Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions
Abstract
:1. Introduction
2. Preparation, Properties, and Applications of Hydrogels
3. Types of Skin Wounds and Wound Healing
3.1. Overview of Skin Trauma
3.2. Types of Wound
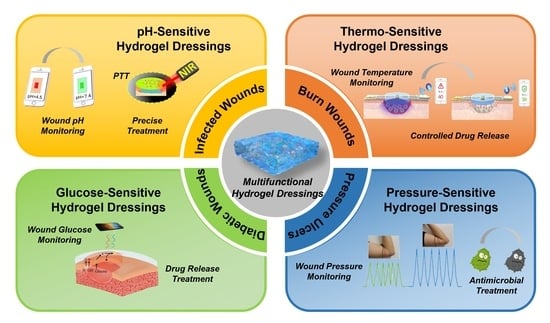
3.2.1. Infected Wounds
3.2.2. Burn Wounds
3.2.3. Diabetic Wounds
3.2.4. Pressure Ulcers
3.3. The Process of Wound Healing
4. Hydrogel Dressings with Wound Microenvironment Monitoring and Treatment Functions
4.1. pH-Sensitive Hydrogel Dressing
4.2. Thermo-Sensitive Hydrogel Dressing
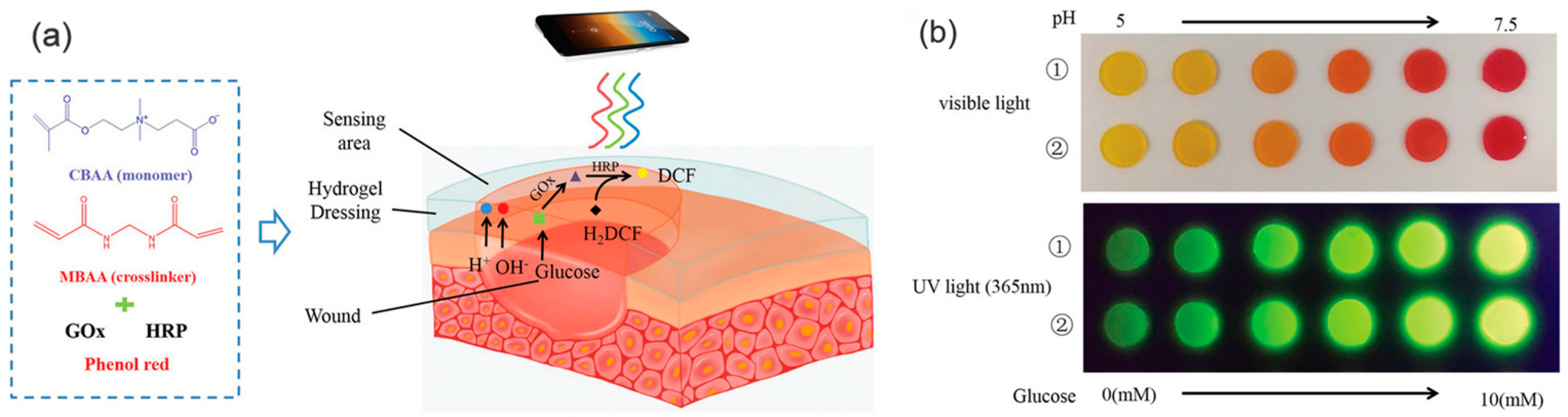
4.3. Blood Glucose-Sensitive Hydrogel Dressing
4.4. Pressure-Sensitive Hydrogel Dressing
4.5. Nano-Composite Hydrogel Dressing
5. Commercial Hydrogel Dressings
6. Conclusions and Prospects
- The development of multifunctional hydrogel dressings is promising. For example, hydrogel dressings can monitor the wound microenvironment and also have excellent antibacterial, anti-inflammatory, antibleeding, mechanical properties, injectable, and self-healing properties;
- The need for the preparation of hydrogel dressings that can meet all the requirements in the whole process of wound healing is urgent. Since the wound repair process is complex and involves dynamic changes in various parameters, providing functionality on demand is a direction for further research;
- The integration of wound microenvironment monitoring and telemedicine is an important direction. It is of great interest to develop a hydrogel wound dressing that can simultaneously monitor the state of the wound’s microenvironment and inform the physician. A hydrogel dressing with wound monitoring and treatment functions can guide doctors to remotely control the treatment of wounds, which may be the future trend.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dabrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.M.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; An, T.Z.; Xian, M.; Luckanagul, J.A.; Su, Z.; Lin, Y.; Wang, Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, P.F.; Zhu, C.L.; Ning, N.; Zhang, S.; Vancso, G.J. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv. Funct. Mater. 2019, 29, 1904402. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.L.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Hu, B.H.; Berkey, C.; Feliciano, T.; Chen, X.; Li, Z.; Chen, C.; Amini, S.; Nai, M.H.; Lei, Q.L.; Ni, R.; et al. Thermal-Disrupting Interface Mitigates Intercellular Cohesion Loss for Accurate Topical Antibacterial Therapy. Adv. Mater. 2020, 32, e1907030. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.T.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Liu, Y.N.; Xiao, Y.Q.; Cao, Y.Y.; Guo, Z.; Li, F.; Wang, L. Construction of Chitosan-Based Hydrogel Incorporated with Antimonene Nanosheets for Rapid Capture and Elimination of Bacteria. Adv. Funct. Mater. 2020, 30, 2003196. [Google Scholar] [CrossRef]
- Sun, M.M.; Xin, T.; Ran, Z.Y.; Pei, X.; Ma, C.; Liu, J.; Cao, M.; Bai, J.; Zhou, M. A Bendable Biofuel Cell-Based Fully Integrated Biomedical Nanodevice for Point-of-Care Diagnosis of Scurvy. ACS Sens. 2021, 6, 275–284. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Liang, Y.P.; Shi, M.T.; Guo, B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Weishaupt, R.; Zund, J.N.; Heuberger, L.; Zuber, F.; Faccio, G.; Robotti, F.; Ferrari, A.; Fortunato, G.; Ren, Q.; Maniura-Weber, K.; et al. Antibacterial, Cytocompatible, Sustainably Sourced: Cellulose Membranes with Bifunctional Peptides for Advanced Wound Dressings. Adv. Healthc. Mater. 2020, 9, e1901850. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Archang, M.M.; Kuan, C.H.; Weaver, W.M.; Weinstein, J.S.; Feng, A.C.; Ruccia, A.; Sideris, E.; Ragkousis, V.; Koh, J.; et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat. Mater. 2021, 20, 560–569. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.P.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Tang, Z.M.; Tang, L.Q.; Zeng, Y.J.; Li, Y.; Wang, L. Research progress of new medical dressings for wound infection detection. J. Donghua Univ. (Nat. Sci.) 2021, 47, 12–18, 48. (In Chinese) [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef]
- Mukherjee, D.; Azamthulla, M.; Santhosh, S.; Dath, G.; Ghosh, A.; Natholia, R.; Anbu, J.; Teja, B.V.; Muzammil, K.M. Development and characterization of chitosan-based hydrogels as wound dressing materials. J. Drug Deliv. Sci. Technol. 2018, 46, 498–510. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Li, Z.; Crago, M.; Schofield, T.; Zeng, H.; Vyas, H.K.N.; Müllner, M.; Mai-Prochnow, A.; Farajikhah, S.; Naficy, S.; Dehghani, F.; et al. Synthesis and Evaluation of Functionalized Polyurethanes for pH-Responsive Delivery of Compounds in Chronic Wounds. Gels 2023, 9, 611. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Gradinaru, V.R.; Bercea, M. Diversity of Bioinspired Hydrogels: From Structure to Applications. Gels 2023, 9, 376. [Google Scholar] [CrossRef]
- Blanco, G.E.D.O.; de Souza, C.W.O.; Bernardo, M.P.; Zenke, M.; Mattoso, L.H.C.; Moreira, F.K.V. Antimicrobially active gelatin/[Mg-Al-CO3]-LDH composite films based on clove essential oil for skin wound healing. Mater. Today Commun. 2021, 27, 102169. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Sun, Y.; Zhao, S.; Feng, M.; Xu, G.; Zhu, H.; Ji, P.; Mao, H.; He, Y.; et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr. Polym. 2021, 261, 117870. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Villa, C.; Russo, E. Hydrogels in Hand Sanitizers. Materials 2021, 14, 1577. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhang, J.M.; Song, J.Y.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2019, 30, 1905493. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent Hybrid Hydrogels for Rapid In Situ Detection and Photothermal Therapy of Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 39685–39694. [Google Scholar] [CrossRef]
- Li, D.R.; Fei, X.; Xu, L.Q.; Wang, Y.; Tian, J.; Li, Y. Pressure-sensitive antibacterial hydrogel dressing for wound monitoring in bed ridden patients. J. Colloid Interface Sci. 2022, 627, 942–955. [Google Scholar] [CrossRef]
- Zhu, S.L.; Zhao, B.J.; Li, M.C.; Wang, H.; Zhu, J.; Li, Q.; Gao, H.; Feng, Q.; Cao, X. Microenvironment responsive nanocomposite hydrogel with NIR photothermal therapy, vascularization and anti-inflammation for diabetic infected wound healing. Bioact. Mater. 2023, 26, 306–320. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Boer-Auer, A.; Schacht, V. Histopathology of the skin-clinically relevant and innovative. Hautarzt 2018, 69, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Xiao, J.S.; Chen, S.Y.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Cai, W.J.; Han, P. Progress in the treatment of chronic wounds with hydrogel dressing. Int. J. Orthop. 2020, 41, 195–198. (In Chinese) [Google Scholar]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Shu, W.T.; Wang, Y.N.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef] [PubMed]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.Y.; Salgado, G.; Lane, E.B.; Hauser, C.A. Transparent crosslinked ultrashort peptide hydrogel dressing with high shape-fidelity accelerates healing of full-thickness excision wounds. Sci. Rep. 2016, 6, 32670. [Google Scholar] [CrossRef] [PubMed]
- Richmond, N.A.; Maderal, A.D.; Vivas, A.C. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 2013, 26, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Barshes, N.R.; Sigireddi, M.; Wrobel, J.S.; Mahankali, A.; Robbins, J.M.; Kougias, P.; Armstrong, D.G. The system of care for the diabetic foot: Objectives, outcomes, and opportunities. Diabet Foot Ankle 2013, 4, 21847. [Google Scholar] [CrossRef]
- Fan, L.P.; Wang, H.S.; Zhang, K.H.; Cai, Z.; He, C.; Sheng, X.; Mo, X. Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv. 2012, 2, 4110–4119. [Google Scholar] [CrossRef]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Lipsky, B.A.; Bakker, K.; International Working Group on the Diabetic, F. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 7–15. [Google Scholar] [CrossRef]
- Wang, H.N.; Xu, Z.J.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Bansal, C.; Scott, R.; Stewart, D.; Cockerell, C.J. Decubitus ulcers: A review of the literature. Int. J. Dermatol. 2005, 44, 805–810. [Google Scholar] [CrossRef]
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2, CD011226. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef]
- Liang, Y.P.; He, J.H.; Guo, B.L. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Ishida, Y.; Kondo, T.; Takayasu, T.; Iwakura, Y.; Mukaida, N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J. Immunol. 2004, 172, 1848–1855. [Google Scholar] [CrossRef]
- Guo, H.S.; Bai, M.; Zhu, Y.N.; Liu, X.; Tian, S.; Long, Y.; Ma, Y.; Wen, C.; Li, Q.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- McLister, A.; McHugh, J.; Cundell, J.; Davis, J. New Developments in Smart Bandage Technologies for Wound Diagnostics. Adv. Mater. 2016, 28, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.R.; Zhou, M.Y.; Xu, T.L.; Zhang, X. Multifunctional hydrogel as wound dressing for intelligent wound monitoring. Chem. Eng. J. 2022, 433, 134625. [Google Scholar] [CrossRef]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Zahran, M.K.; Hakeim, O.A. Greener therapeutic pH-sensing wound dressing based on Curcuma Longa and cellulose hydrogel. Eur. Polym. J. 2021, 159, 110744. [Google Scholar] [CrossRef]
- Fierheller, M.; Sibbald, R.G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Skin Wound Care 2010, 23, 369–379, quiz 380–361. [Google Scholar] [CrossRef]
- Nakagami, G.; Sanada, H.; Iizaka, S.; Kadono, T.; Higashino, T.; Koyanagi, H.; Haga, N. Predicting delayed pressure ulcer healing using thermography: A prospective cohort study. J. Wound Care 2010, 19, 465–466, 468, 470. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.N.; Guo, B.L. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.F.; Zhang, S.M.; Qu, C.L.; Li, C.B.; Gao, Y.L. Research progress of hydrogel wound dressings based on natural polysaccharides. Mater. Rep. 2022, 36, 182–190. (In Chinese) [Google Scholar]
- Montaser, A.S.; Rehan, M.; El-Naggar, M.E. pH-Thermosensitive hydrogel based on polyvinyl alcohol/sodium alginate/N-isopropyl acrylamide composite for treating re-infected wounds. Int. J. Biol. Macromol. 2019, 124, 1016–1024. [Google Scholar] [CrossRef]
- Pang, Q.; Lou, D.; Li, S.J.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, e1703509. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Blatchley, M.R.; Duh, E.J.; Gerecht, S. Acellular and cellular approaches to improve diabetic wound healing. Adv. Drug Del. Rev. 2019, 146, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Niu, L.J.; Liang, H.Z.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.F.; Wang, L.Y.; Du, L.; Wang, X.; Li, Q.; Wang, X.; Zhang, J.; Nie, J.; Ma, G. Smart Polycationic Hydrogel Dressing for Dynamic Wound Healing. Small 2022, 18, e2201620. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, 1901408. [Google Scholar] [CrossRef]
- Basuki, J.S.; Qie, F.; Mulet, X.; Suryadinata, R.; Vashi, A.V.; Peng, Y.Y.; Li, L.; Hao, X.; Tan, T.; Hughes, T.C. Photo-Modulated Therapeutic Protein Release from a Hydrogel Depot Using Visible Light. Angew. Chem. Int. Ed. 2017, 56, 966–971. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, C.; Hu, Y.; Ge, S.; Wang, J.; Jin, Z. In Situ Self-Assembly of Nanoparticles into Waxberry-Like Starch Microspheres Enhanced the Mechanical Strength, Fatigue Resistance, and Adhesiveness of Hydrogels. ACS Appl. Mater. Interfaces 2020, 12, 46609–46620. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef]
- Xu, M.-L.; Guan, L.-Y.; Li, S.-K.; Chen, L.; Chen, Z. Stable gold graphitic nanocapsule doped hydrogels for efficient photothermal antibacterial applications. Chem. Commun. 2019, 55, 5359–5362. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ding, J.; Wu, X.; Zeng, M.; Tian, Y.; Wu, K.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Flexible and temperature-responsive hydrogel dressing for real-time and remote wound healing monitoring. J. Mater. Chem. B 2023, 11, 4934–4945. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Ma, C.; Li, S.; Liu, H.; Xia, C.; Li, J.; Zhang, S.; Zhang, W.; Cai, L.; Huang, Z. Tough thermosensitive hydrogel with excellent adhesion to low-energy surface developed via nanoparticle-induced dynamic crosslinking. Appl. Surf. Sci. 2021, 560, 149935. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Gheybi, H.; Adeli, M. Efficient wound healing by antibacterial property: Advances and trends of hydrogels, hydrogel-metal NP composites and photothermal therapy platforms. J. Drug Deliv. Sci. Technol. 2022, 73, 103458. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, X.; Hu, X.; Xiao, L.; Zhang, L.; Song, L.; Xu, M.; Zou, Y.; Chen, L.; Chen, Z.; et al. Simultaneous Application of Photothermal Therapy and an Anti-inflammatory Prodrug using Pyrene-Aspirin-Loaded Gold Nanorod Graphitic Nanocapsules. Angew. Chem. Int. Ed. 2018, 57, 177–181. [Google Scholar] [CrossRef]
- Luo, W.; Hu, B.; Zhang, H.-L.; Li, C.; Shi, Y.; Li, X.; Jin, L. Antibacterial, photothermal and stable Ag-titanium-oxo-clusters hydrogel designed for wound healing. Mater. Des. 2023, 226, 111674. [Google Scholar] [CrossRef]
- Ma, K.; Li, Y.; Wang, Z.; Chen, Y.; Zhang, X.; Chen, C.; Yu, H.; Huang, J.; Yang, Z.; Wang, X.; et al. Core–Shell Gold Nanorod@Layered Double Hydroxide Nanomaterial with Highly Efficient Photothermal Conversion and Its Application in Antibacterial and Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 29630–29640. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Xie, X.; Liao, J. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722. [Google Scholar] [CrossRef]
- Gan, Y.; Lin, C.; Zhu, H.; Cheng, X.; Liu, C.; Shi, J. An injectable self-healing CS/PDA–AgNPs hybrid hydrogel for mild and highly-efficient photothermal sterilization. New J. Chem. 2022, 46, 8043–8052. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2021, 18, 2104368. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A Review on Chitosan and Cellulose Hydrogels for Wound Dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef] [PubMed]















| Types of Chronic Wound | Types of Monitoring | Monitoring and Treatment Components | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Infected wound | pH | Brilliant Yellow, cabbage juice, and gentamicin-loaded alginate fibers | Offer comprehensive monitoring and treatment based on accurate and timely wound information | The exudate itself may be affected by color | [36] |
| pH | Litmus, convolutional neural network | Prevent or reduce the risk of bacterial infection | The wound cannot be treated accurately, and the solvent replacement method used in the preparation of hydrogels is time-consuming | [73] | |
| pH | Bromothymol blue, near-infrared absorption conjugated polymers, chitosan | In situ visual diagnosis of bacterial infection and photothermal therapy | Easy leaching of dyes | [38] | |
| pH | Curcuma longa extract | Visual detection, antibacterial | Inaccurate monitoring ability, easy leaching of dye, and poor wound healing effect | [74] | |
| Temperature | Temperature sensor and four UV-LEDs, gentamicin | Flexibility, compatibility, high monitoring sensitivity, and durability | High cost, short service life of response elements, and can only be used as disposable dressings | [81] | |
| pH, temperature | Potentiometric pH sensors, NIPAM | Dual response of pH and temperature, precise monitoring, and treatment | High-cost and complex preparation | [82] | |
| Burn wound | Temperature | Silver nanoparticles | Excellent antibacterial activity | Cannot monitor the wound microenvironment | [78] |
| Temperature | NIPAM, diclofenac sodium | Controlled and targeted drug delivery | Inaccurate perception of temperature changes | [80] | |
| Diabetic wound | pH, glucose | Schiff base, phenyl borate base, insulin, fibroblasts | Effective control of blood glucose levels treats diabetic wounds | The rate of insulin release is not well controlled | [84] |
| pH, glucose | Phenyl borate ester bond, metformin, copper-loaded dopamine nanoparticles | pH and glucose responses to controlled treatment, self-healing, good injectability, and adhesive properties | The rate of metformin release is not well controlled | [40] | |
| pH, glucose | Phenol red, glucose oxidase (GOx), horseradish peroxidase | Effectively monitor pH and glucose changes | Horseradish peroxidase is needed | [37] | |
| Temperature, glucose | NIPAM, methyl acrylamide phenyl boric acid | Continuous real-time monitoring and differentiation of temperature, glucose concentration, and wound strain | Inaccurate perception of temperature changes | [69] | |
| Pressure ulcers | Pressure | Imidazolidine ionic liquids, polyvinyl alcohol, acrylamide | Excellent pressure sensitivity, real-time responsiveness, stable signal output, and superior mechanical properties | Complex preparation process | [39] |
| Pressure | 2-(methacryloyloxy)-N,N,N-trimethyl ethylamine chloride, dopamine hydrochloride | Effectively monitor human health | The solvent displacement method is time-consuming | [85] |
| Hydrogel | Company | City and Country | Main Constituent |
|---|---|---|---|
| Algisite M | Smith & Nephew | London, United Kingdom | Alginate |
| Amniomatrix®4 | Derma Sciences Inc. | Pennsylvania, United States | Amniotic membrane and fluid constituents |
| Comfeel® Plus Contour Dressing | Coloplast Corp. | Guangdong, China | Carboxymethylcellulose |
| CovaWound™ Hydrocolloid dressing | Covalon Technologies, Ltd. | Georgia, United States | Hydrocolloids |
| Cutimed® Gel | Bsn Medical Gmbh | Hamburg, Germany | Carbomer 940 |
| DermaFilm® | DermaRite Industries, LLC | New Jersey, United States | Hydrocolloids |
| Helix3-cm® | Amerx Health Care Corp. | Florida, United States | Collagen |
| Inadine™ | Systagenix | Nevada, United States | Polyethylene Glycol |
| Kaltostat® | Convatec | Shanghai, China | Alginate |
| Kendall™ Hydrogel Dressing | Cardinal Health | Ohio, United States | Glycerin formulation |
| Sofargel | Sofar | Guangdong, China | Carbopol 974P |
| Tegaderm™ Hydrocolloid Dressing | 3 M Health Care | Leicestershire, United Kingdom | Hydrocolloids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. https://doi.org/10.3390/gels9090694
Jin S, Newton MAA, Cheng H, Zhang Q, Gao W, Zheng Y, Lu Z, Dai Z, Zhu J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels. 2023; 9(9):694. https://doi.org/10.3390/gels9090694
Chicago/Turabian StyleJin, Shanshan, Md All Amin Newton, Hongju Cheng, Qinchen Zhang, Weihong Gao, Yuansheng Zheng, Zan Lu, Zijian Dai, and Jie Zhu. 2023. "Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions" Gels 9, no. 9: 694. https://doi.org/10.3390/gels9090694
APA StyleJin, S., Newton, M. A. A., Cheng, H., Zhang, Q., Gao, W., Zheng, Y., Lu, Z., Dai, Z., & Zhu, J. (2023). Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels, 9(9), 694. https://doi.org/10.3390/gels9090694