Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems
Abstract
1. Introduction
2. Results and Discussion
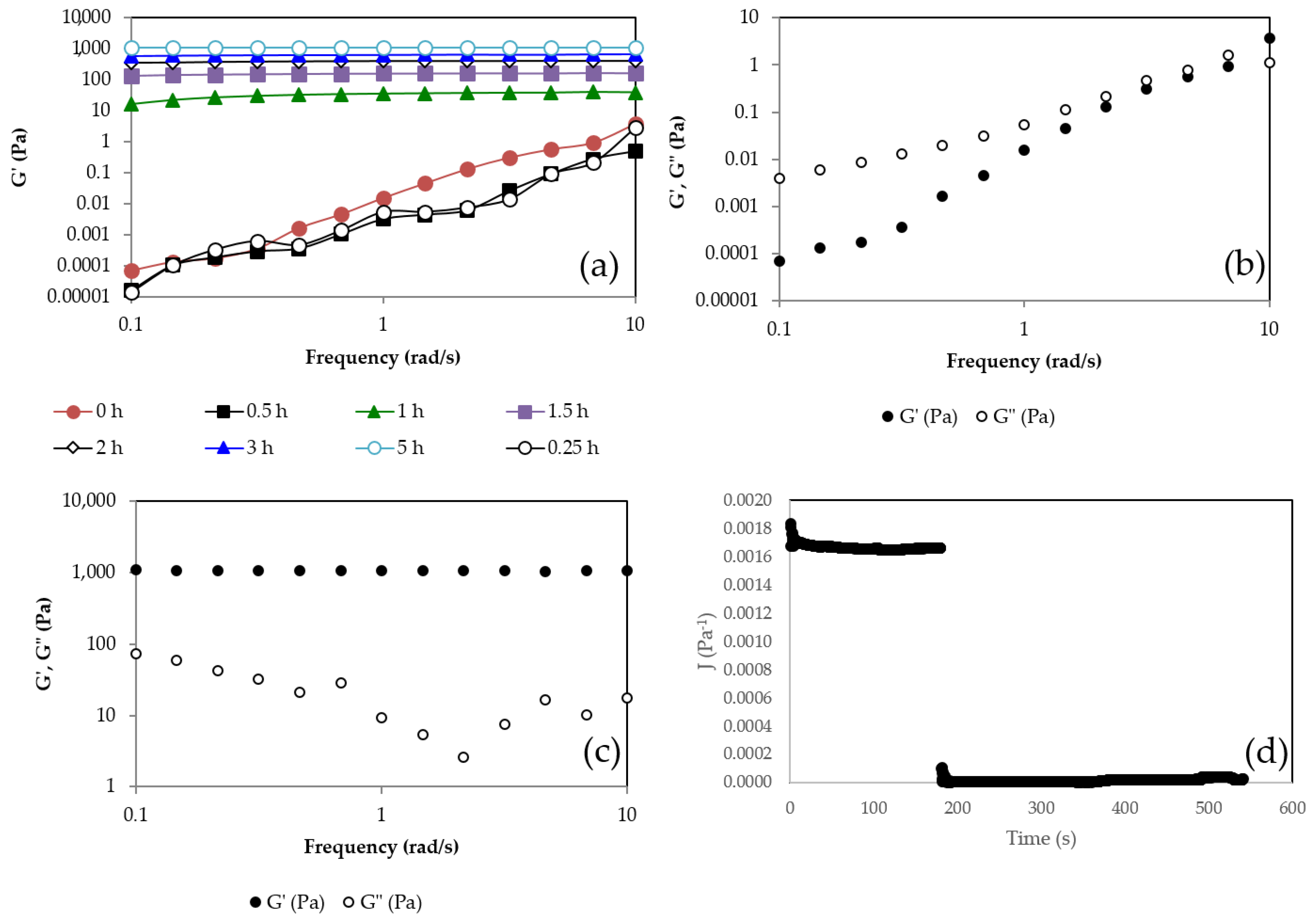
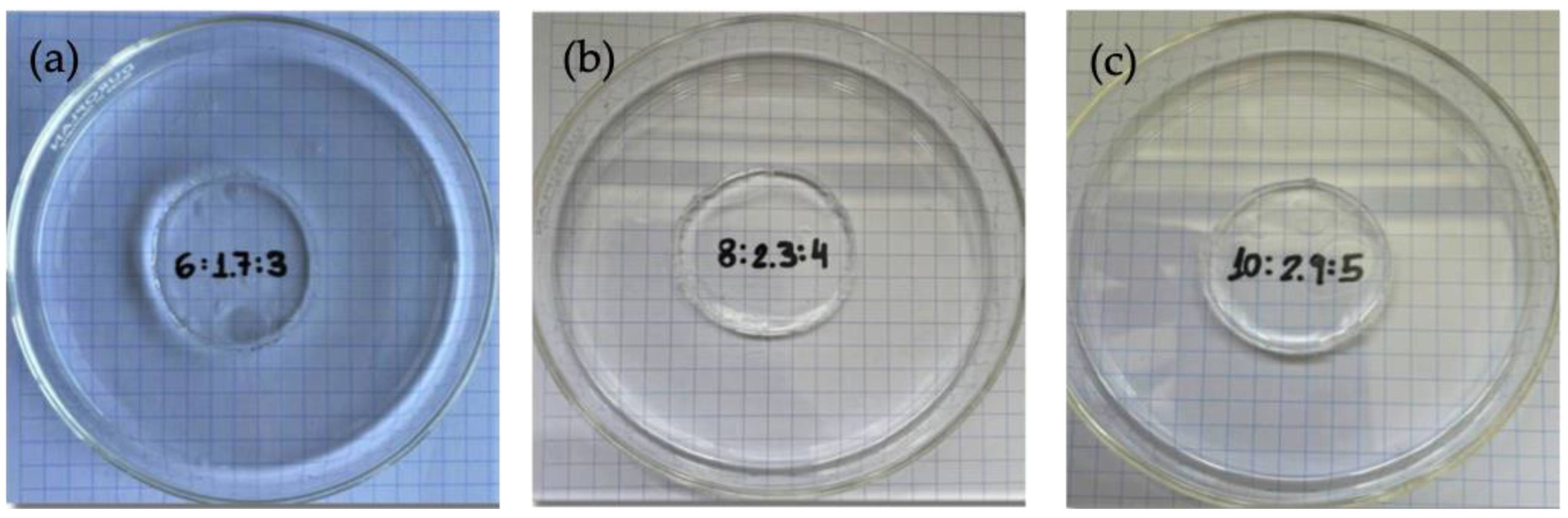
2.1. Gelation Kinetics
2.2. Swelling Degree
2.3. Rehydration Behavior
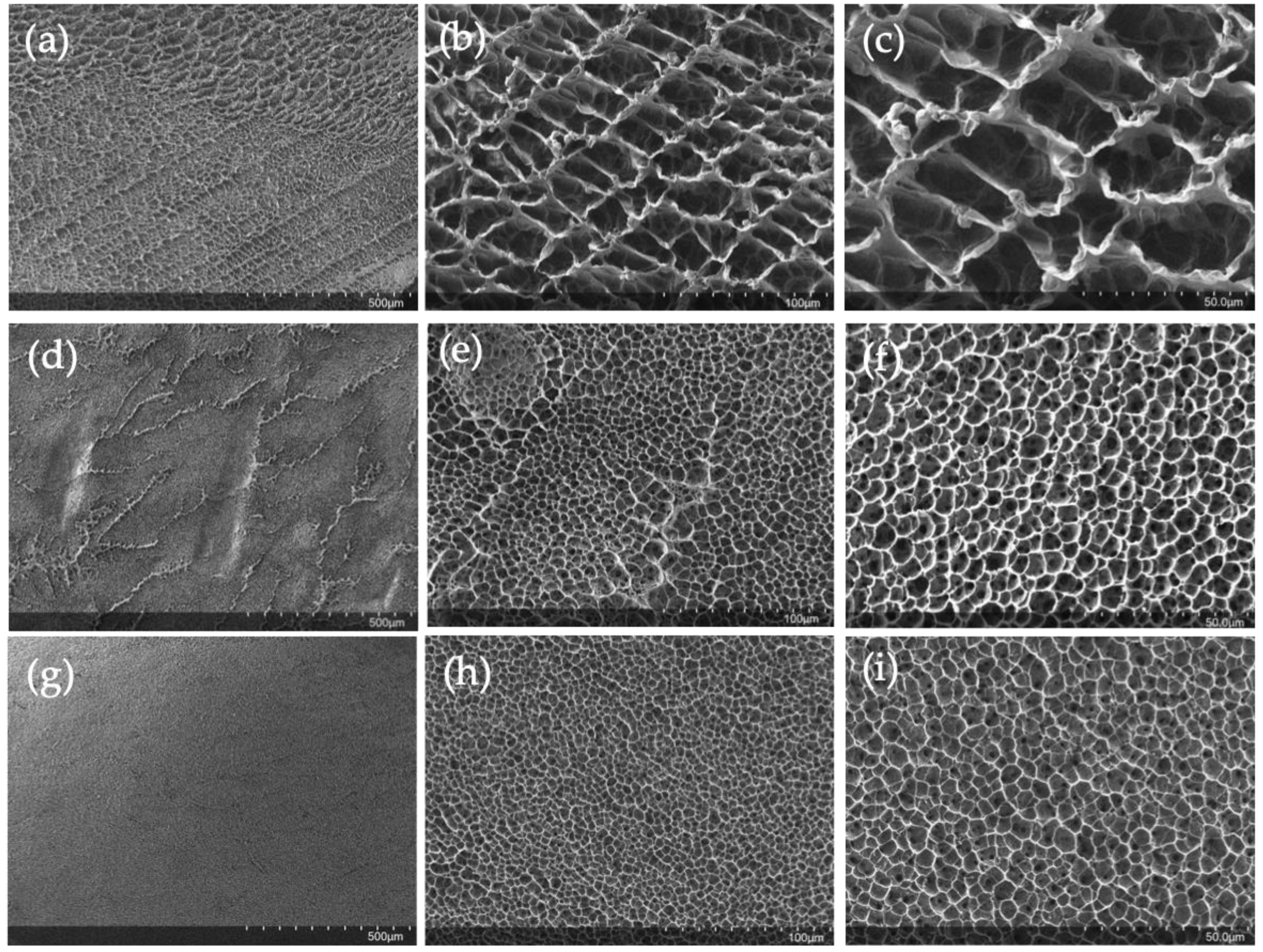
2.4. SEM Characterization
2.5. Carvacrol Release Kinetics
3. Conclusions
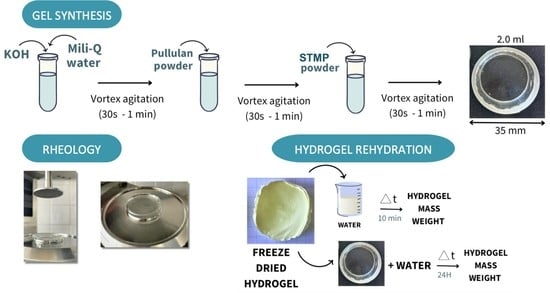
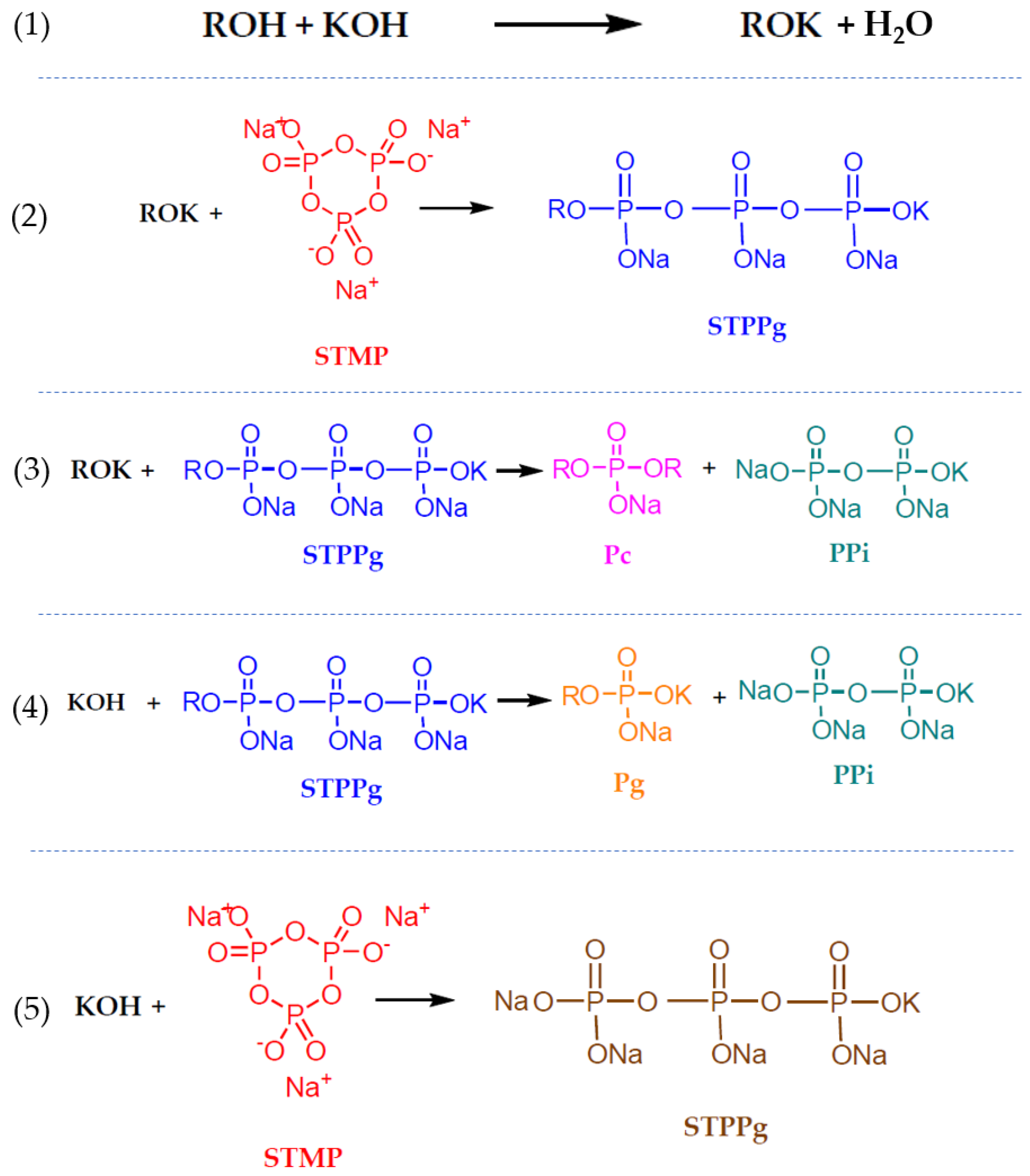
4. Materials and Methods
4.1. Materials
4.2. Formation of the Gels
4.3. Carvacrol Nanoemulsion Loaded into Pullulan Hydrogels
4.4. Rheological Measurements
4.5. Swelling Degree and Water Retention
4.6. SEM Measurements
4.7. Carvacrol Release Kinetics Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Rafe, A.; Razavi, S.M.A. Scaling law, fractal analysis and rheological characteristics of physical gels cross-linked with sodium trimetaphosphate. Food Hydrocoll. 2017, 62, 58–65. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Q.; Zhang, X.; Tsou, C.; Reyes De Guzman, M.; Li, X.; Yuan, L.; Xia, Y.; Sheng, Y.; Li, Q.; et al. A tough conductive hydrogel with triple physical cross-linking, pH-Responsive swelling behaviors, and excellent strain sensitivity. Polymer 2023, 273, 125887. [Google Scholar] [CrossRef]
- Yan, J.; Krasowska, M.; Ge, W.; Platts, K.; Facal-Marina, P.; Blencowe, A. Combining thermosensitive physical self-assembly and covalent cycloaddition chemistry as simultaneous dual cross-linking mechanisms for the preparation of injectable hydrogels with tuneable properties. Eur. Polym. J. 2023, 183, 111761. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, J.; Hong, Y.; Shen, L. Recent advances in design strategies of tough hydrogels. Maxcromol. Rapid Commun. 2022, 43, e2200075. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly(acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]
- Ye, D.; Chang, C.; Zhang, L. High strength and tough cellulose hydrogels chemically dual cross-linked by using low and high molecular weight cross-linkers. Macromolecules 2019, 20, 5. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z. Tough, swelling-resistant, self-healing, and adhesive dual-cross-linked hydrogels based on polymer-tannic acid multiple hydrogen bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 190–201. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Sangeetha Priyaa, V.; Iyappanb, K.; Gayathric, V.S.; Williamd, S.; Suguna, L. Influence of pullulan hydrogel on sutureless wound healing in rats. Wound Med. 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.-J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Liparoti, S.; Speranza, V.; Marra, F. Alginate hydrogel: The influence of the hardening on the rheological behaviour. J. Mech. Behav. Biomed. Mater. 2021, 116, 104341. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Galic, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef]
- Cofelice, M.; Messia, M.C.; Marconi, E.; Cuomo, F.; Lopez, F. Effect of the xanthan gum on the rheological properties of alginate hydrogels. Food Hydrocoll. 2023, 142, 108768. [Google Scholar] [CrossRef]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable pectin hydrogels produced by internal gelation: pH dependence of gelling and rheological properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Zhang, P.; Zhang, G.; Han, J. Rheological insight of polysaccharide/protein based hydrogels in recent food and biomedical fields: A review. Int. J. Biol. Macromol. 2022, 222, 1642–1664. [Google Scholar] [CrossRef]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef]
- Feng Tang, Y.; Min Du, Y.; Wen Hu, X.; Wen Shi, X.; Kennedy, J.F. Rheological characterisation of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohydr. Polym. 2007, 67, 491–499. [Google Scholar] [CrossRef]
- Montembaulta, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.-H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Su, W.; Mather, P.T.; Bunning, T.J. Rheology of highly swollen chitosan/polyacrylate hydrogels. Polymer 1999, 40, 4593–4602. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr. Polym. 2007, 67, 586–595. [Google Scholar] [CrossRef]
- Richa, K.; Choudhury, R.A. Synthesis and rheological characterization of a novel thermostable quick setting composite hydrogel of gellan and pullulan. Int. J. Biol. Macromol. 2019, 125, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Tongb, Q.; Zhoua, Y.; Denga, F. Rheological properties of pullulan-sodium alginate based solutions during film formation. Carbohydr. Polym. 2015, 130, 49–56. [Google Scholar] [CrossRef]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Lack, S.; Dulong, V.; Le Cerf, D.; Picton, L.; Argillier, J.F.; Muller, G. Hydrogels Based on Pullulan Crosslinked with sodium trimetaphosphate (STMP): Rheological study. Polym. Bull. 2004, 52, 429–436. [Google Scholar] [CrossRef]
- Dulong, V.; Forbice, R.; Condamine, E.; Le Cerf, D.; Picton, L. Pullulan–STMP hydrogels: A way to correlate crosslinking mechanism, structure and physicochemical properties. Polym. Bull. 2011, 67, 455–466. [Google Scholar] [CrossRef]
- Mocanu, G.; Mihai, D.; Le Cerf, D.; Picton, L.; Muller, G. Synthesis of new associative gel microspheres from carboxymethyl pullulan and their interactions with lysozyme. Eur. Polym. J. 2004, 40, 283–289. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in biomedical research and development—A review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Rafe, A.; Yeganehzad, S. Structure-rheology relationships of composite gels: Alginate and Basil seed gum/guar gum. Carbohydr. Polym. 2020, 232, 115809. [Google Scholar] [CrossRef]
- Ambebila, E.N.; Santamaría, E.; Maestro, A.; Gutiérrez, J.M.; González, C. Gellan Hydrogels: Preparation, Rheological Characterization and Application in Encapsulation of Curcumin. Food Biophys. 2019, 14, 154–163. [Google Scholar] [CrossRef]
- Núñez-Santiago, M.-C.; Pérez-López, A.; Espinosa-Solares, T.; Nicolás-Vázquez, M.-I.; Laureano-López, B. Sol-gel transition diagram and theoretical study of κ-carrageenan in the presence of calcium ions. LWT 2023, 182, 114867. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Lin, H. Design and fabrication of TPP cross-linked chitosan hydrogels with tunable stimuli-responsive properties and their application on drug delivery. J. Control. Release 2017, 259, e177. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulated by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Liyan, L.; Shiliang, Y.; Jing, W.; Yun, S.; Shuwen, D.; Luyang, X.; Qixiang, Y. The formation mechanism of hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear viscoelasticity at the gel point of a crosslinking PDMS with imbalanced stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Atencio, S.; Maestro, A.; Santamaría, E.; Gutiérrez, J.M.; González, C. Encapsulation of ginger oil in alginate-based shell materials. Food Biosci. 2020, 37, 100714. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Food Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Manzoor, M.; Sharma, P.; Murtaza, M.; Jaiswal, A.K.; Jaglan, S. Fabrication, characterization, and interventions of protein, polysaccharide and lipid-based nanoemulsions in food and nutraceutical delivery applications: A review. Int. J. Biol. Macromol. 2023, 241, 124485. [Google Scholar] [CrossRef]
- Mushtaq, A.; Wani, S.M.; Malik, A.R.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, A.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; He, H.; Wu, S.; Li, B.; Li, Y. Fabrication of nanoemulsion-filled alginate hydrogel to control the digestion behavior of hydrophobic nobiletin. LWT—Food Sci. Technol. 2017, 82, 260–267. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Winter, H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Metze, F.K.; Sant, S.; Meng, Z.; Klok, H.A.; Kaur, K. swelling-avtivated, soft mechanochemistry in polymer materials. Langmuir 2023, 39, 3546–3557. [Google Scholar] [CrossRef]
- Zawani, M.; Maarof, M.; Tabata, Y.; Motta, A.; Fauzi, M.B. Quercetin-Embedded Gelatin Injectable Hydrogel as Provisional Biotemplate for Future Cutaneous Application: Optimization and In Vitro Evaluation. Gels 2022, 8, 623. [Google Scholar] [CrossRef]
- Brett, D.W.A. A review of moisture-control dressing in wound care. J. Wound Ostomy Cont. Nurs. 2006, 33, 3–8. [Google Scholar] [CrossRef]
- Mert, H.; Özkahraman, B.; Damar, H. A novel wound dressing material: Pullulan grafted copolymer hydrogel via UV copolymerization and crosslinking. J. Drug Deliv. Sci. Technol. 2020, 60, 101962. [Google Scholar] [CrossRef]
- Kalia, S.; Choudhury, A.R. Synthesis and rheological studies of a novel composite hydrogel of xanthan, gellan and pullulan. Int. J. Biol. Macromol. 2019, 137, 475–482. [Google Scholar] [CrossRef]
- Sornkamnerd, S.; Okajima, M.K.; Kaneko, T. Tough and Porous Hydrogels Prepared by Simple Lyophilization of LC Gels. ACS Omega 2017, 2, 5304–5314. [Google Scholar] [CrossRef]
- Valot, L.; Maumus, M.; Brunel, L.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G.A. Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels 2021, 7, 73. [Google Scholar] [CrossRef]
- Khan, Y.A.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102126. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Ghaffar, A.; Gull, N.; Azam, M.; Khan, R.U. Novel pH-responsive chitosan/sodium alginate/PEG based hydrogels for release of sodium ceftriaxone. Mater. Chem. Phys. 2022, 277, 125456. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.; Guo, Y.; Zhao, P.; Wang, J.; Zhang, R.; Wang, X. Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation release kinetics. Food Chem. 2023, 405, 134856. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, E.; Maestro, A.; González, C. Encapsulation of Carvacrol-Loaded Nanoemulsion Obtained Using Phase Inversion Composition Method in Alginate Beads and Polysaccharide-Coated Alginate Beads. Foods 2023, 12, 1874. [Google Scholar] [CrossRef]
- Veres, P.; Kéri, M.; Bányai, I.; Lázár, I.; Fábián, I.; Domingo, C.; Kalmár, J. Mechanism of drug release from silica-gelatin aerogel—Relationship between matrix structure and release kinetics. Colloids Surf. B Biointerfaces 2017, 152, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, E.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Preparation of structured meso–macroporous silica materials: Influence of composition variables on material characteristics. J. Porous Mater. 2014, 21, 263–274. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Wan, L.S.; Heng, P.W.; Wong, L.F. Relationship between swelling and frug release in a hydrophilic matrix. Drug Deliv. Ind. Pharm. 1993, 19, 1201–1210. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; De Ascentiis, A.; Peppas, N.A. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J. Control. Release 1996, 39, 231–237. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modelling of diffusion controlled drug delivery. J. Control. Release 2012, 16, 351–362. [Google Scholar] [CrossRef]
- Chalier, P.; Arfa, A.B.; Guillard, V.; Gontard, N. Moisture and Temperature Triggered Release of a Volatile Active Agent from Soy Protein Coated Paper: Effect of Glass Transition Phenomena on Carvacrol Diffusion Coefficient. J. Agric. Food Chem. 2009, 57, 658–665. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Incorporation of hydroxypropyl-b-cyclodextrins into chitosan films to tailor loading capacity for active aroma compound carvacrol. Food Hydrocoll. 2015, 43, 603–611. [Google Scholar] [CrossRef]
- Santamaría, E.; Maestro, A.; Vilchez, S.; González, C. Study of nanoemulsions using carvacrol/MCT-(Oleic acid-potassium oleate)/Tween 80®-water system by low energy method. Heliyon 2023, 9, e16967. [Google Scholar] [CrossRef] [PubMed]



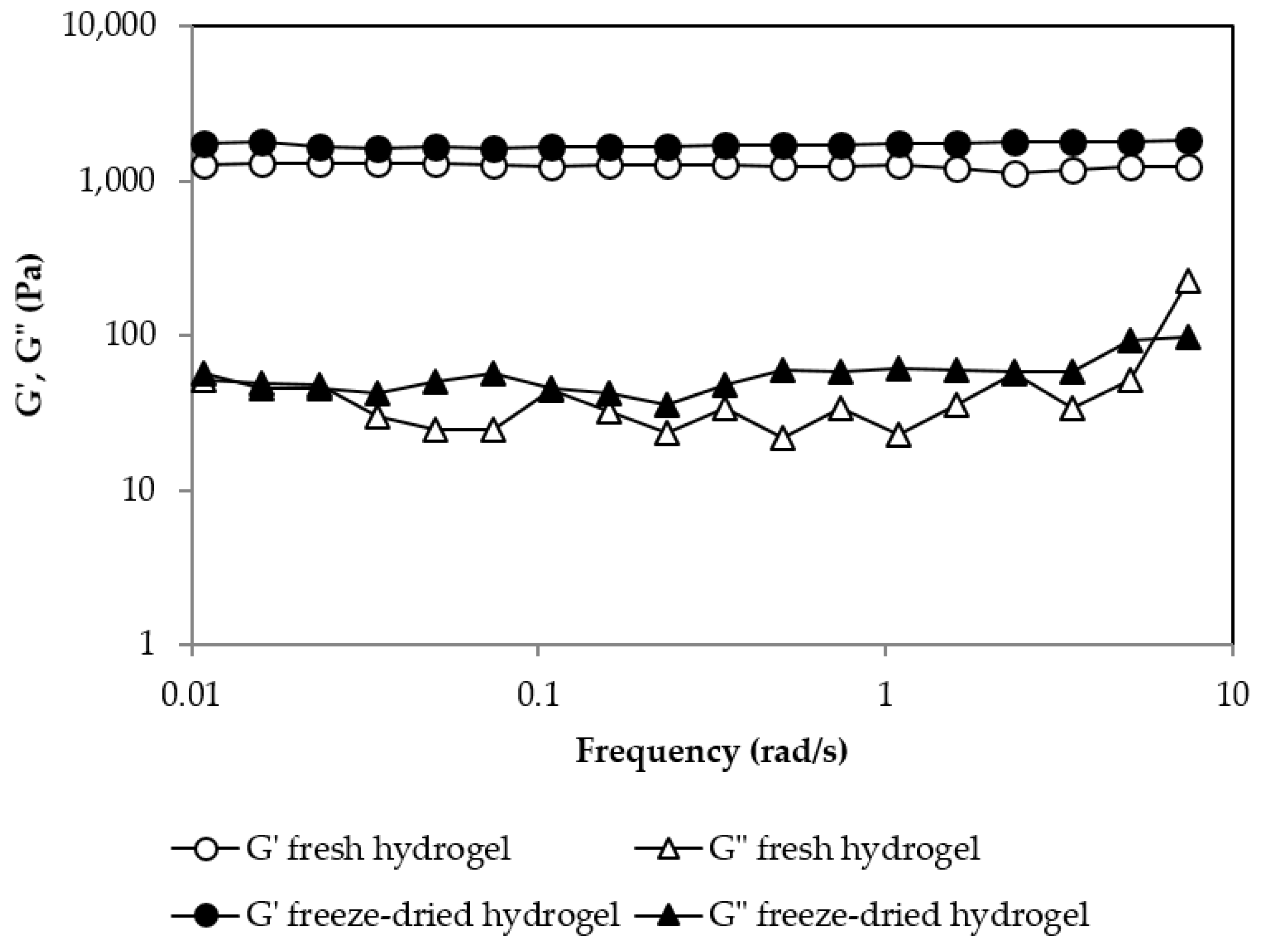

| [Pullulan] (%w/w) | [KOH] (%w/w) | [Pullulan]/[KOH] | [Pullulan]/[STMP] | G′ (Pa) at 24 h | Gel’s Appareance |
|---|---|---|---|---|---|
| 6 | 5.0 | 1.20 | 2 | 514 | Transparent slightly yellow |
| 4.5 | 1.33 | 2 | 630 | Transparent slightly yellow | |
| 4.0 | 1.50 | 2 | 348 | Transparent slightly yellow | |
| 3.5 | 1.70 | 2 | 1532 | Transparent slightly yellow | |
| 3.0 | 2.00 | 2 | 1243 | Transparent slightly yellow | |
| 2.5 | 2.40 | 2 | 1370 | Transparent | |
| 2.0 | 3.00 | 2 | 1276 | Transparent | |
| 1.7 | 3.50 | 2 | 1873 | Transparent | |
| 1.5 | 4.00 | 2 | 826 | Transparent | |
| 8 | 5.0 | 1.20 | 2 | * | Not gel |
| 4.5 | 1.33 | 2 | 76 | Transparent slightly yellow | |
| 4.0 | 1.50 | 2 | 426 | Transparent slightly yellow | |
| 3.5 | 1.70 | 2 | 385 | Transparent slightly yellow | |
| 3.0 | 2.00 | 2 | 752 | Transparent | |
| 2.5 | 2.40 | 2 | 2120 | Transparent | |
| 2.0 | 3.00 | 2 | 1856 | Transparent | |
| 1.7 | 3.50 | 2 | 2300 | Transparent | |
| 1.5 | 4.00 | 2 | 2170 | Transparent | |
| 10 | 5.0 | 1.20 | 2 | * | Not gel |
| 4.5 | 1.33 | 2 | * | Not gel | |
| 4.0 | 1.50 | 2 | 298 | Transparent | |
| 3.5 | 1.70 | 2 | 527 | Transparent | |
| 3.0 | 2.00 | 2 | 2059 | Transparent | |
| 2.5 | 2.40 | 2 | 2222 | Transparent | |
| 2.0 | 3.00 | 2 | 2243 | Transparent | |
| 1.7 | 3.50 | 2 | 5521 | Transparent | |
| 1.5 | 4.00 | 2 | 3465 | Transparent |
| pH | [Pullulan] (%w/w) | Ratio [Pullulan]/[KOH] | Ratio [Pulllan]/[STMP] | KH | R2 | Ds (m2/s) |
|---|---|---|---|---|---|---|
| 2 | 6 | 3.5 | 2 | 0.0147 | 0.9556 | 7.74 × 10−13 |
| 8 | 3.5 | 2 | 0.0239 | 0.9478 | 9.38 × 10−13 | |
| 10 | 3.5 | 2 | 0.0565 | 0.9690 | 2.12 × 10−12 | |
| 7 | 6 | 3.5 | 2 | 0.0956 | 0.9937 | 2.37 × 10−12 |
| 8 | 3.5 | 2 | 0.0348 | 0.9135 | 1.92 × 10−12 | |
| 10 | 3.5 | 2 | 0.0552 | 0.9587 | 1.07 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaría, E.; Anjinho de Barros, L.; González, C.; Maestro, A. Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems. Gels 2023, 9, 644. https://doi.org/10.3390/gels9080644
Santamaría E, Anjinho de Barros L, González C, Maestro A. Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems. Gels. 2023; 9(8):644. https://doi.org/10.3390/gels9080644
Chicago/Turabian StyleSantamaría, Esther, Leticia Anjinho de Barros, Carme González, and Alicia Maestro. 2023. "Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems" Gels 9, no. 8: 644. https://doi.org/10.3390/gels9080644
APA StyleSantamaría, E., Anjinho de Barros, L., González, C., & Maestro, A. (2023). Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems. Gels, 9(8), 644. https://doi.org/10.3390/gels9080644