Mechanistic Study of the Formation of Multicomponent Oxide Porous Microspheres (MICROSCAFS®) by Cryo-Scanning Electron Microscopy
Abstract
:1. Introduction
2. Results and Discussion
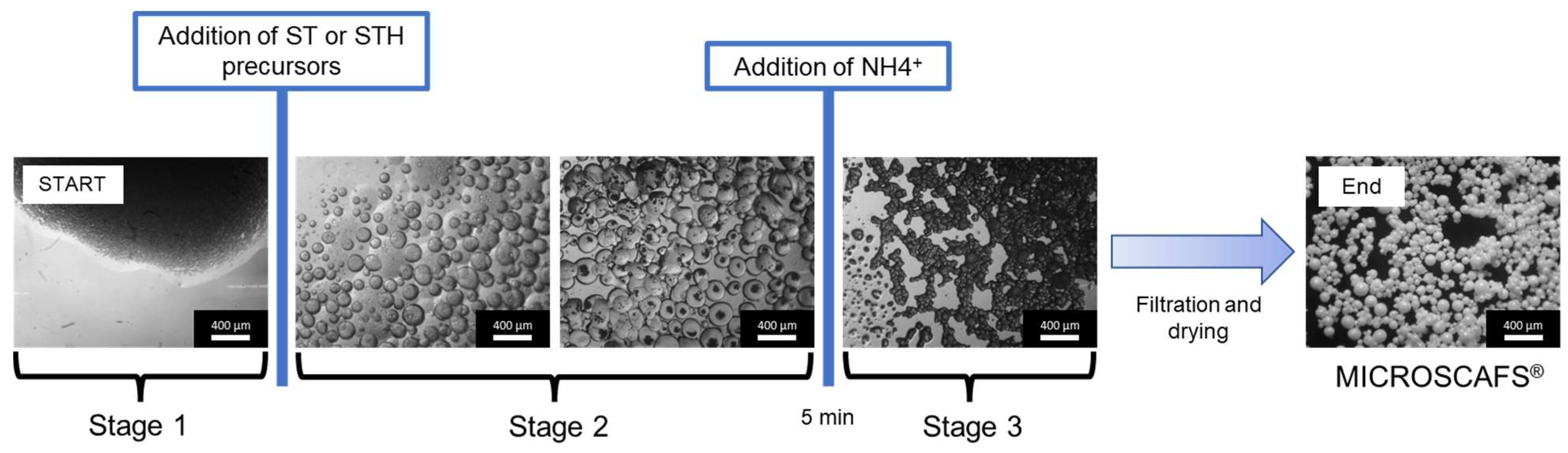
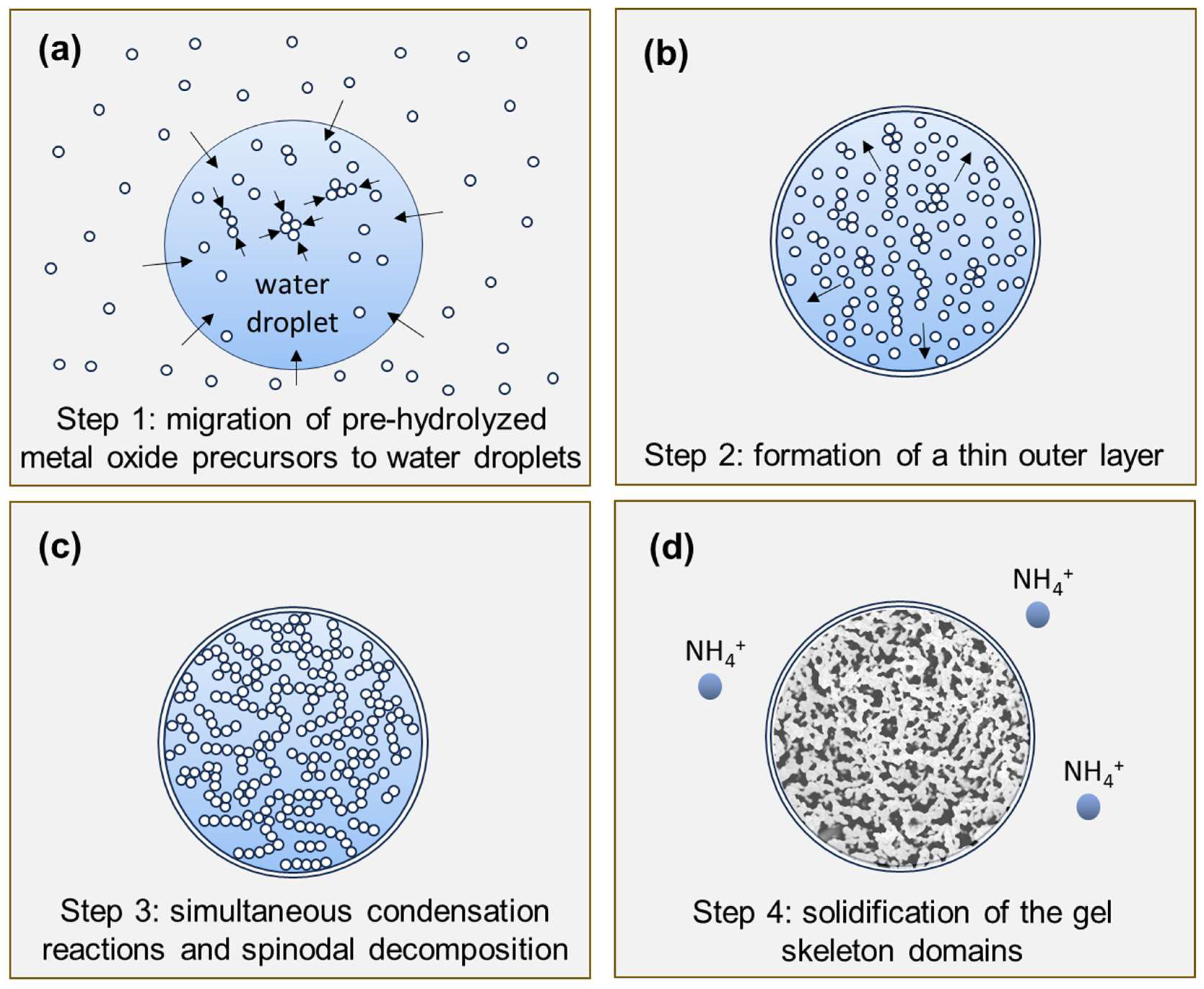
- Step 1
- the pre-hydrolyzed metal oxide precursors (sol) migrate to the water phase agglomerated droplets of the emulsion with the formation of oligomeric clusters (Figure 6a).
- Step 2
- accumulation of the oligomers at the water–oil interface, either by migration or kinetically trapping, and formation of a nanometric outer layer prior to the development of the internal interconnected gel skeleton, isolating the internal water/oligomer system from the outside (oil phase) and preventing particle coalescence (Figure 6b).
- Step 3
- condensation reactions lead to the formation of Si-O-Si, Si-O-Ti bonds at the expense of Si-OH and Ti-OH, with the formation of Si- and Ti-rich skeleton domains and their separation from the water phase inside the droplets (Figure 6c), typically by spinodal decomposition.
- Step 4
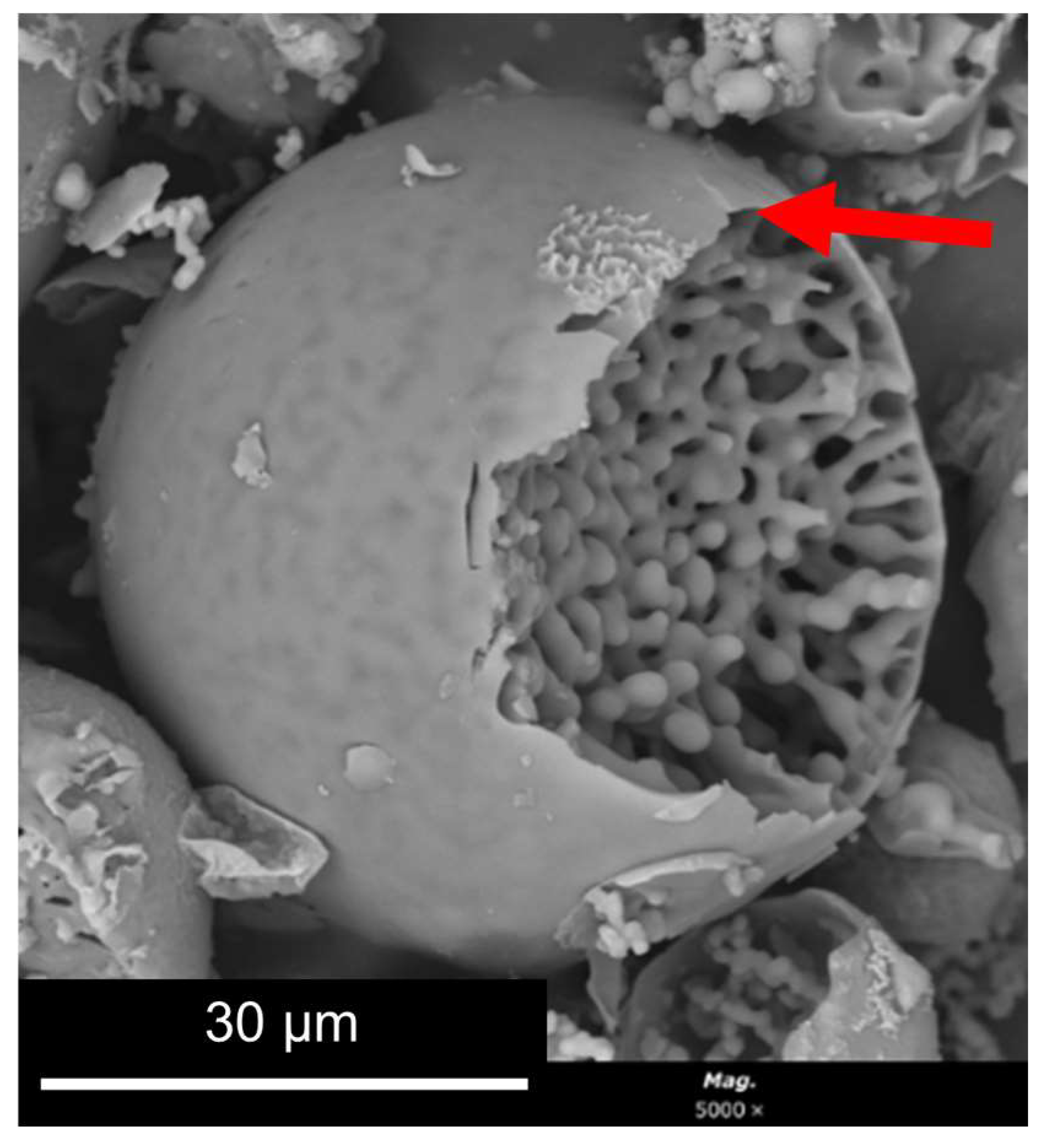
- concomitant phase separation and gelation with the system quickly maturing after the addition of the ammonia catalyst, producing the final MICROSCAFS® with interconnected macroporosity (Figure 6d). The typical mesoporosity observed in the MICROSCAFS® arises mainly from the binding of oligomeric clusters, forming the skeleton domains, while interconnected macroporosity is due to the phase separation process by spinodal decomposition. The size of the oligomeric clusters affects the size of the mesopores and can be influenced by the pH of the reaction medium, temperature, or the use of specific surfactants, such as the amphiphilic Pluronic® P123. Post-synthesis procedures, based on solvent exchange, consist of another alternative.
3. Conclusions
4. Materials and Methods
4.1. Synthesis of the ST and STH MICROSCAFS®
4.2. Optical Microscopy
4.3. Scanning Electron Microscopy
4.4. Cryo-Scanning Electron Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loureiro, M.V.; Vale, M.; De Schrijver, A.; Bordado, J.C.; Silva, E.; Marques, A.C. Hybrid Custom-Tailored Sol-Gel Derived Microscaffold for Biocides Immobilization. Microporous Mesoporous Mater. 2018, 261, 252–258. [Google Scholar] [CrossRef]
- Vale, M.; Loureiro, M.V.; Ferreira, M.J.; Marques, A.C. Silica-Based Microspheres with Interconnected Macroporosity by Phase Separation. J. Sol-Gel Sci. Technol. 2020, 95, 746–759. [Google Scholar] [CrossRef]
- Vale, M.; Orišková, S.; Mariquito, A.; Reis, L.; Pinto, M.; Marques, A.C. Multicomponent Oxide Microspheres with Designed Macroporosity (MICROSCAFS®): A Customized Platform for Chemicals Immobilization. RSC Adv. 2023, 13, 12951–12965. [Google Scholar] [CrossRef] [PubMed]
- Goi, Y.; Fujisawa, S.; Saito, T.; Yamane, K.; Kuroda, K.; Isogai, A. Dual Functions of TEMPO-Oxidized Cellulose Nanofibers in Oil-in-Water Emulsions: A Pickering Emulsifier and a Unique Dispersion Stabilizer. Langmuir 2019, 35, 10920–10926. [Google Scholar] [CrossRef]
- Zamani, S.; Malchione, N.; Selig, M.J.; Abbaspourrad, A. Formation of Shelf Stable Pickering High Internal Phase Emulsions (HIPE) through the Inclusion of Whey Protein Microgels. Food Funct. 2018, 9, 982–990. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Wang, Y.; Boesch, C.; Zhao, Y.; Sarkar, A. Pickering Emulsions Stabilized by Colloidal Gel Particles Complexed or Conjugated with Biopolymers to Enhance Bioaccessibility and Cellular Uptake of Curcumin. Curr. Res. Food Sci. 2020, 3, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Li, B. Novel Food-Grade Pickering Emulsions Stabilized by Tea Water-Insoluble Protein Nanoparticles from Tea Residues. Food Hydrocoll. 2019, 96, 322–330. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, Y.; Jin, H.; Sui, X.; Jiang, L. Deciphering the Structural Network That Confers Stability to High Internal Phase Pickering Emulsions by Cross-Linked Soy Protein Microgels and Their in Vitro Digestion Profiles. J. Agric. Food Chem. 2020, 68, 9796–9803. [Google Scholar] [CrossRef] [PubMed]
- Lorignon, F.; Gossard, A.; Carboni, M.; Meyer, D. Microstructural and Rheological Investigation of Upcycled Metal-Organic Frameworks Stabilized Pickering Emulsions. J. Colloid Interface Sci. 2021, 586, 305–314. [Google Scholar] [CrossRef]
- Omarova, M.; Swientoniewski, L.T.; Tsengam, I.K.M.; Panchal, A.; Yu, T.; Blake, D.A.; Lvov, Y.M.; Zhang, D.; John, V. Engineered Clays as Sustainable Oil Dispersants in the Presence of Model Hydrocarbon Degrading Bacteria: The Role of Bacterial Sequestration and Biofilm Formation. ACS Sustain. Chem. Eng. 2018, 6, 14143–14153. [Google Scholar] [CrossRef]
- Song, P.; Natale, G.; Wang, J.; Bond, T.; Hejazi, H.; de la Hoz Siegler, H.; Gates, I.; Lu, Q. 2D and 3D Metal–Organic Framework at the Oil/Water Interface: A Case Study of Copper Benzenedicarboxylate. Adv. Mater. Interfaces 2019, 6, 1801139. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and Evaluation of a Novel High Internal Phase Pickering Emulsion Based on Whey Protein Isolate Nanofibrils Derived by Hydrothermal Method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Derakhshandeh, M.; Pilapil, B.K.; Workman, B.; Trifkovic, M.; Bryant, S.L. Analysis of Network Formation and Long-Term Stability in Silica Nanoparticle Stabilized Emulsions. Soft Matter 2018, 14, 4268–4277. [Google Scholar] [CrossRef]
- Cameron, N.R.; Sherrington, D.C. High Internal Phase Emulsions (HIPEs)—Structure, Properties and Use in Polymer Preparation. Adv. Polym. Sci. 1996, 126, 162–214. [Google Scholar] [CrossRef]
- Galvão, K.C.S.; Vicente, A.A.; Sobral, P.J.A. Development, Characterization, and Stability of O/W Pepper Nanoemulsions Produced by High-Pressure Homogenization. Food Bioprocess Technol. 2018, 11, 355–367. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Q.; Cao, S.; Wang, Y.; Zhou, G.; Zhang, W. Effect of Regenerated Cellulose Fiber on the Properties and Microstructure of Emulsion Model System from Meat Batters. Food Hydrocoll. 2019, 87, 83–89. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, J.; Xu, D.; Cao, Y.; Sun, B. The Effect of Unadsorbed Proteins on the Physiochemical Properties of the Heteroaggregates of Oppositely Charged Lactoferrin Coated Lutein Droplets and Whey Protein Isolate Coated DHA Droplets. Food Funct. 2018, 9, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, Y.; Tian, T.; Wang, Z.Z.; Qi, B.; Zhang, X.; Liu, J.; Li, Y.; Jiang, L.; Wang, Z.Z. In Vitro Simulated Digestion and Microstructure of Peppermint Oil Nanoemulsion. J. Oleo Sci. 2019, 68, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Moens, K.; Tzompa-Sosa, D.A.; Van de Walle, D.; Van der Meeren, P.; Dewettinck, K. Influence of Cooling Rate on Partial Coalescence in Natural Dairy Cream. Food Res. Int. 2019, 120, 819–828. [Google Scholar] [CrossRef]
- Safieh, P.; Pensini, E.; Marangoni, A.; Lamont, K.; Ghazani, S.M.; Callaghan-Patrachar, N.; Strüder-Kypke, M.; Peyronel, F.; Chen, J.; Rodriguez, B.M. Natural Emulsion Gels and Lecithin-Based Sorbents: A Potential Treatment Method for Organic Spills on Surface Waters. Colloids Surf A Physicochem Eng Asp 2019, 574, 245–259. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Zhou, H.; Ouyang, X.; Jiang, D.; Li, J.; Guo, Q.; Tang, J.; Yang, C. Effect of the Chain Structure of Self-Emulsifying Polyester Sizing Agent on ILSS of Carbon Fiber/Unsaturated Polyester Resin Composites. Polymers 2019, 11, 1528. [Google Scholar] [CrossRef]
- Haridas, M.M.; Menon, A.; Goyal, N.; Chandran, S.; Bellare, J.R. Cryo-SEM Imaging of Microstructures in a Chelated Sol-Gel Process. Trans. Indian Ceram. Soc. 1995, 54, 152–155. [Google Scholar] [CrossRef]
- Oliveira, F.; Monteiro, S.R.; Barros-Timmons, A.; Lopes-Da-Silva, J.A. Weak-Gel Formation in Dispersions of Silica Particles in a Matrix of a Non-Ionic Polysaccharide: Structure and Rheological Characterization. Carbohydr. Polym. 2010, 82, 1219–1227. [Google Scholar] [CrossRef]
- Erlich, M.; Arie, T.; Koifman, N.; Talmon, Y. Structure Elucidation of Silica-Based Core–Shell Microencapsulated Drugs for Topical Applications by Cryogenic Scanning Electron Microscopy. J. Colloid Interface Sci. 2020, 579, 778–785. [Google Scholar] [CrossRef] [PubMed]
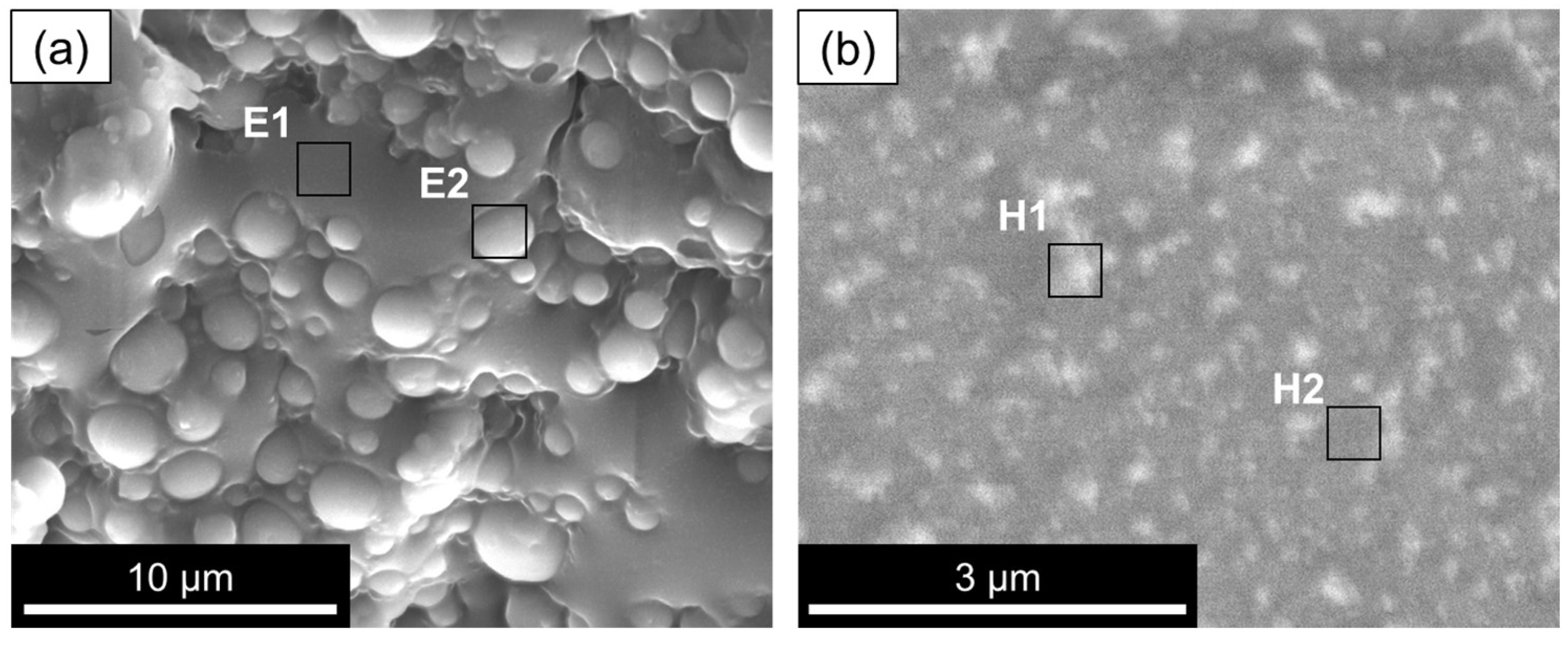


| Cryo-SEM Photomicrograph Region | Stage of the Synthesis | Atomic Concentration, % | |||||
|---|---|---|---|---|---|---|---|
| C | O | Si | Cl | Ti | Hf | ||
| E1 | Stage 1: Emulsion stage | 92.8 | 7.2 | - | - | - | - |
| E2 | 9.8 | 90.2 | - | - | - | - | |
| H1 | Stage 1: Pre-hydrolysate (precursors colloidal solution, before adding to the emulsion) | 35.2 | 46.5 | 14.8 | - | 3.5 | - |
| H2 | 36.4 | 47.7 | 13.0 | 0.5 | 2.4 | - | |
| EH1 | Stage 2: After addition of the pre-hydrolysate to the emulsion (embryonic stage) | 24.4 | 43.1 | 24.1 | - | 8.4 | - |
| EH2 | 26.7 | 48.2 | 21.6 | - | 3.5 | - | |
| EH3 | 29.7 | 65.6 | 2.9 | 1.1 | 0.2 | 0.5 | |
| EH4 | 96.4 | 3.6 | - | - | - | - | |
| EH5 | 29.8 | 60.1 | 6.4 | 1.4 | 1.5 | 0.8 | |
| EH6 | 19.8 | 73.4 | 5.1 | - | 0.9 | 0.8 | |
| G1 | Stage 3: After addition of the ammonia solution to catalyze the gelation (final stage) | 21.5 | 72.3 | 4.7 | - | 1.5 | - |
| G2 | 94.8 | 5.2 | - | - | - | - | |
| G3 | 24.5 | 67.6 | 5 | 1.4 | 1.1 | 0.4 | |
| G4 | 92.8 | 7.2 | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vale, M.; Marques, A.C. Mechanistic Study of the Formation of Multicomponent Oxide Porous Microspheres (MICROSCAFS®) by Cryo-Scanning Electron Microscopy. Gels 2023, 9, 704. https://doi.org/10.3390/gels9090704
Vale M, Marques AC. Mechanistic Study of the Formation of Multicomponent Oxide Porous Microspheres (MICROSCAFS®) by Cryo-Scanning Electron Microscopy. Gels. 2023; 9(9):704. https://doi.org/10.3390/gels9090704
Chicago/Turabian StyleVale, Mário, and Ana C. Marques. 2023. "Mechanistic Study of the Formation of Multicomponent Oxide Porous Microspheres (MICROSCAFS®) by Cryo-Scanning Electron Microscopy" Gels 9, no. 9: 704. https://doi.org/10.3390/gels9090704
APA StyleVale, M., & Marques, A. C. (2023). Mechanistic Study of the Formation of Multicomponent Oxide Porous Microspheres (MICROSCAFS®) by Cryo-Scanning Electron Microscopy. Gels, 9(9), 704. https://doi.org/10.3390/gels9090704








