H.; Li, X.; Hao, C. Polyacrylic acid/carboxymethyl cellulose/activated carbon composite hydrogel for removal of heavy metal ion and cationic dye. Cellulose 2022, 29, 483–501. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Bo, Y.; Chen, D.; Wang, Y.; Wang, H.; He, Y.; Qin, J. Cellulose-based self-healing hydrogel through boronic ester bonds with excellent biocompatibility and conductivity. RSC Adv. 2020, 10, 11300–11310. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, A.; Aggelopoulos, C.A.; Tsakiroglou, C.D. Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: Adsorption kinetics and equilibrium isotherms as a tool. J. Environ. Chem. Eng. 2018, 6, 6958–6970. [Google Scholar] [CrossRef]
- Yin, X.C.; Zhang, N.D.; Du, M.X.; Zhu, H.; Ke, T. Preparation of bio-absorbents by modifying Liquorice residue via chemical methods and removal of copper ions from wastewater. Water Sci. Technol. 2021, 84, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hu, Z.; Peng, C.; Deng, L.; Liu, S. Effective Adsorption and Sensitive Detection of Cr(VI) by Chitosan/Cellulose Nanocrystals Grafted with Carbon Dots Composite Hydrogel. Polymers 2021, 13, 3788–3798. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Sun, X.F.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper (II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Luo, Y. Adsorption mechanisms of hydrogels for heavy metal and organic dyes removal: A short review. J. Agric. Food Res. 2023, 12, 100552. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A. Assessing of phosphorus removal by polymeric anion exchangers. Desalination 2011, 281, 111–117. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Chinese medicinal herbal residues as a bulking agent for food waste composting. Bioresour. Technol. 2018, 249, 182–188. [Google Scholar] [CrossRef]
- Nagara, V.N.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of heavy metals from stormwater runoff using granulated drinking water treatment residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Xu, L. Biochar from Chinese herb residues as adsorbent for toxic metals removal. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12147–12151. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yin, X.C.; Si, G.H.; Zhang, N.D.; Wang, X.H. Bio-adsorbent preparation based on Chinese radix isatidis residue for Pb(II) removal. Water Pract. Technol. 2020, 15, 1202–1212. [Google Scholar] [CrossRef]
- Qiu, Z.; Fu, K.; Yu, D.; Luo, J.; Shang, J.; Luo, S.; Crittenden, J.C. Radix Astragali residue-derived porous amino-laced double-network hydrogel for efficient Pb (II) removal: Performance and modeling. J. Hazard. Mater. 2022, 438, 129418. [Google Scholar] [CrossRef] [PubMed]
- Debamita, C.; Nakul, R.; Gautham, J.P.; Vairavel, P. Process optimization, isotherm, kinetics, and thermodynamics studies for removal of Remazol Brilliant Blue—R dye from contaminated water using adsorption on guava leaf powder. Desalin. Water Treat. 2020, 185, 318–343. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Zong, L.; Kang, Y.; Wang, A. Fast and Highly Efficient Adsorption Removal of Toxic Pb(II) by a Reusable Porous Semi-IPN Hydrogel Based on Alginate and Poly(Vinyl Alcohol). Front. Chem. 2021, 9, 662482. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Bundjaja, V.; Kurniawan, A.; Yuliana, M.; Hsieh, C.W.; Go, A.W.; Cheng, K.C.; Soetaredjo, F.E.; Ismadji, S. Investigation of the influence of crosslinking activation methods on the physicochemical and Cu (II) adsorption characteristics of cellulose hydrogels. J. Environ. Chem. Eng. 2022, 10, 106971. [Google Scholar] [CrossRef]
- Divya, J.M.; Palak, K.; Vairavel, P. Optimization, kinetics, equilibrium isotherms, and thermodynamics studies of Coomassie violet dye adsorption using Azadirachta indica (neem) leaf adsorbent. Desalin. Water Treat. 2020, 190, 353–382. [Google Scholar] [CrossRef]
- Long, X.; Lu, Y.L.; Guo, H.; Tang, Y.P. Recent Advances in Solid Residues Resource Utilization in Traditional Chinese Medicine. ChemistrySelect 2023, 8, e202300383. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Y.; Qu, G.; Ning, P.; Ren, N.; Chen, X.; Wang, Z.; Hu, Y. HCl-assisted KOH activated Chinese medicine residue biochar as a new method to improve the purification capacity of lead-containing wastewater: Experimental and mechanism studies. Sustain. Chem. Pharm. 2023, 33, 101063. [Google Scholar] [CrossRef]
- Sahu, S.; Bishoyi, N.; Sahu, M.K.; Patel, R.K. Investigating the selectivity and interference behavior for detoxification of Cr (VI) using lanthanum phosphate polyaniline nanocomposite via adsorption-reduction mechanism. Chemosphere 2021, 278, 130507. [Google Scholar] [CrossRef]
- Lu, W.; Shi, X.; Zhou, H.; Luo, W.; Wang, L.; He, H. Tailoring and properties of a novel solar energy-triggered regenerative bionic fiber adsorbent for CO2 capture. Chem. Eng. J. 2022, 449, 137885. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Y.; Wang, F.; Zhang, J.; Li, D. Arsenic (V) removal by granular adsorbents made from water treatment residuals materials and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124036. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Gsweeney, D.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green. Energy. Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Liu, H.; Lei, H.; Jian, B.; Liu, R.; Li, X.; Wang, Y.; Zhou, B. Preparation of green magnetic hydrogel from soybean residue cellulose for effective and rapid removal of copper ions from wastewater. J. Environ. Chem. Eng. 2022, 10, 108213. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Xu, M.; Liu, B.; Yang, M.; Chen, Z.; Gao, T.; James, T.D.; Wang, L.; Xiao, H. A ratiometric fluorescent hydrogel of controlled thickness prepared continuously using microtomy for the detection and removal of Hg (II). Chem. Eng. J. 2021, 426, 131296. [Google Scholar] [CrossRef]
- Yin, X.C.; Zhu, H.; Ke, T.; Gu, Y.E.; Wang, H.Y.; Xu, P. Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals. Water 2022, 14, 3811. [Google Scholar] [CrossRef]
- Van Rie, J.; Thielemans, W. Cellulose-gold nanoparticle hybrid materials. Nanoscale 2017, 9, 8525–8554. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Chen, C.; Zhang, X.; Zhou, K.; Long, X. Banana stem and leaf biochar as an effective adsorbent for cadmium and lead in aqueous solution. Sci. Rep. 2022, 12, 1584–1597. [Google Scholar] [CrossRef]
- Sheng, C.; Zhou, Y.; Lu, J.; Zhang, X.; Xue, G. Preparation and characterization of chitosan based hydrogels chemical cross-linked by oxidized cellulose nanowhiskers. Polym. Compos. 2019, 40, 2432–2440. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Felekori, M.; Nezafati, N.; Moraveji, M.; Hesaraki, S.; Ramezani, T. Bioorthogonal hydroxyethyl cellulose-based scaffold crosslinked via click chemistry for cartilage tissue engineering applications. Int. J. Biol. Macromol. 2021, 183, 2030–2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, B.; Wang, L. Adsorption/desorption performance of Pb2+ and Cd2+ with super adsorption capacity of PASP/CMS hydrogel. Water Sci. Technol. 2021, 84, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Azevedo, E. Functionalization and crosslinking of microcrystalline cellulose in aqueous media: A safe and economic approach. Int. J. Pharm. Sci. Rev. Res. 2011, 8, 28–36. [Google Scholar]
- Ciolacu, D.E.; Rusu, D.; Darie-Niţă, R.N.; Tîmpu, D.; Ciolacu, F. Influence of Gel Stage from Cellulose Dissolution in NaOH-Water System on the Performances of Cellulose Allomorphs-Based Hydrogels. Gels 2022, 8, 410. [Google Scholar] [CrossRef]
- Bai, Y.X.; Li, Y.F. Preparation and characterization of crosslinked porous cellulose beads. Carbohydr. Polym. 2006, 64, 402–407. [Google Scholar] [CrossRef]
- Dehabadi, L.; Wilson, L.D. Polysaccharide-based materials and their adsorption properties in aqueous solution. Carbohydr. Polym. 2014, 113, 471–479. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Al-Muhtaseb, A.H.; Kumar, A.; Khan, M.R.; Kalia, S.; Shweta Bala, M.; Sharma, A. Fabrication and characterization of chitosan-crosslinked-poly (alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int. J. Biol. Macromol. 2017, 95, 484–493. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Peng, H.; Wu, J.; Liu, Z.; Guo, X. Removal of Anionic Dyes from Aqueous Solutions by Cellulose-Based Adsorbents: Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2016, 61, 3266–3276. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically crosslinked hydrogel and its driving force towards superabsorbent behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hua, W.Q.; Li, L.; Zhou, Z.H.; Xu, L.; Bian, F.G.; Ji, X.; Zhong, G.J.; Li, Z.M. Robust hydrogel of regenerated cellulose by chemical crosslinking coupled with polyacrylamide network. J. Appl. Polym. Sci. 2019, 136, 47811–47822. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Wang, C.C. Pseudo-isotherms for the sorption of cadmium ion onto tree fern. Process. Biochem. 2004, 39, 759–763. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Wang, J.; Hu, N.; Liu, M.; Sun, J.; Xu, Y. A novel core–shell structured biosorbent derived from chemi-mechanical pulp for heavy metal ion removal. Cellulose 2019, 26, 8789–8799. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Liu, G.; Yu, R.; Lan, T.; Liu, Z.; Zhang, P.; Liang, R. Gallic acid-functionalized graphene hydrogel as adsorbent for removal of chromium (iii) and organic dye pollutants from tannery wastewater. RSC Adv. 2019, 9, 27060–27068. [Google Scholar] [CrossRef]
- Dakova, I.; Vasileva, P.; Karadjova, I. Cr (III) Ion-Imprinted Hydrogel Membrane for Chromium Speciation Analysis in Water Samples. Gels 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Guo, J.; Wang, Y.; Huang, C.; Hu, Y. Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution. J. Mater. Sci. Mater. 2021, 56, 13096–13107. [Google Scholar] [CrossRef]
- Song, Q.; Gao, J.; Lin, Y.; Zhang, Z.; Xiang, Y. Synthesis of cross-linking chitosan-PVA composite hydrogel and adsorption of Cu(II) ions. Water Sci. Technol. 2020, 81, 1063–1070. [Google Scholar] [CrossRef]
- Qu, W.; He, D.L.; Guo, Y.N.; Tang, Y.N.; Shang, J.; Zhou, L.; Zhu, R.L.; Song, R.J. Adsorption of Ni2+ and Pb2+ from water using diethylenetriamine-grafted Spirodela polyrhiza: Behavior and mechanism studies. Environ. Sci. Pollut. Res. Int. 2019, 26, 34562–34574. [Google Scholar] [CrossRef]
- Fei, Y.H.; Deng, H.; Wu, G.; Luo, M.; Chen, Y.; Wang, X.; Ye, H.; Liu, T. Insight into adsorption process and mechanisms of Cr (III) using carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/attapulgite composite hydrogel. Environ. Technol. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Yuan, Y.; Fan, M.; Zhang, D.; Wang, D.; Xu, Y. Selective, highly efficient extraction of Cr(III), Pb(II) and Fe(III) from complex water environment with a tea residue derived porous gel adsorbent. Bioresour. Technol. 2020, 311, 123520. [Google Scholar] [CrossRef]
- Han, C.L.; Cao, M.M.; Yu, J.; Wang, S.T.; Zhou, X.L.; Chen, Y.H.; Yang, F. Carboxymethyl Cellulose-Based Composite Polymer Hydrogels Cross-Linked with Epichlorohydrin and Application for Cu (II) Removal. ACS Appl. Polym. Mater. 2023, 5, 2070–2078. [Google Scholar] [CrossRef]
- Chen, J.J.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Adsorption-desorption characteristic of thermo-magneto-responsive poly (N-isopropylacrylamide)-co-acrylic acid composite hydrogel towards chromium (III) ions. J. Water Process. Eng. 2019, 32, 100957. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Jovanović-Malinovska, R.; Cvetkovska, M.; Kuzmanova, S.; Tsvetanov, C.; Winkelhausen, E. Immobilization of saccharomyces cerevisiae in novel hydrogels based on hybrid networks of poly (ethylene oxide), alginate and chitosan for ethanol production. Maced. J. Chem. Chem. Eng. 2010, 29, 169–179. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Shen, J.; Zhang, S.; Zhang, S.; Liu, X.; Chen, X.; Ma, Y.; Ren, S.; Fang, G.; et al. Simultaneous removal of Pb2+, Cu2+ and Cd2+ ions from wastewater using hierarchical porous polyacrylic acid grafted with lignin. J. Hazard. Mater. 2020, 392, 122208. [Google Scholar] [CrossRef] [PubMed]













| Adsorbent | qm (mg/g) | References | ||
|---|---|---|---|---|
| Pb2+ | Cr3+ | Cu2+ | ||
| Licorice/epichlorohydrin | 591.75 | 583.5 | 134.25 | Present study |
| Chitosan/calcium alginate/bentonite | 434.89 | - | 115.30 | [62] |
| Chitosan/poly(N-isopropylacrylamide) | 172.0 | - | 115.1 | [63] |
| Chitosan/polyvinyl alcohol | - | - | 62.1 | [64] |
| Spirodela polyrhiza/epichlorohydrin | 51.75 | - | - | [65] |
| N,N-dimethylformamide | ||||
| Carboxymethyl cellulose/attapulgite/acrylic acid-co-acrylamide | - | 74.8 | - | [66] |
| Cellulose/epichlorohydrin/graphene oxide | - | - | 94.34 | [9] |
| Tea residue/acrylic acid | 253.16 | 206.1 | - | [67] |
| Carboxymethyl cellulose/epichlorohydrin/polyacrylamide | - | - | 75.93 | [68] |
| Silica/poly(N-isopropylacrylamide) | ||||
| N,N′-methylenebis-acrylamide | - | 243.9 | - | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Ke, T.; Zhu, H.; Xu, P.; Wang, H. Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent. Gels 2023, 9, 559. https://doi.org/10.3390/gels9070559
Yin X, Ke T, Zhu H, Xu P, Wang H. Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent. Gels. 2023; 9(7):559. https://doi.org/10.3390/gels9070559
Chicago/Turabian StyleYin, Xiaochun, Ting Ke, Hai Zhu, Pei Xu, and Huiyao Wang. 2023. "Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent" Gels 9, no. 7: 559. https://doi.org/10.3390/gels9070559
APA StyleYin, X., Ke, T., Zhu, H., Xu, P., & Wang, H. (2023). Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent. Gels, 9(7), 559. https://doi.org/10.3390/gels9070559