Novel Hydrophilic Oligomer-Crosslinked Gelatin-Based Hydrogels for Biomedical Applications
Abstract
:1. Introduction
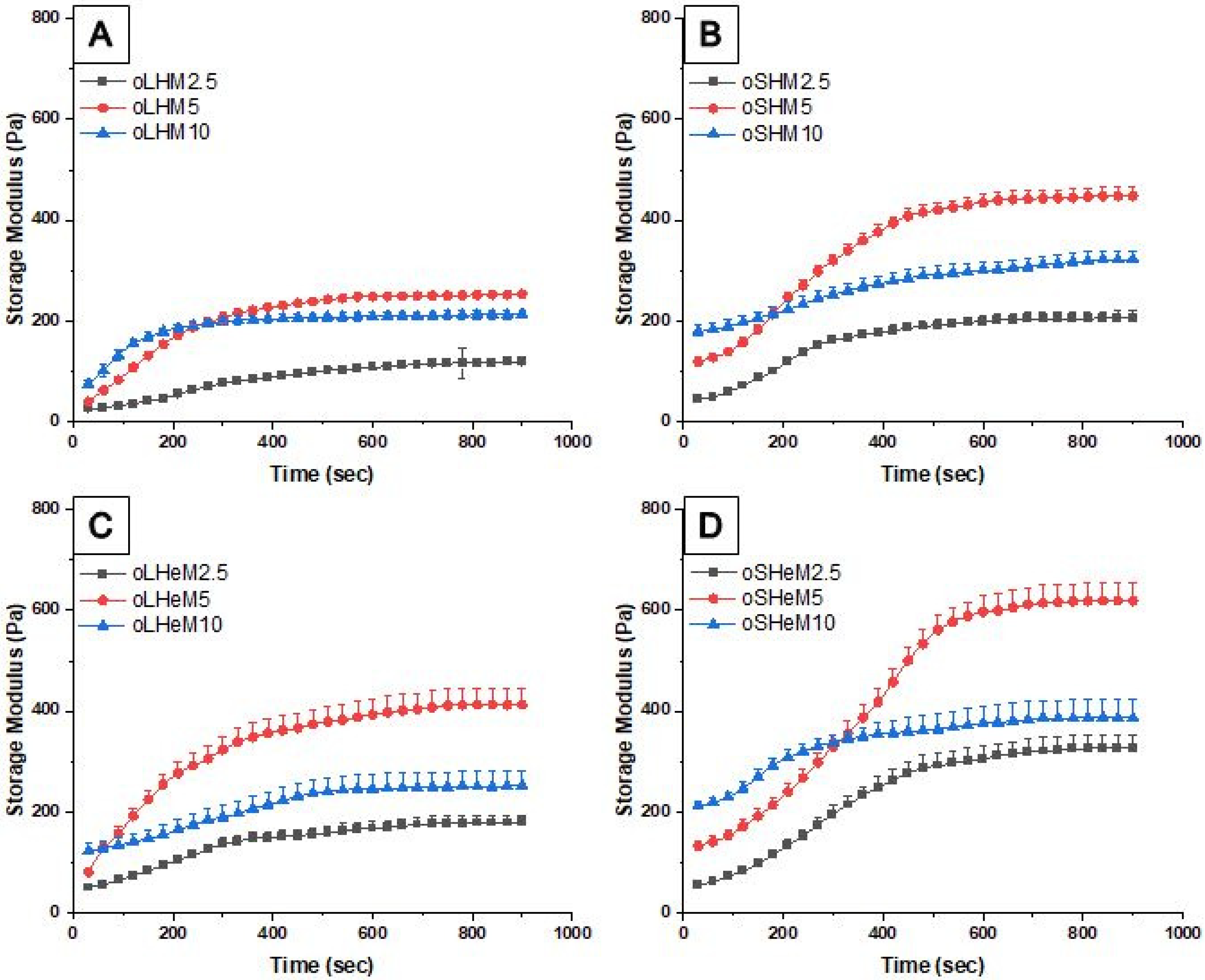
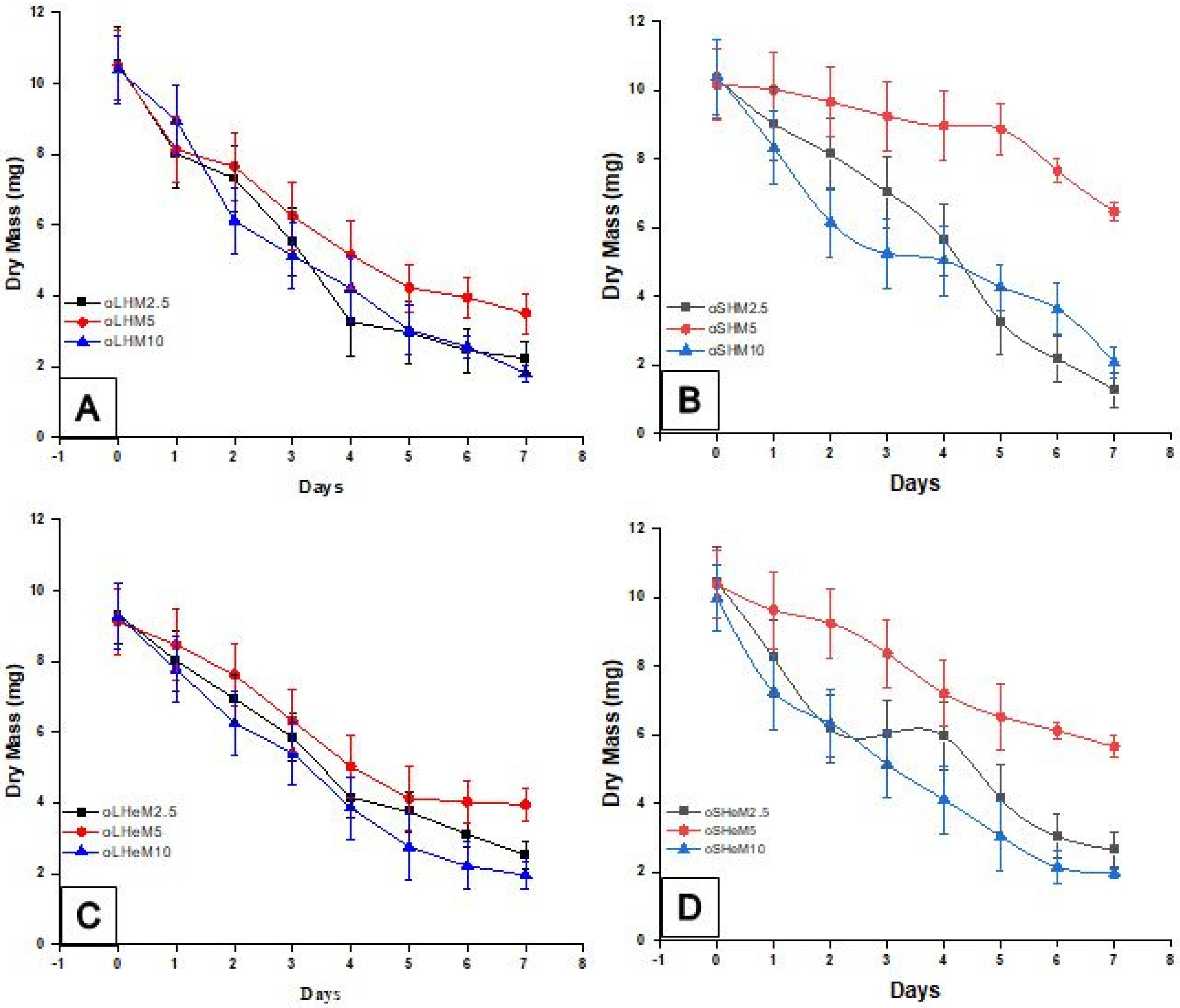
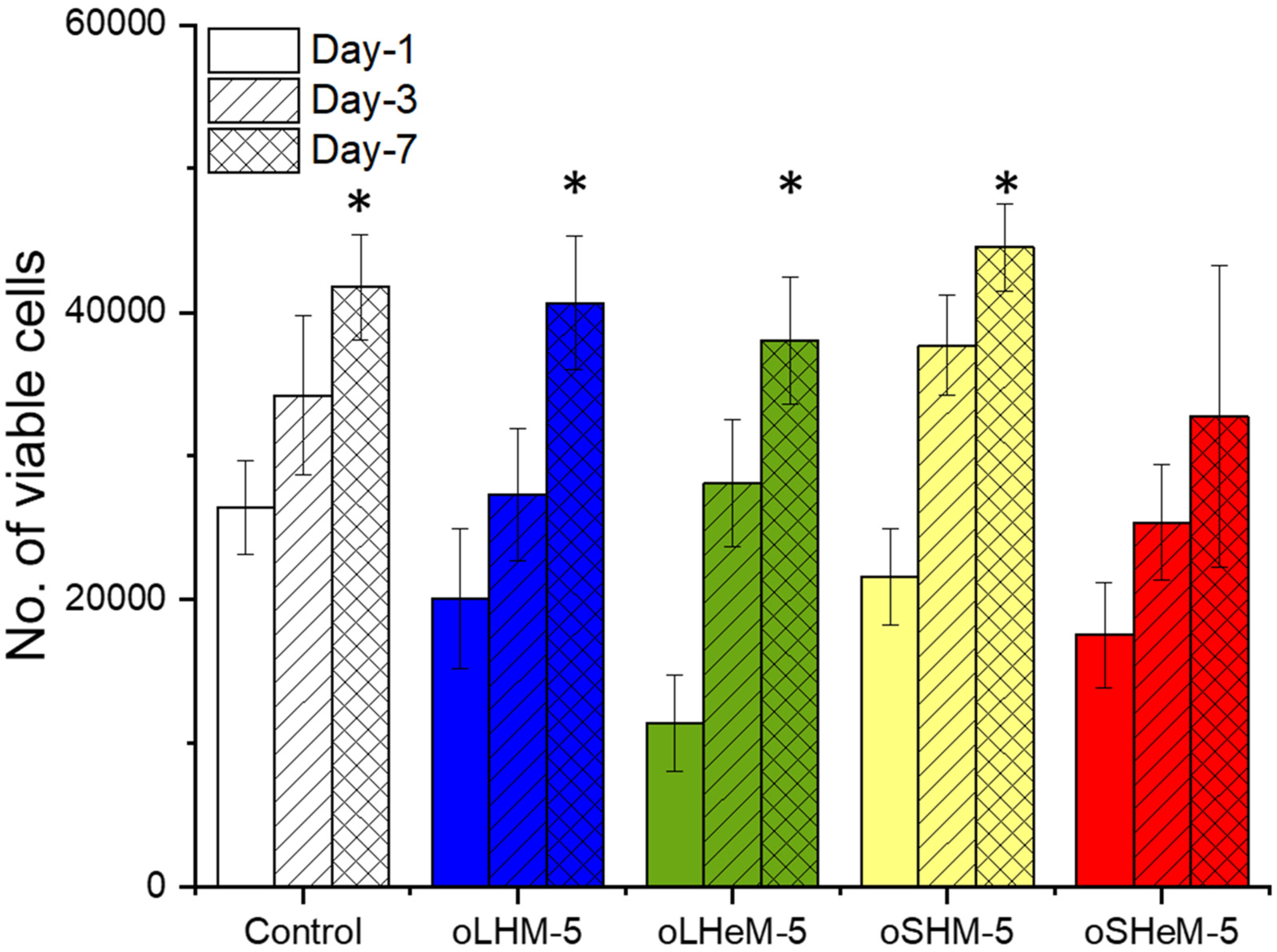
2. Results and Discussion
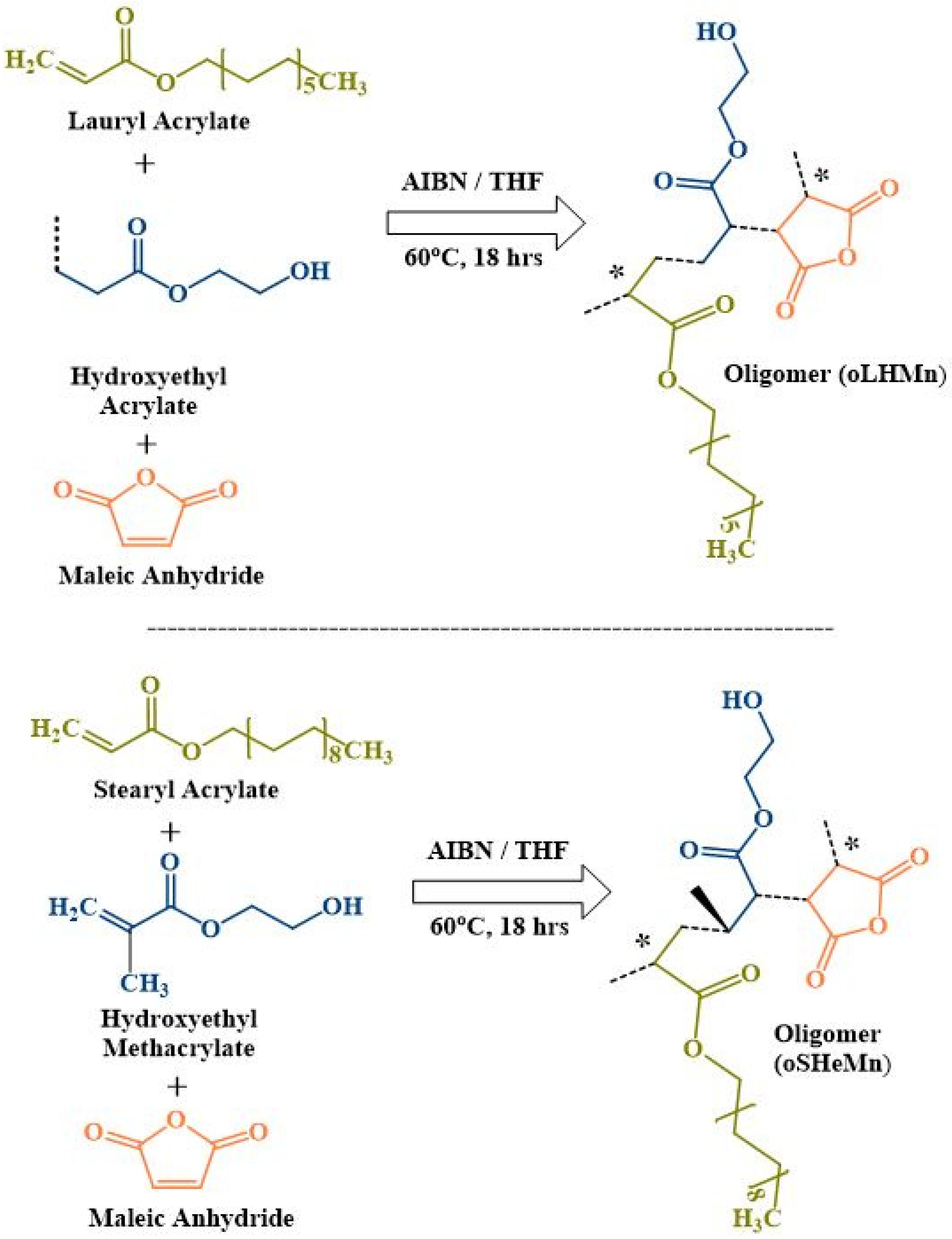
2.1. Synthesis of Two Novel Sets of Oligomers, i.e., oligo LA-co-HEA-co-MA (oLHM), oligo LA-co-HEMA-co-MA (oLHeM), and oligo SA-co-HEA-co-MA (oSHM), oligo SA-co-HEMA-co-MA (oSHeM)
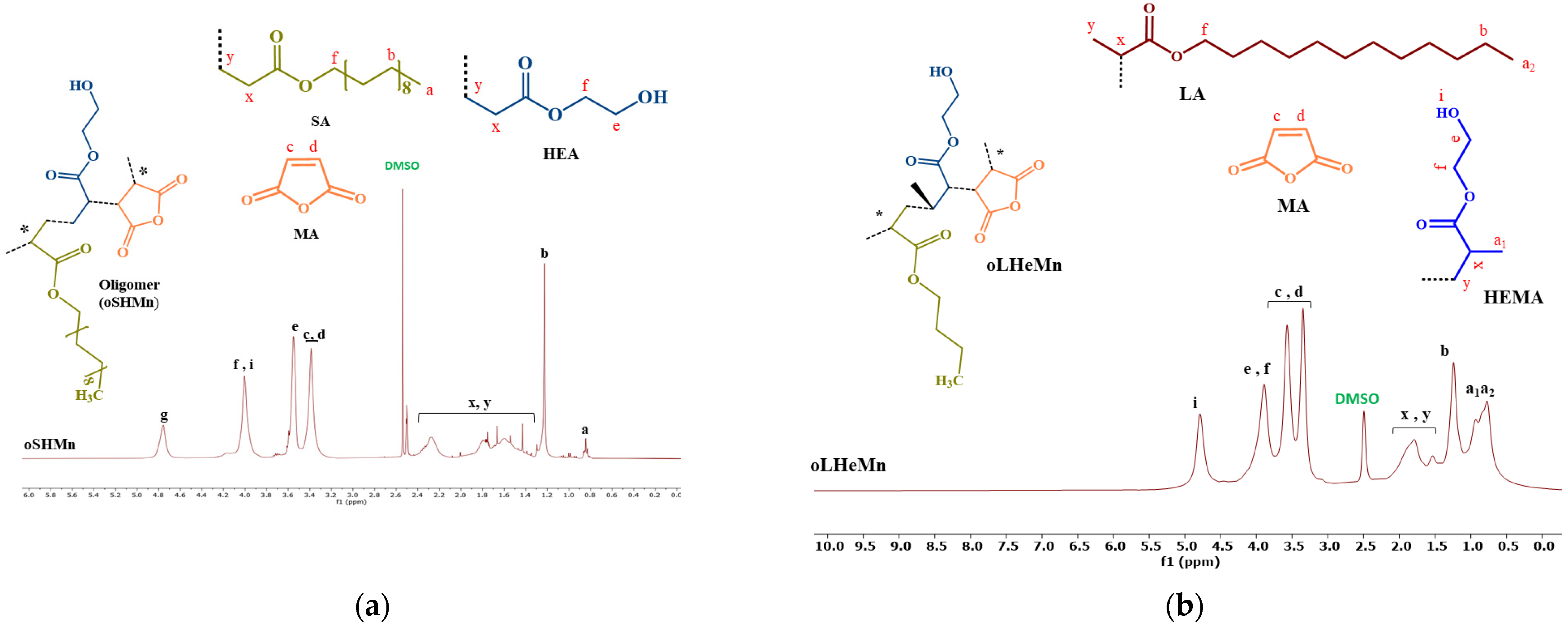
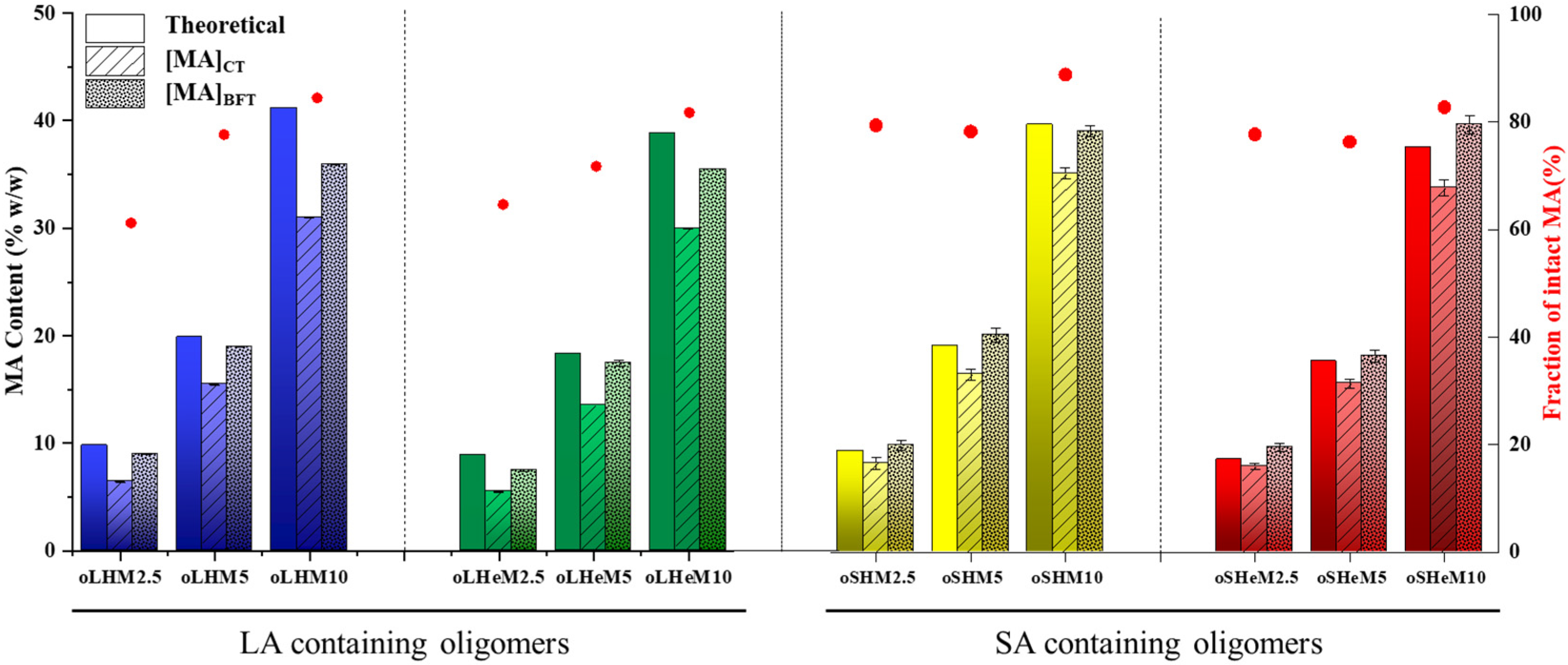
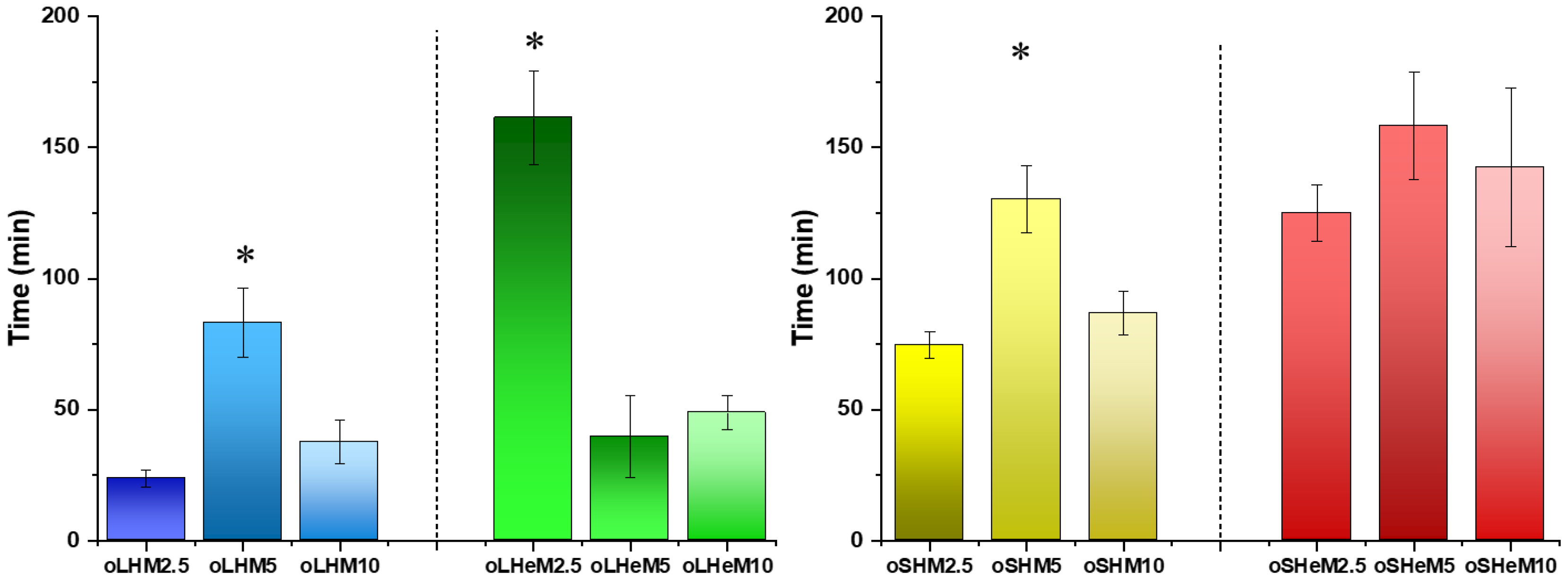
2.2. Characterization of Oligomers
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis
4.3. Oligomer Characterization
4.3.1. Titration
4.3.2. Gel Permeation Chromatography (GPC)
4.3.3. Nuclear Magnetic Resonance (NMR)
4.3.4. Oligomer Dissolution Characteristics
4.3.5. Hydrogel Fabrication
4.3.6. Rheological Characterization
4.3.7. In-Vitro Gel Degradation
4.3.8. In Vitro Cell Experiments
4.3.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y. Smart Hydrogels in Tissue Engineering and. Materials 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; You, J.; Zhang, Y.; Yang, Q.; Jiao, J.; Zhang, H. Recent Studies on Hydrogels Based on H2O2-Responsive Moieties: Mechanism, Preparation and Application. Gels 2022, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, A.H.; Cengiz, I.F.; Silva-Correia, J.; Sousa, R.A.; Oliveira, J.M.; Reis, R.L. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. In Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine; Khang, G., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017; pp. 327–361. ISBN 9789814745123. [Google Scholar] [CrossRef]
- Chen, H.; Feng, R.; Xia, T.; Wen, Z.; Li, Q.; Qiu, X.; Huang, B.; Li, Y. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels 2023, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Osetrov, K.; Uspenskaya, M.; Zaripova, F.; Olekhnovich, R. Nanoarchitectonics of a Skin-Adhesive Hydrogel Based on the Gelatin Resuscitation Fluid Gelatinol®. Gels 2023, 9, 330. [Google Scholar] [CrossRef]
- Luca, A.; Nacu, I.; Tanasache, S.; Peptu, C.A.; Butnaru, M.; Verestiuc, L. New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy. Gels 2023, 9, 371. [Google Scholar] [CrossRef]
- Kim, S.; Lee, W.; Park, H.; Kim, K. Tumor Microenvironment-Responsive 6-Mercaptopurine-Releasing Injectable Hydrogel for Colon Cancer Treatment. Gels 2023, 9, 319. [Google Scholar] [CrossRef]
- Nawaz, H.A.; Schröck, K.; Schmid, M.; Krieghoff, J.; Maqsood, I.; Kascholke, C.; Kohn-Polster, C.; Schulz-Siegmund, M.; Hacker, M.C. Injectable oligomer-cross-linked gelatine hydrogelsviaanhydride-amine-conjugation. J. Mater. Chem. B 2021, 9, 2295–2307. [Google Scholar] [CrossRef]
- Chang, S.; Wang, J.; Xu, N.; Wang, S.; Cai, H.; Liu, Z.; Wang, X. Facile Construction of Hybrid Hydrogels with High Strength and Biocompatibility for Cranial Bone Regeneration. Gels 2022, 8, 745. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Dinu, A.I.; Dinescu, S.; Lungu, A.; Stancu, I.C.; Iovu, H. Inorganic/Biopolymers Hybrid Hydrogels Dual Cross-Linked for Bone Tissue Regeneration. Gels 2022, 8, 762. [Google Scholar] [CrossRef]
- Albrecht, F.B.; Schmidt, F.F.; Volz, A.-C.; Kluger, P.J. Bioprinting of 3D Adipose Tissue Models Using a GelMA-Bioink with Human Mature Adipocytes or Human Adipose-Derived Stem Cells. Gels 2022, 8, 611. [Google Scholar] [CrossRef]
- Mohan, A.; Girdhar, M.; Kumar, R.; Chaturvedi, H.S.; Vadhel, A.; Solanki, P.R.; Kumar, A.; Kumar, D.; Mamidi, N. Polyhydroxybutyrate-based nanocomposites for bone tissue engineering. Pharmaceuticals 2021, 14, 1163. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-functional macromers for hydrogel design in biomedical engineering and regenerative medicine. Int. J. Mol. Sci. 2015, 16, 27677–27706. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Lee, Y.; Son, J.Y.; Oh, D.H.; Lee, J.S.; Park, K.D. Synthesis and characterizations of in situ cross-linkable gelatin and 4-arm-PPO-PEO hybrid hydrogels via enzymatic reaction for tissue regenerative medicine. Biomacromolecules 2012, 13, 604–611. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Van Genabeek, B.; Lamers, B.A.G.; Hawker, C.J.; Meijer, E.W.; Gutekunst, W.R.; Schmidt, B.V.K.J. Properties and applications of precision oligomer materials; where organic and polymer chemistry join forces. J. Polym. Sci. 2021, 59, 373–403. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Stepto, R.F.T.; Kratochvíl, P.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Loth, T.; Hennig, R.; Kascholke, C.; Hötzel, R.; Hacker, M.C. Reactive and stimuli-responsive maleic anhydride containing macromers—Multi-functional cross-linkers and building blocks for hydrogel fabrication. React. Funct. Polym. 2013, 73, 1480–1492. [Google Scholar] [CrossRef]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-based biomaterial engineering with anhydride-containing oligomeric cross-linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef]
- Hacker, M.C.; Klouda, L.; Ma, B.B.; Kretlow, J.D.; Mikos, A.G. Synthesis and Characterization of Injectable, Thermally and Chemically Gelable, Amphiphilic Poly ( N -isopropylacrylamide ) -Based Macromers. Biomacromolecules 2008, 9, 1558–1570. [Google Scholar] [CrossRef]
- Kohn-Polster, C.; Bhatnagar, D.; Woloszyn, D.J.; Richtmyer, M.; Starke, A.; Springwald, A.H.; Franz, S.; Schulz-Siegmund, M.; Kaplan, H.M.; Kohn, J.; et al. Dual-component gelatinous peptide/reactive oligomer formulations as conduit material and luminal filler for peripheral nerve regeneration. Int. J. Mol. Sci. 2017, 18, 1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, C.; Klemens, J.M.; Kascholke, C.; Murthy, N.S.; Kohn, J.; Brandenburger, M.; Hacker, M.C. Dual-component collagenous peptide/reactive oligomer hydrogels as potential nerve guidance materials-from characterization to functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Kascholke, C.; Loth, T.; Kohn-Polster, C.; Möller, S.; Bellstedt, P.; Schulz-Siegmund, M.; Schnabelrauch, M.; Hacker, M.C. Dual-Functional Hydrazide-Reactive and Anhydride-Containing Oligomeric Hydrogel Building Blocks. Biomacromolecules 2017, 18, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, K.; Ewe, A.; Kohn, C.; Loth, T.; Aigner, A.; Hacker, M.C.; Schulz-Siegmund, M. Sustained delivery of siRNA poly- and lipopolyplexes from porous macromer-crosslinked gelatin gels. Int. J. Pharm. 2017, 526, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wölk, C.; Nawaz, H.A.; Maqsood, I.; Strati, F.; Brezesinski, G.; Hause, G.; Schulz-Siegmund, M.; Hacker, M.C. Amphiphilic Functionalized Oligomers: A Promising Strategy for the Postfabrication Functionalization of Liposomes. Adv. Mater. Interfaces 2020, 2001168, 2001168. [Google Scholar] [CrossRef]
- Klouda, L.; Hacker, M.C.; Kretlow, J.D.; Mikos, A.G. Cytocompatibility evaluation of amphiphilic, thermally responsive and chemically crosslinkable macromers for in situ forming hydrogels. Biomaterials 2009, 30, 4558–4566. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, D. Modifications of Hydroxyl-Functionalized HEA/HEMA and Their Polymers in the Synthesis of Functional and Graft Copolymers. Curr. Org. Synth. 2017, 14, 798–809. [Google Scholar] [CrossRef]
- Ewe, A.; Przybylski, S.; Burkhardt, J.; Janke, A.; Appelhans, D.; Aigner, A. A novel tyrosine-modified low molecular weight polyethylenimine (P10Y) for efficient siRNA delivery in vitro and in vivo. J. Control. Release 2016, 230, 13–25. [Google Scholar] [CrossRef]
- Backlund, C.M.; Sgolastra, F.; Otter, R.; Minter, L.M.; Takeuchi, T.; Futaki, S.; Tew, G.N. Increased hydrophobic block length of PTDMs promotes protein internalization. Polym. Chem. 2016, 7, 7514–7521. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Z.; Chen, M. The length of hydrophobic chain in amphiphilic polypeptides regulates the efficiency of gene delivery. Polymers 2018, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Chitanu, G.C.; Zaharia, L.I.; Carpov, A. Review: Analysis and characterization of maleic copolymers. Int. J. Polym. Anal. Charact. 1997, 4, 1–20. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; Elias, A. Amine responsive poly(lactic acid) (PLA) and succinic anhydride (SAh) graft-polymer: Synthesis and characterization. Polymers 2019, 11, 1466. [Google Scholar] [CrossRef] [Green Version]
- Popescu, I.; Suflet, D.M.; Pelin, I.M.; Chiţanu, G.C. Biomedical applications of maleic anhydride copolymers. Rev. Roum. Chim. 2011, 56, 173–188. [Google Scholar]
- Hammad, M.A.; Müller, B.W. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998, 46, 361–367. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-based biomaterial for skin wound healing (Gelatin vs. collagen): Expert review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Moreno-Castellanos, N.; Cuartas-Gómez, E.; Vargas-Ceballos, O. Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance. Gels 2023, 9, 496. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Pablos, J.L.; Jiménez-Holguín, J.; Salcedo, S.S.; Salinas, A.J.; Corrales, T.; Vallet-Regí, M. New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering. Gels 2023, 9, 403. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Hipwood, L.; Clegg, J.; Weekes, A.; Davern, J.W.; Dargaville, T.R.; Meinert, C.; Bock, N. Semi-Synthetic Click-Gelatin Hydrogels as Tunable Platforms for 3D Cancer Cell Culture. Gels 2022, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Tarus, D.; Hamard, L.; Caraguel, F.; Wion, D.; Szarpak-Jankowska, A.; Van Der Sanden, B.; Auzély-Velty, R. Design of Hyaluronic Acid Hydrogels to Promote Neurite Outgrowth in Three Dimensions. ACS Appl. Mater. Interfaces 2016, 8, 25051–25059. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.N.; Deijs, G.S.; Manuguri, S.; Ting, M.S.H.; Williams, M.A.K.; Malmström, J. A comparative study of tough hydrogen bonding dissipating hydrogels made with different network structures. Nanoscale Adv. 2021, 3, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.; Patlay, A.; Silant’ev, V.; Kovalev, V.V.; Kumeiko, V. Preparation of Hydrogels Based on Modified Pectins by Tuning Their Properties for Anti-Glioma Therapy. Int. J. Mol. Sci. 2023, 24, 630. [Google Scholar] [CrossRef]
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef]
- Dromel, P.C.; Singh, D.; Alexander-Katz, A.; Kurisawa, M.; Spector, M.; Young, M. Mechano-Chemical Effect of Gelatin- and HA-Based Hydrogels on Human Retinal Progenitor Cells. Gels 2023, 9, 58. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [Green Version]

| General Name | Oligomer Name | Lauryl Acrylate (LA) | Hydroxyethyl Acrylate (HEA) | Hydroxyethyl Methacrylate (HEMA) | Maleic Anhydride (MA) |
|---|---|---|---|---|---|
| Ratio: 1: 20 (LA: HEA/HEMA + MA) | |||||
| oLHMn | oLHM2.5 | 1 | 17.5 | - | 2.5 |
| oLHM5 | 1 | 15.0 | - | 5.0 | |
| oLHM10 | 1 | 10.0 | - | 10.0 | |
| oLHeMn | oLHeM2.5 | 1 | - | 17.5 | 2.5 |
| oLHeM5 | 1 | - | 15.0 | 5.0 | |
| oLHeM10 | 1 | - | 10.0 | 10.0 | |
| General Name | Oligomer Name | Stearyl Acrylate (SA) | Hydroxyethyl Acrylate (HEA) | Hydroxyethyl Methacrylate (HEMA) | Maleic Anhydride (MA) |
|---|---|---|---|---|---|
| Ratio: 1: 20 (LA: HEA/HEMA + MA) | |||||
| oSHM | oSHM2.5 | 1 | 16 | - | 4 |
| oSHM5 | 1 | 15 | - | 5 | |
| oSHM10 | 1 | 10 | - | 10 | |
| oSHeM | oSHeM2.5 | 1 | - | 17.5 | 2.5 |
| oSHeM5 | 1 | - | 16 | 4 | |
| oSHeM10 | 1 | - | 10 | 10 | |
| Ter-Oligomer Groups | Oligomer | Comonomers in the Feed | Hydrophilic Comonomer (HEA or HEMA) | MA | ||
|---|---|---|---|---|---|---|
| HEA | HEMA | MA | ||||
| oLHM | oLHM2.5 | 17.5 | --- | 2.5 | 1.15 | 1.12 |
| oLHM5 | 15 | --- | 5 | 1.24 | 1.23 | |
| oLHM10 | 10 | --- | 10 | 1.39 | 1.31 | |
| oLHeM | oLHeM2.5 | --- | 17.5 | 2.5 | 1.08 | 1.10 |
| oLHeM5 | --- | 15 | 5 | 1.13 | 1.13 | |
| oLHeM10 | --- | 10 | 10 | 1.16 | 1.28 | |
| oSHM | oSHM2.5 | 17.5 | --- | 2.5 | 1.05 | 1.07 |
| oSHM5 | 15 | --- | 5 | 1.11 | 1.13 | |
| oSHM10 | 10 | --- | 10 | 1.06 | 1.09 | |
| oSHeM | oSHeM2.5 | --- | 17.5 | 2.5 | 1.08 | 1.02 |
| oSHeM5 | --- | 15 | 5 | 1.12 | 1.04 | |
| oSHeM10 | --- | 10 | 10 | 1.07 | 1.07 | |
| Ter-Oligomer Groups | Oligomer | Mn (103 Da) | SD | Mw (103 Da) | SD | Ð (Mw/Mn) | SD |
|---|---|---|---|---|---|---|---|
| oLHM | oLHM2.5 | 2.39 | 0.21 | 3.42 | 0.17 | 1.45 | 0.16 |
| oLHM5 | 1.93 | 0.20 | 3.20 | 0.13 | 1.67 | 0.12 | |
| oLHM10 | 1.81 | 0.14 | 4.54 | 0.08 | 2.53 | 0.16 | |
| oLHeM | oLHeM2.5 | 2.64 | 0.20 | 4.49 | 0.16 | 1.71 | 0.17 |
| oLHeM5 | 2.24 | 0.31 | 3.33 | 0.26 | 1.51 | 0.22 | |
| oLHeM10 | 2.12 | 0.35 | 3.17 | 0.18 | 1.71 | 0.42 | |
| oSHM | oSHM2.5 | 3.61 | 0.26 | 4.90 | 0.29 | 1.36 | 0.02 |
| oSHM5 | 3.08 | 0.17 | 4.14 | 0.12 | 1.34 | 0.03 | |
| oSHM10 | 2.41 | 0.42 | 3.69 | 0.20 | 1.53 | 0.28 | |
| oSHeM | oSHeM2.5 | 3.76 | 0.51 | 5.15 | 0.27 | 1.37 | 0.32 |
| oSHeM5 | 3.13 | 0.21 | 5.09 | 0.16 | 1.63 | 0.38 | |
| oSHeM10 | 2.83 | 0.26 | 4.93 | 0.42 | 1.74 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, M.; Khokhar, R.; Javed, A.; Usman, M.; Anjum, S.M.M.; Rasheed, H.; Bukhari, N.I.; Yan, C.; Nawaz, H.A. Novel Hydrophilic Oligomer-Crosslinked Gelatin-Based Hydrogels for Biomedical Applications. Gels 2023, 9, 564. https://doi.org/10.3390/gels9070564
Tariq M, Khokhar R, Javed A, Usman M, Anjum SMM, Rasheed H, Bukhari NI, Yan C, Nawaz HA. Novel Hydrophilic Oligomer-Crosslinked Gelatin-Based Hydrogels for Biomedical Applications. Gels. 2023; 9(7):564. https://doi.org/10.3390/gels9070564
Chicago/Turabian StyleTariq, Mamoona, Rabia Khokhar, Arslan Javed, Muhammad Usman, Syed Muhammad Muneeb Anjum, Huma Rasheed, Nadeem Irfan Bukhari, Chao Yan, and Hafiz Awais Nawaz. 2023. "Novel Hydrophilic Oligomer-Crosslinked Gelatin-Based Hydrogels for Biomedical Applications" Gels 9, no. 7: 564. https://doi.org/10.3390/gels9070564






