Carrageenan-Based Crowding and Confinement Combination Approach to Increase Collagen Deposition for In Vitro Tissue Development
Abstract
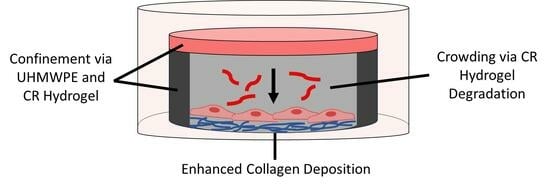
1. Introduction
2. Results
2.1. Hydrogel Degradation
2.2. Fluorescence Correlation Spectroscopy (FCS)
2.3. CR Gel Confinement Effects on 2D Cell Cultures
2.4. Comparison of Soluble CR Isoforms on Collagen Deposition
2.5. Computaional Modeling of Glucose and Oxygen Transport
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. CR Hydrogel Formation
5.3. Characterization of Hydrogel Degradation
5.4. Florescence Correlation Spectroscopy
5.5. Cellular Experiments with CR Hydrogels
5.6. Biochemical Analyses for Collagen and DNA Content
5.7. Resazurin Assay for CR Gel Toxicity
5.8. Soluble Crowding Experiments
5.9. Computational Modeling of Glucose and Oxygen Transport
5.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artegiani, B.; Clevers, H. Use and application of 3D-organoid technology. Hum. Mol. Genet. 2018, 27, R99–R107. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.F.; Cole, W.G.; Pillow, J.J.; Ramshaw, J.A. Induction of procollagen processing in fibroblast cultures by neutral polymers. J. Biol. Chem. 1986, 261, 4198–4203. [Google Scholar] [CrossRef]
- Rivas, G.; Minton, A.P. Macromolecular Crowding In Vitro, In Vivo, and In Between. Trends Biochem. Sci. 2016, 41, 970–981. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Zaslavsky, B.Y.; Breydo, L.; Turoverov, K.K.; Uversky, V.N. Beyond the excluded volume effects: Mechanistic complexity of the crowded milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef]
- Prewitz, M.C.; Stißel, A.; Friedrichs, J.; Träber, N.; Vogler, S.; Bornhäuser, M.; Werner, C. Extracellular matrix deposition of bone marrow stroma enhanced by macromolecular crowding. Biomaterials 2015, 73, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Etheredge, L.; Kane, B.P.; Valkov, N.; Adams, S.; Birk, D.E.; Hassell, J.R. Enhanced cell accumulation and collagen processing by keratocytes cultured under agarose and in media containing IGF-I, TGF-β or PDGF. Matrix Biol. 2010, 29, 519–524. [Google Scholar] [CrossRef][Green Version]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate--carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Satyam, A.; Kumar, P.; Fan, X.; Gorelov, A.; Rochev, Y.; Joshi, L.; Peinado, H.; Lyden, D.; Thomas, B.; Rodriguez, B.; et al. Macromolecular crowding meets tissue engineering by self-assembly: A paradigm shift in regenerative medicine. Adv. Mater. 2014, 26, 3024–3034. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Fuller, K.P.; Zeugolis, D.I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 2019, 88, 197–210. [Google Scholar] [CrossRef]
- De Pieri, A.; Rana, S.; Korntner, S.; Zeugolis, D.I. Seaweed polysaccharides as macromolecular crowding agents. Int. J. Biol. Macromol. 2020, 164, 434–446. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Lajeunesse, D.; Frondoza, C.; Schoffield, B.; Sacktor, B. Osteocalcin secretion by the human osteosarcoma cell line MG-63. J. Bone Miner. Res. 1990, 5, 915–922. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Romano, P.R.; Park, K.Y. Regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D3 in human osteosarcoma cells. J. Biol. Chem. 1988, 263, 18938–18945. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tan, S.F.; Wei, K.L.; Tsai, W.B. Modulation of the functions of osteoblast-like cells on poly(allylamine hydrochloride) and poly(acrylic acid) multilayer films. Colloids Surf. B Biointerfaces 2011, 88, 297–303. [Google Scholar] [CrossRef]
- Tsai, S.W.; Chen, C.C.; Chen, P.L.; Hsu, F.Y. Influence of topography of nanofibrils of three-dimensional collagen gel beads on the phenotype, proliferation, and maturation of osteoblasts. J. Biomed. Mater. Res. A 2009, 91, 985–993. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release from Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Antczak, A.; Samborska, K.; Lenart, A. Acid hydrolysis of kappa-carrageenan as a way of gaining new substances for freezing process modification and protection from excessive recrystallisation of ice. Int. J. Food Sci. Technol. 2015, 50, 1799–1806. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M. Mechanism of gel formation in κ-carrageenan. Biopolymers 1984, 23, 735–745. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Cui, B.; Liu, Y. Influence of cations on texture, compressive elastic modulus, sol-gel transition and freeze-thaw properties of kappa-carrageenan gel. Carbohydr. Polym. 2018, 202, 530–535. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Li, L. Thermoreversible gelation and viscoelasticity of κ-carrageenan hydrogels. J. Rheol. 2016, 60, 203–214. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, S.; Feng, Q.; Dong, C.; Yang, Y.; Li, G.; Bian, L. Nanocomposite hydrogels stabilized by self-assembled multivalent bisphosphonate-magnesium nanoparticles mediate sustained release of magnesium ion and promote in-situ bone regeneration. Acta Biomater. 2017, 64, 389–400. [Google Scholar] [CrossRef]
- Wieczorek, A.; Rezaei, N.; Chan, C.K.; Xu, C.; Panwar, P.; Brömme, D.; Merschrod, S.E.; Forde, N.R. Development and characterization of a eukaryotic expression system for human type II procollagen. BMC Biotechnol. 2015, 15, 112. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Boukari, H.; Leach, J.B. Solute diffusion and interactions in cross-linked poly(ethylene glycol) hydrogels studied by Fluorescence Correlation Spectroscopy. Soft Matter 2010, 6, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Lareu, R.R.; Arsianti, I.; Subramhanya, H.K.; Yanxian, P.; Raghunath, M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: A preliminary study. Tissue Eng. 2007, 13, 385–391. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Cigognini, D.; Pandit, A.; Zeugolis, D.I. Low oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human corneal fibroblast culture. J. Tissue Eng. Regen. Med. 2018, 12, 6–18. [Google Scholar] [CrossRef]
- Satyam, A.; Kumar, P.; Cigognini, D.; Pandit, A.; Zeugolis, D.I. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 2016, 44, 221–231. [Google Scholar] [CrossRef]
- Zeiger, A.S.; Loe, F.C.; Li, R.; Raghunath, M.; Van Vliet, K.J. Macromolecular crowding directs extracellular matrix organization and mesenchymal stem cell behavior. PLoS ONE 2012, 7, e37904. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Capretto, L.; Quinci, F.; Piva, R.; Nastruzzi, C. Preparation of cell-encapsulation devices in confined microenvironment. Adv. Drug Deliv. Rev. 2013, 65, 1533–1555. [Google Scholar] [CrossRef]
- Mosier, J.A.; Rahman-Zaman, A.; Zanotelli, M.R.; VanderBurgh, J.A.; Bordeleau, F.; Hoffman, B.D.; Reinhart-King, C.A. Extent of Cell Confinement in Microtracks Affects Speed and Results in Differential Matrix Strains. Biophys. J. 2019, 117, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Tsiapalis, D.; Zeugolis, D.I. It is time to crowd your cell culture media—Physicochemical considerations with biological consequences. Biomaterials 2021, 275, 120943. [Google Scholar] [CrossRef]
- Graceffa, V.; Zeugolis, D.I. Carrageenan enhances chondrogenesis and osteogenesis in human bone marrow stem cell culture. Eur. Cell Mater. 2019, 37, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, C.; Liu, Y.; Cui, B. Fabrication of kappa–carrageenan hydrogels with cinnamon essential oil/hydroxypropyl–β–cyclodextrin composite: Evaluation of physicochemical properties, release kinetics and antimicrobial activity. Int. J. Biol. Macromol. 2021, 170, 593–601. [Google Scholar] [CrossRef]
- Joy, R.; Vigneshkumar, P.N.; John, F.; George, J. Chapter 9—Hydrogels based on carrageenan. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Giri, T.K., Ghosh, B., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 293–325. [Google Scholar] [CrossRef]
- Islam, M.A. Einstein–Smoluchowski Diffusion Equation: A Discussion. Phys. Scr. 2004, 70, 120. [Google Scholar] [CrossRef]
- Anderson, D.; Athanasiou, K. A comparison of primary and passaged chondrocytes for use in engineering the temporomandibular joint. Arch. Oral Biol. 2009, 54, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zustiak, S.; Nossal, R.; Sackett, D. 279198 High-Throughput Stiffness Assay for the Study of Cancer Cell Susceptibility to Anti-Cancer Drugs. In Proceedings of the 2012 AIChE Annual Meeting, Pittsburgh, PA, USA, 28 October–2 November 2012. [Google Scholar]
- Zhou, S.; Cui, Z.; Urban, J.P. Nutrient gradients in engineered cartilage: Metabolic kinetics measurement and mass transfer modeling. Biotechnol. Bioeng. 2008, 101, 408–421. [Google Scholar] [CrossRef]
- Brown, D.A.; MacLellan, W.R.; Laks, H.; Dunn, J.C.; Wu, B.M.; Beygui, R.E. Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol. Bioeng. 2007, 97, 962–975. [Google Scholar] [CrossRef]
- Komarova, S.V.; Ataullakhanov, F.I.; Globus, R.K. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am. J. Physiol. Cell Physiol. 2000, 279, C1220–C1229. [Google Scholar] [CrossRef]
- Mohebbi-Kalhori, D.; Behzadmehr, A.; Doillon, C.J.; Hadjizadeh, A. Computational modeling of adherent cell growth in a hollow-fiber membrane bioreactor for large-scale 3-D bone tissue engineering. J. Artif. Organs 2012, 15, 250–265. [Google Scholar] [CrossRef]
- Pattappa, G.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krebs, J.; Stealey, S.; Brown, A.; Krohn, A.; Zustiak, S.P.; Case, N. Carrageenan-Based Crowding and Confinement Combination Approach to Increase Collagen Deposition for In Vitro Tissue Development. Gels 2023, 9, 705. https://doi.org/10.3390/gels9090705
Krebs J, Stealey S, Brown A, Krohn A, Zustiak SP, Case N. Carrageenan-Based Crowding and Confinement Combination Approach to Increase Collagen Deposition for In Vitro Tissue Development. Gels. 2023; 9(9):705. https://doi.org/10.3390/gels9090705
Chicago/Turabian StyleKrebs, Joseph, Samuel Stealey, Alyssa Brown, Austin Krohn, Silviya Petrova Zustiak, and Natasha Case. 2023. "Carrageenan-Based Crowding and Confinement Combination Approach to Increase Collagen Deposition for In Vitro Tissue Development" Gels 9, no. 9: 705. https://doi.org/10.3390/gels9090705
APA StyleKrebs, J., Stealey, S., Brown, A., Krohn, A., Zustiak, S. P., & Case, N. (2023). Carrageenan-Based Crowding and Confinement Combination Approach to Increase Collagen Deposition for In Vitro Tissue Development. Gels, 9(9), 705. https://doi.org/10.3390/gels9090705