Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management
Abstract
:1. Introduction
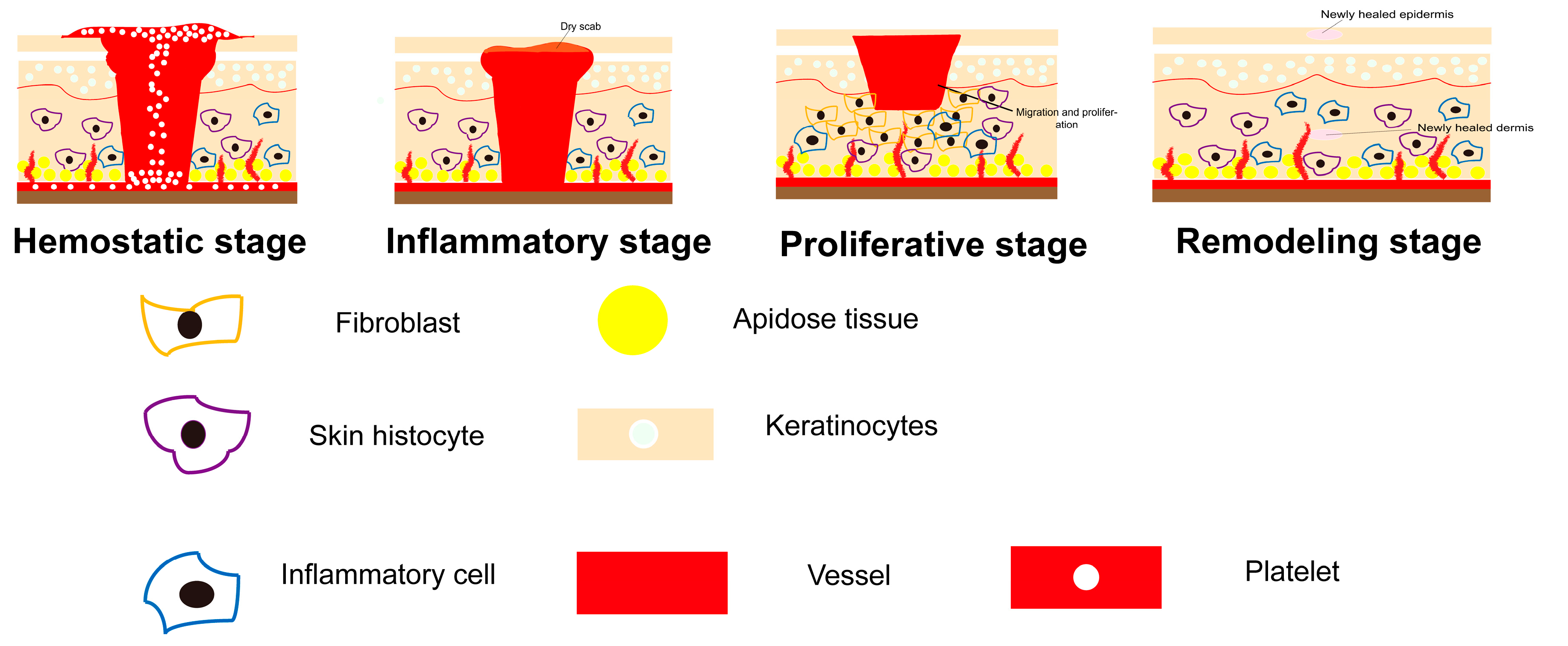
1.1. Chronic Wound
1.2. Healing Conditions
1.2.1. Suitable Healing Environment
1.2.2. Sustained Delivery of Growth Factor
2. Growth Factor Delivery Systems to Heal Chronic Wounds
2.1. Existing Delivery Systems
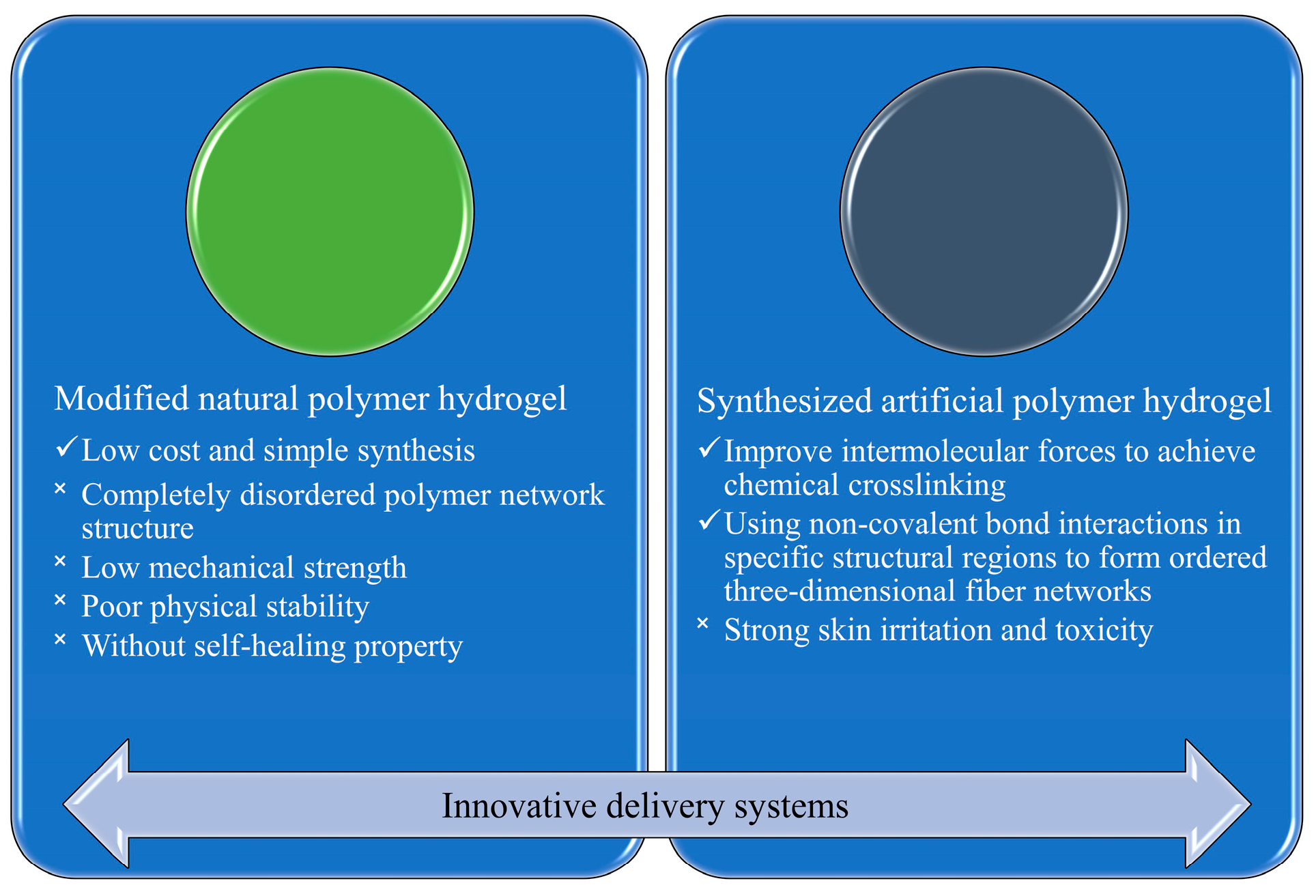
2.2. Innovative Delivery Systems
2.2.1. Hydrogels of Modified Natural Polymers
2.2.2. Hydrogels of Synthesized Polymers
3. Lyotropic Liquid Crystalline Might Be a Promising Candidate for Chronic Wound Healing
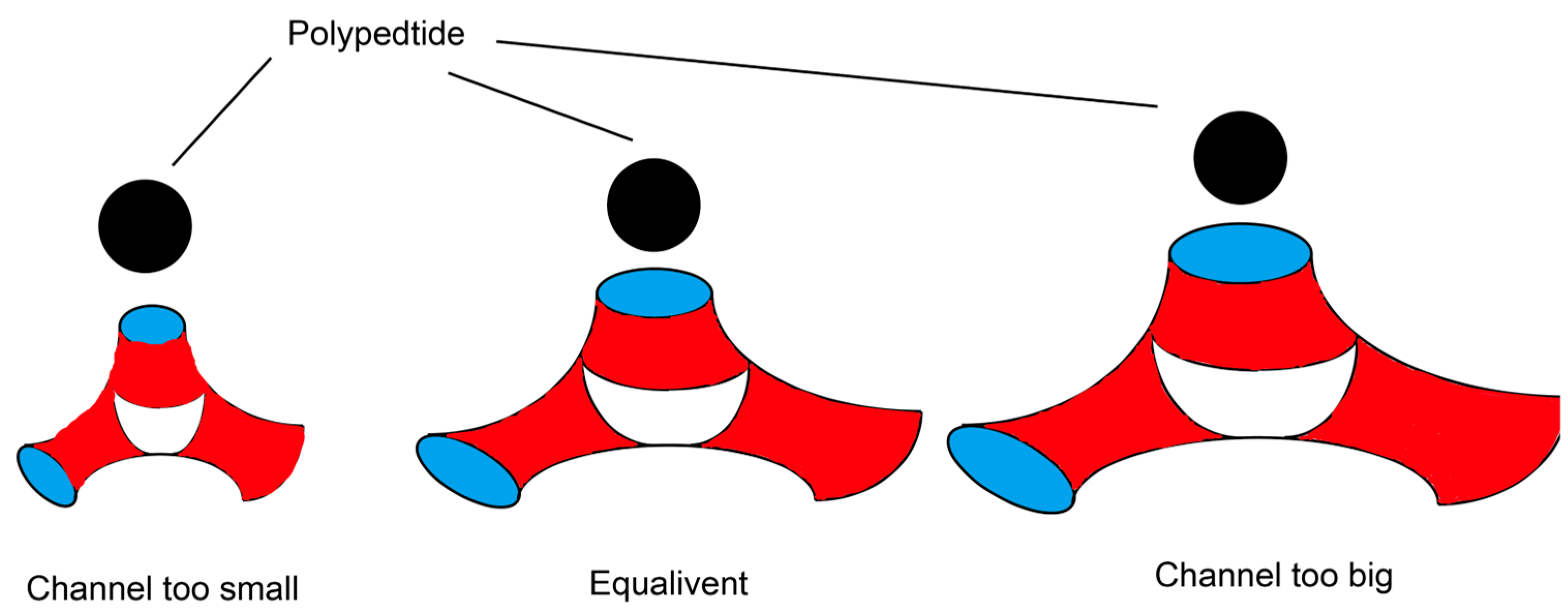
3.1. LLC Can Provide Sustained and Stable Growth Factor Delivery
3.2. LLC Can Provide a Suitable Healing Environment for Wounds
3.3. LLC Application in Wound Healing
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Skrepnek, G.H.; Mills, J.L., Sr.; Lavery, L.A.; Armstrong, D.G. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the US. Diabetes Care 2017, 40, 936–942. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory microenvironment of skin wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Qi, X.; Cai, E.; Zhang, C.; Wang, J.; Lan, Y.; Deng, H.; Shen, J.; Hu, R. Highly efficient bacteria-infected diabetic wound healing employing a melanin-reinforced biopolymer hydrogel. Chem. Eng. J. 2023, 460, 141852. [Google Scholar] [CrossRef]
- Bayat, M.; Sarojini, H.; Chien, S. The role of cluster of differentiation 163-positive macrophages in wound healing: A preliminary study and a systematic review. Arch. Dermatol. Res. 2023, 315, 359–370. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y.; He, G.; Ma, S.; Wang, Y.; Sun, Y. Pilose antler extract restores type I and III collagen to accelerate wound healing. Biomed. Pharmacother. 2023, 161, 114510. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Morello, S.; Petrella, A. The combination of mesoglycan and VEGF promotes skin wound repair by enhancing the activation of endothelial cells and fibroblasts and their cross-talk. Sci. Rep. 2022, 12, 11041. [Google Scholar] [CrossRef]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Stahl, H.C.; Ahmadi, F.; Nahzat, S.M.; Dong, H.J.; Stahl, K.W.; Sauerborn, R. Health economic evaluation of moist wound care in chronic cutaneous leishmaniasis ulcers in Afghanistan. Infect. Dis. Poverty 2018, 7, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Men, J.; Zhang, L.T.; Cheng, L.F.; Yang, W.B.; Zhang, W.H. Microstructural evolution and self-healing mechanism of a 2D C/SiC-BCx composite under constant load in static wet oxygen and dynamic combustion atmosphere. Mater. Corros. 2015, 66, 128–136. [Google Scholar] [CrossRef]
- Steiner, C.A.; Cartwright, I.M.; Taylor, C.T.; Colgan, S.P. Hypoxia-inducible factor as a bridge between healthy barrier function, wound healing, and fibrosis. Am. J. Physiol.—Cell Physiol. 2022, 323, C866–C878. [Google Scholar] [CrossRef] [PubMed]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef]
- Luan, X.; Li, W.; Lou, F. Applied analysis of humanized nursing combined with wet healing therapy to prevent bedsore. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4162–4166. [Google Scholar]
- Zahid, M.; Lodhi, M.; Rehan, Z.A.; Tayyab, H.; Javed, T.; Shabbir, R.; Mukhtar, A.; El Sabagh, A.; Adamski, R.; Sakran, M.I. Sustainable development of chitosan/Calotropis procera-based hydrogels to stimulate formation of granulation tissue and angiogenesis in wound healing applications. Molecules 2021, 26, 3284. [Google Scholar] [CrossRef]
- Han, X.; Ju, L.S.; Irudayaraj, J. Oxygenated Wound Dressings for Hypoxia Mitigation and Enhanced Wound Healing. Mol. Pharm. 2023, 20, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K. Innovations and Advances in Wound Healing; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Wang, X.-Z.; Tan, W.-Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2021, 6, 230–243. [Google Scholar] [CrossRef]
- Rodrigues, M.; Govindharajan, T. Study of hydrocellular functional material as microbicidal wound dressing for diabetic wound healing. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211054930. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yan, Y.; Qi, J.; Deng, L.; Shao, Z.-W.; Zhang, K.-Q.; Li, B.; Sun, Z.; Li, X. Cooperative assembly of a peptide gelator and silk fibroin afford an injectable hydrogel for tissue engineering. ACS Appl. Mater. Interfaces 2018, 10, 12474–12484. [Google Scholar] [CrossRef]
- Gragnani, A.; Tonarelli, E.; Chomiski, V.; Daher, R.P.; Ferreira, L. Fibroblast growth factor in the treatment of burns: A systematic review. Burns 2022, 48, 104–110. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mishra, A.; Agrawal, G.; Agrawal, A.; Mishra, S. Platelet Derived Growth Factor in Healing of Large Diabetic Foot Ulcers in Indian Clinical Set-Up: A Protocol-Based Approach. Available online: http://static.webmedcentral.com/article_view/3985 (accessed on 1 February 2013).
- Landi, F.; Aloe, L.; Russo, A.; Cesari, M.; Onder, G.; Bonini, S.; Carbonin, P.U.; Bernabei, R. Topical treatment of pressure ulcers with nerve growth factor: A randomized clinical trial. Ann. Intern. Med. 2003, 139, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Papanas, D.; Maltezos, E. Benefit-risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Saf. 2010, 33, 455–461. [Google Scholar] [CrossRef]
- Ziyadeh, N.; Fife, D.; Walker, A.M.; Wilkinson, G.S.; Seeger, J.D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Ski. Wound Care 2011, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Igarashi, A.; Urabe, K.; Koga, T.; Nakayama, J.; Kawamori, R.; Tamaki, K.; Hirakata, H.; Ohura, T.; Furue, M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur. J. Dermatol. 2009, 19, 461–468. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Huda, F.; Pant, J.; Ghosh Kar, A.; Banerjee, T.; Shukla, V.K. An appraisal of vascular endothelial growth factor (VEGF): The dynamic molecule of wound healing and its current clinical applications. Growth Factors 2022, 40, 73–88. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, J.; Feng, W.; Xu, B.; Zhang, K.; Ma, G.; Li, Y.; Yang, M.; Xu, F.-J. Glycosaminoglycan-based hydrogel delivery system regulates the wound microenvironment to rescue chronic wound healing. ACS Appl. Mater. Interfaces 2022, 14, 31737–31750. [Google Scholar] [CrossRef]
- Lau, H.-C.; Kim, A. Pharmaceutical perspectives of impaired wound healing in diabetic foot ulcer. J. Pharm. Investig. 2016, 46, 403–423. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Xia, G.; Liu, Y.; Tian, M.; Gao, P.; Bao, Z.; Bai, X.; Yu, X.; Lang, X.; Hu, S.; Chen, X. Nanoparticles/thermosensitive hydrogel reinforced with chitin whiskers as a wound dressing for treating chronic wounds. J. Mater. Chem. B 2017, 5, 3172–3185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Stojanović, G.M.; Hassan, R.; Anand, T.J.S.; Al-Ejji, M.; Hasan, A. Role of Graphene Oxide in Bacterial Cellulose− Gelatin Hydrogels for Wound Dressing Applications. ACS Omega 2023, 8, 15909–15919. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO dressing materials for burn and chronic wound healing with curcumin release kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Li, J.-Y.; Lin, Y.-T.; Wang, D.K.; Tseng, H.-H.; Wey, M.-Y. Planetary cross-linked structure design of hybrid organosilica membrane by amine-driven polymerization for CO2 separation. J. Clean. Prod. 2023, 398, 136568. [Google Scholar] [CrossRef]
- Williams, P.A.; Campbell, K.T.; Silva, E.A. Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis. J. Biomed. Mater. Res. Part A 2018, 106, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Jang, Y.-M.; Kim, Y.-M.; Park, W.S.; Choi, I.-W.; Jung, W.-K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018, 198, 197–205. [Google Scholar] [CrossRef]
- Zepon, K.M.; Marques, M.S.; da Silva Paula, M.M.; Morisso, F.D.P.; Kanis, L.A. Facile, green and scalable method to produce carrageenan-based hydrogel containing in situ synthesized AgNPs for application as wound dressing. Int. J. Biol. Macromol. 2018, 113, 51–58. [Google Scholar] [CrossRef]
- Wang, Z.; An, G.; Zhu, Y.; Liu, X.; Chen, Y.; Wu, H.; Wang, Y.; Shi, X.; Mao, C. 3D-printable self-healing and mechanically reinforced hydrogels with host–guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz. 2019, 6, 733–742. [Google Scholar] [CrossRef]
- Peppas, N.A. Hydrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 1997, 2, 531–537. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, Q.; Liu, C.; Yan, J.; Cha, D.; Liang, S.; Li, X.; Fan, B. Synthesis, characterization, and evaluation of poly (aminoethyl) modified chitosan and its hydrogel used as antibacterial wound dressing. Int. J. Biol. Macromol. 2017, 102, 457–467. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Wang, Y. Swelling behavior of konjac glucomannan/N, n-dimethylene bisacrylamide hydrogel. Anhui Agron. Bull. 2015, 21, 26–27. [Google Scholar]
- Wang, Y.; Zhang, B.; Ma, M.; Lu, W. Preparation of gelma/PEGDA hydrogel by UV copolymerization and crosslinking. Imaging Sci. Photochem. 2017, 35, 574–580. [Google Scholar]
- Wang, Q.; Ren, L.; Wang, Y.; Yao, Y. Preparation and characterization of photocrosslinked n-acryloyl glucosamine/PEGDA hydrogel. J. South China Univ. Technol. 2013, 41, 62–67. [Google Scholar]
- Su, J.; Satchell, S.C.; Wertheim, J.A.; Shah, R.N. Poly (ethylene glycol)-crosslinked gelatin hydrogel substrates with conjugated bioactive peptides influence endothelial cell behavior. Biomaterials 2019, 201, 99–112. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva Jr, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, characterization and safety evaluation of sericin-based hydrogels for controlled delivery of acyclovir. Pharmaceuticals 2021, 14, 234. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, P.X.; D’Acierno, F.; Hamad, W.Y.; Michal, C.A.; MacLachlan, M.J. Tunable diffraction gratings from biosourced lyotropic liquid crystals. Adv. Mater. 2020, 32, 1907376. [Google Scholar] [CrossRef]
- Ye, T.-J.; Qian, S.; Gao, Y.; Yu, M.-J.; Wei, Y.-F. Research Status, Problems and Countermeasures of Lyotropic Liquid Crystal in New Drug Delivery Systems of Traditional Chinese Medicine. Chin. J. Exp. Tradit. Med. Formulae 2019, 24, 229–234. [Google Scholar] [CrossRef]
- De Souza, J.F.; Pontes, K.d.S.; Alves, T.F.; Amaral, V.A.; Rebelo, M.d.A.; Hausen, M.A.; Chaud, M.V. Spotlight on biomimetic systems based on lyotropic liquid crystal. Molecules 2017, 22, 419. [Google Scholar] [CrossRef]
- Shan, X.; Li, X.; Luo, Z.; Lin, Q.; Lu, Y.; Jiang, M.; Zhang, J.; Huang, J.; Xie, L.; Guo, X. A Clinically-Achievable Injectable and Sprayable in Situ Lyotropic Liquid Crystalline Platform in Treating Hormone-Sensitive and Castration-Resistant Prostate Cancer. ACS Nano 2023, 17, 6045–6061. [Google Scholar] [CrossRef]
- Mancuso, A.; Cianflone, E.; Cristiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic liquid crystals: A biocompatible and safe material for local cardiac application. Pharmaceutics 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef]
- Silvestrini, A.V.P.; Caron, A.L.; Viegas, J.; Praca, F.G.; Bentley, M.V.L.B. Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin. Drug Deliv. 2020, 17, 1781–1805. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, P.; Swami, R.; Swarnakar, N.K.; Kushwah, V.; Katiyar, S.S. Lyotropic liquid crystalline nanoparticles of amphotericin B: Implication of phytantriol and glyceryl monooleate on bioavailability enhancement. AAPS PharmSciTech 2018, 19, 1699–1711. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; El-Megrab, N.A.; El-Nahas, H.M. An overview of PLGA in-situ forming implants based on solvent exchange technique: Effect of formulation components and characterization. Pharm. Dev. Technol. 2021, 26, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roy, B.; Satpathi, S.; Hazra, P. Impact of topology on the characteristics of water inside cubic lyotropic liquid crystalline systems. J. Phys. Chem. B 2019, 123, 4118–4128. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. Diffusion, molecular separation, and drug delivery from lipid mesophases with tunable water channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef]
- Ghanbari, R.; Assenza, S.; Saha, A.; Mezzenga, R. Diffusion of polymers through periodic networks of lipid-based nanochannels. Langmuir 2017, 33, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Meikle, T.G.; Yao, S.; Zabara, A.; Conn, C.E.; Drummond, C.J.; Separovic, F. Predicting the release profile of small molecules from within the ordered nanostructured lipidic bicontinuous cubic phase using translational diffusion coefficients determined by PFG-NMR. Nanoscale 2017, 9, 2471–2478. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Waghule, T.; Hans, N.; Mahmood, A.; Gorantla, S.; Dubey, S.K.; Singhvi, G. Insights of lyotropic liquid crystals in topical drug delivery for targeting various skin disorders. J. Mol. Liq. 2020, 315, 113771. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skja, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Hag, L.; Li, X.; Meikle, T.G.; Hoffmann, S.V.; Jones, N.C.; Pedersen, J.S.; Hawley, A.M.; Gras, S.L.; Conn, C.E.; Drummond, C.J. How peptide molecular structure and charge influence the nanostructure of lipid bicontinuous cubic mesophases: Model synthetic WALP peptides provide insights. Langmuir 2016, 32, 6882–6894. [Google Scholar] [CrossRef]
- Clapper, J.D.; Guymon, C.A. Nanostructured biodegradable polymer composites generated using lyotropic liquid crystalline media. Macromolecules 2007, 40, 7951–7959. [Google Scholar] [CrossRef]
- Wang, H.; Peng, T.; Wu, H.; Chen, J.; Chen, M.; Mei, L.; Li, F.; Wang, W.; Wu, C.; Pan, X. In situ biomimetic lyotropic liquid crystal gel for full-thickness cartilage defect regeneration. J. Control. Release 2021, 338, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Z.; Huang, Y.; Wang, B.; Yang, P.; Fan, Y.; Hou, A.; Yang, B.; Zhao, Z.; Quan, G.; et al. In situ gelation of rhEGF-containing liquid crystalline precursor with good cargo stability and system mechanical properties: A novel delivery system for chronic wounds treatment. Biomater. Sci. 2019, 7, 995–1010. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, X.; Wang, C.; Huang, Y.; Hu, P.; Wang, G.; Cui, Y.; Xia, X.; Zhou, Z.; Pan, X.; et al. A bacteria-resistant and self-healing spray dressing based on lyotropic liquid crystals to treat infected post-operative wounds. J. Mater. Chem. B 2021, 9, 8121–8137. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Mei, L.; Wang, B.; Huang, Y.; Quan, G.; Lu, C.; Peng, T.; Pan, X.; Wu, C. A pirfenidone loaded spray dressing based on lyotropic liquid crystals for deep partial thickness burn treatment: Healing promotion and scar prophylaxis. J. Mater. Chem. B 2020, 8, 2573–2588. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Guan, S.; Witte, F. The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Eng. Regen. 2021, 2, 182–194. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef]



| Growth Factor | Number of Amino Acid Residue | Commercial/Clinical Products for Wound Treatment | Dosage Form | Matrix Material |
|---|---|---|---|---|
| Epidermal growth factor | 53 | HEBERPROT-P® (Heber, Biotech, Havana, Cuba) EASYEF® (Daewoong Pharmaceutical, Seoul, Republic of Korea) REGEN-DTM150 (Bharat Biotech International Limited, Hyderabad, India) | Solution Solution Gel | Normal saline Normal saline — |
| Platelet-derived growth factor | 196–370 | Regranex® (Smith and Nephew, Inc., London, UK) | Gel | Carboxymethyl cellulose |
| Basic fibroblast growth factor | 155 | Fiblast®Spray (Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) | Solution | Normal saline |
| Vascular endothelial growth factor | 121–206 | Telbermin (Genentech, South San Francisco, CA, USA), phase I | Gel | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Shu, L.; Huang, Z.; Huang, Y.; Wu, C.; Pan, X.; Hu, P. Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management. Gels 2023, 9, 738. https://doi.org/10.3390/gels9090738
Luo P, Shu L, Huang Z, Huang Y, Wu C, Pan X, Hu P. Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management. Gels. 2023; 9(9):738. https://doi.org/10.3390/gels9090738
Chicago/Turabian StyleLuo, Peili, Lei Shu, Zhengwei Huang, Ying Huang, Chuanbin Wu, Xin Pan, and Ping Hu. 2023. "Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management" Gels 9, no. 9: 738. https://doi.org/10.3390/gels9090738
APA StyleLuo, P., Shu, L., Huang, Z., Huang, Y., Wu, C., Pan, X., & Hu, P. (2023). Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management. Gels, 9(9), 738. https://doi.org/10.3390/gels9090738