Valorization of Lignocellulosic Wastes Material for Efficient Adsorption of a Cationic Azo Dye and Sludge Recycling as a Reinforcement of Thermoplastic Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Adsorbent
2.3. Isotherm and Kinetic Studies
2.4. Preparation of Polystyrene Composite
3. Results and Discussions
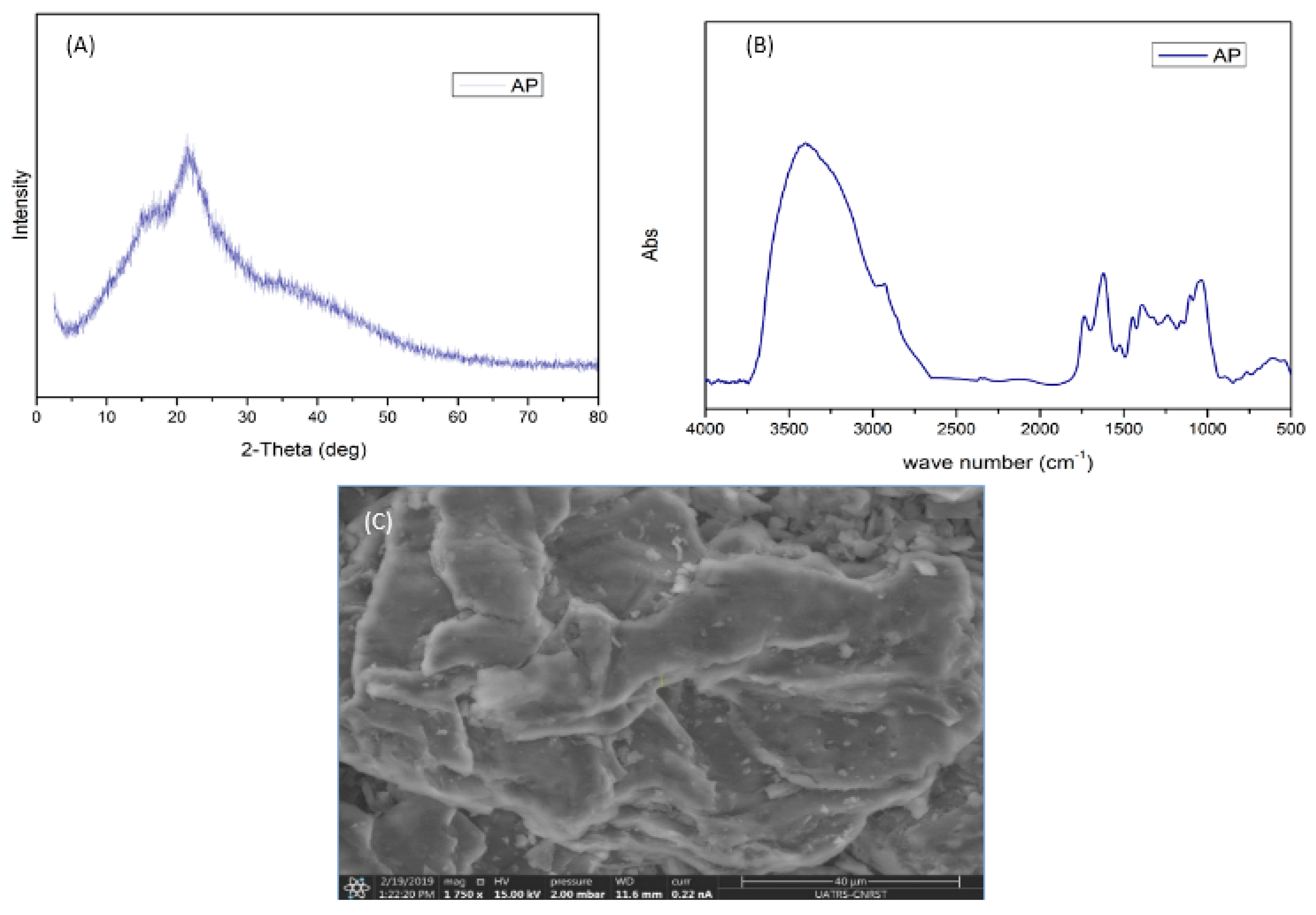
3.1. Characterization of Adsorbent
3.2. Adsorption of Malachite Green: Influencing Factors
3.3. Adsorption Kinetics and Isotherm Study
3.3.1. Adsorption Kinetics
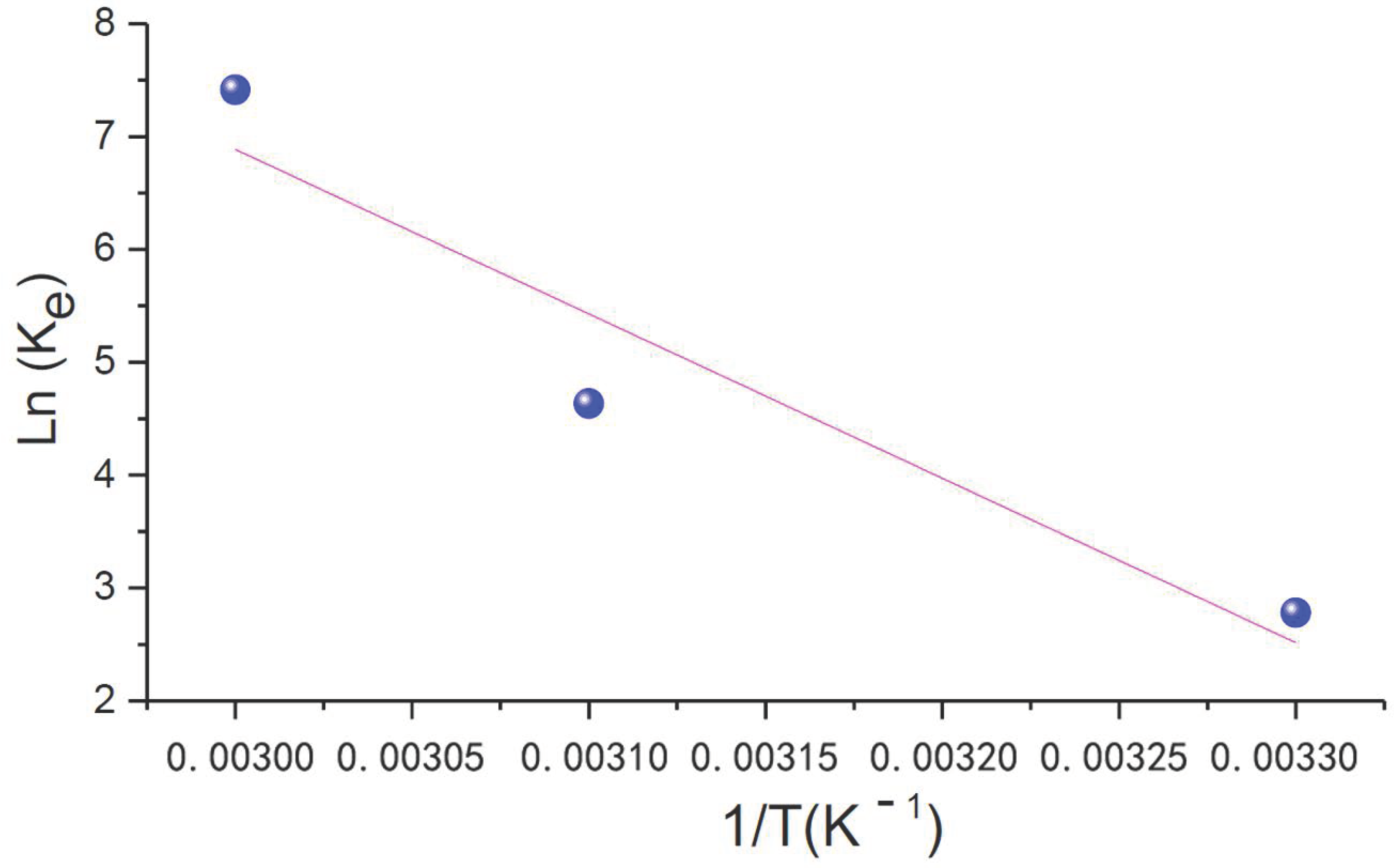
3.3.2. Adsorption Isotherms
3.3.3. Mechanism of Adsorption
- -
- Diffusion of the dye from the bulk solution to the boundary layer.
- -
- Through the boundary layer, dye molecules can be diffused into the surface of the adsorbent.
3.3.4. Comparison of Other Studies
3.4. Preparation of Polystyrene/AP Sludge Composites
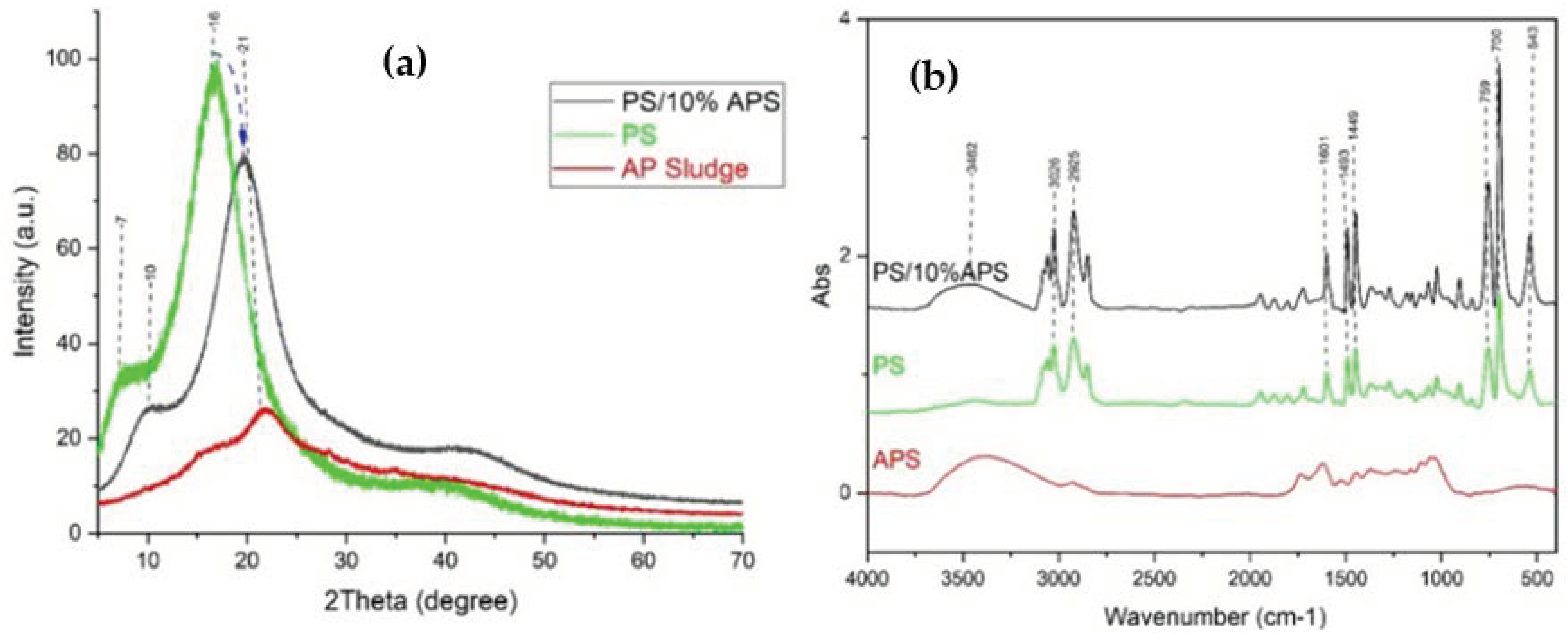
3.4.1. Morphological Analysis of Composite
3.4.2. Analyses Structural of Polystyrene Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhami, D.; Homagai, P.L. Adsorptive Removal of Malachite Green Dye from Aqueous Solution Using Chemically Modified Charred and Xanthated Wheat Bran. J. Nepal Chem. Soc. 2020, 41, 103–109. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A.; Jabri, M.; Kali, A.; Chraibi, S.; Hadey, C.; Aziz, F. Adsorption of Crystal Violand onto an Agricultural Waste Residue: Kinetics, Isotherm, Thermodynamics, and Mechanism of Adsorption. Sci. World J. 2020, 2020, 5873521. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mondal, A. Kinetics, isotherm, and thermodynamic studies of methylene blue selective adsorption and photocatalysis of malachite green from aqueous solution using layered Na-intercalated Cu-doped Titania. Appl. Clay Sci. 2019, 183, 105323. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, X.; Li, X.; Jia, X.; Liang, S.; Qian, L. Synthesis of MgO/Fe3O4 nanoparticles embedded activated carbon from biomass for high-efficient adsorption of malachite green. Mater. Chem. Phys. 2020, 240, 122240. [Google Scholar] [CrossRef]
- Mkrtchyan, E.; Burakov, A.; Burakova, I. The Adsorption of Malachite Green on Graphene Nanocomposites: A Study on Kinetics under Dynamic Conditions. Mater. Today: Proc. 2019, 11, 404–409. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abouarnadasse, S.; Abdallaoui, A. Comparative study of malachite green and phenol adsorption on synthetic hematite iron oxide nanoparticles (α-Fe2O3). Surf. Interfaces 2020, 21, 100637. [Google Scholar] [CrossRef]
- Iwaszczuk, N.; Szyba, M.; Iwaszczuk, A.; Yakubiv, V. Production of agricultural biogas from waste—An element of socially responsible actions in the food sector. Acta Innov. 2019, 33, 52–62. [Google Scholar] [CrossRef]
- Rodríguez, H. Ionic liquids in the pretreatment of lignocellulosic biomass. Acta Innov. 2021, 38, 23–36. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Hennequin, L.M.; Brandt-Talbot, A.; Bedoya-Lora, F.E.; Kelsall, G.H.; Polizzi, K.; Fennell, P.S.; Hallett, J.P. Towards an environmentally and economically sustainable biorefinery: Heavy metal contaminated waste wood as a low-cost feedstock in a low-cost ionic liquid process. Green Chem. 2020, 22, 5032–5041. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Lopes, T.F.; Łukasik, R.M. Economic, social and environmental impacts attained by the use of the effluents generated within a small-scale biorefinery concept. Acta Innov. 2020, 36, 57–63. [Google Scholar] [CrossRef]
- Plastics-the-facts-2021.pdf. Consulté le: 17 janvier 2023. [En ligne]. Available online: https://dein-kunststoff.de/wp-content/uploads/2022/08/Plastics-the-facts-2021.pdf (accessed on 6 October 2022).
- Nair, K.C.M.; Diwan, S.M.; Thomas, S. Tensile Properties of Short Sisal Fiber Reinforced Polystyrene Composites. J. Appl. Polym. Sci. 1996, 60, 1483–1497. [Google Scholar] [CrossRef]
- Masri, T.; Ounis, H.; Sedira, L.; Kaci, A.; Benchabane, A. Characterization of new composite material based on date palm leaflets and expanded polystyrene wastes. Constr. Build. Mater. 2018, 164, 410–418. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Adeoye, A.S.; Ighalo, J.O.; Onifade, D.V. FEA of effective elastic properties of banana fiber-reinforced polystyrene composite. Mech. Adv. Mater. Struct. 2021, 28, 1869–1877. [Google Scholar] [CrossRef]
- Jabri, M.; Oulidi, O.; Loulidi, I.; Amar, A.; Kali, A.; Boukhlifi, F. Acorn Waste Valorization as Reinforcement in Polystyrene Composite: A Comparative Study. In Artificial Intelligence of Things for Smart Green Energy Management; El Himer, S., Ouaissa, M., Emhemed, A.A.A., Ouaissa, M., Boulouard, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 213–224. [Google Scholar] [CrossRef]
- Hijab, M.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Minimizing adsorbent requirements using multi-stage batch adsorption for malachite green removal using microwave date-stone activated carbons. Chem. Eng. Process.-Process Intensif. 2021, 167, 108318. [Google Scholar] [CrossRef]
- Fan, X.; Deng, L.; Li, K.; Lu, H.; Wang, R.; Li, W. Adsorption of malachite green in aqueous solution using sugarcane bagasse-barium carbonate composite. Colloid Interface Sci. Commun. 2021, 44, 100485. [Google Scholar] [CrossRef]
- Giri, B.S.; Sonwani, R.K.; Varjani, S.; Chaurasia, D.; Varadavenkatesan, T.; Chaturvedi, P.; Yadav, S.; Katiyar, V.; Singh, R.S.; Pandey, A. Highly efficient bio-adsorption of Malachite green using Chinese Fan-Palm Biochar (Livistona chinensis). Chemosphere 2022, 287, 132282. [Google Scholar] [CrossRef]
- Ruihao, T.; Wei, H.; Srinivasakannan, C.; Xuelin, L.; Xin, W.; Xinhui, D. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2021, 281, 119950. [Google Scholar] [CrossRef]
- Asnaoui, H.; Dehmani, Y.; Khalis, M.; Hachem, E.-K. Adsorption of phenol from aqueous solutions by Na–bentonite: Kinetic, equilibrium and thermodynamic studies. Int. J. Environ. Anal. Chem. 2022, 102, 3043–3057. [Google Scholar] [CrossRef]
- Thiruchelvi, R.; Venkataraghavan, R.; Sharmila, D. Optimization of environmental parameters by Plackett-Burman design and response surface methodology for the adsorption of Malachite green onto Gracilaria edulis. Mater. Today Proc. 2021, 37, 1859–1864. [Google Scholar] [CrossRef]
- Kim, M.; Sharma, N.; Chung, J.; Yun, K. Activated graphene with fractal structure for the adsorption of malachite green with high removal rate. Microporous Mesoporous Mater. 2021, 322, 111166. [Google Scholar] [CrossRef]
- Ojediran, J.O.; Dada, A.O.; Aniyi, S.O.; David, R.O. Functionalized Zea Mays Cob (FZMC) as low-cost agrowaste for effective adsorption of malachite green dyes data set. Chem. Data Collect. 2020, 30, 100563. [Google Scholar] [CrossRef]
- Baskaran, P.K.; Venkatraman, B.R.; Arivoli, S. Adsorption of Malachite Green Dye by Acid Activated Carbon—Kinetic, Thermodynamic and Equilibrium Studies. J. Chem. 2011, 8, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Sundararaman, T.R.; Saravanan, A.; Kumar, P.S.; Mabel, M.M.; Hemavathy, R.V.; Karishma, S.; Jeevanantham, S.; Hemavathi, R.; Ishwariya, A.; Kowsalya, S. Adsorptive Removal of Malachite Green Dye onto Coal-Associated Soil and Conditions Optimization. Adsorpt. Sci. Technol. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Gebreslassie, Y.T. Equilibrium, Kinetics, and Thermodynamic Studies of Malachite Green Adsorption onto Fig (Ficus cartia) Leaves. J. Anal. Methods Chem. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Miyar, H.K.; Pai, A.; Goveas, L.C. Adsorption of Malachite Green by extracellular polymeric substance of Lysinibacillus sSS1: Kinetics and isotherms. Heliyon 2021, 7, e07169. [Google Scholar] [CrossRef]
- Li, W.; Xie, Z.; Xue, S.; Ye, H.; Liu, M.; Shi, W.; Liu, Y. Studies on the adsorption of dyes, Methylene blue, Safranin T, and Malachite green onto Polystyrene foam. Sep. Purif. Technol. 2021, 276, 119435. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Gutiérrez, J.; Henao, H.; Melo, A.M.; Pérez, J.F.; Chica, E. Adsorption capacity of the biochar obtained from Pinus patula wood micro-gasification for the treatment of polluted water containing malachite green dye. J. King Saud Univ.-Eng. Sci. 2021, S1018363921000982. [Google Scholar] [CrossRef]
- Bathla, A.; Singla, D.; Pal, B. Highly efficient CaCO3-CaO extracted from tap water distillation for effective adsorption and photocatalytic degradation of malachite green dye. Mater. Res. Bull. 2019, 116, 1–7. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Ahmad, M.A.; Yahaya, N.K.E.M.; Karim, J. Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arab. J. Chem. 2021, 14, 103104. [Google Scholar] [CrossRef]
- Lin, L.; Tang, S.; Wang, X.; Sun, X.; Yu, A. Adsorption of malachite green from aqueous solution by nylon microplastics: Reaction mechanism and the optimum conditions by response surface methodology. Process Saf. Environ. Prot. 2020, 140, 339–347. [Google Scholar] [CrossRef]
- Alorabi, A.Q. Effective Removal of Malachite Green from Aqueous Solutions Using Magnetic Nanocomposite: Synthesis, Characterization, and Equilibrium Study. Adsorpt. Sci. Technol. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Sevim, F.; Lacin, O.; Ediz, E.F.; Demir, F. Adsorption capacity, isotherm, kinetic, and thermodynamic studies on adsorption behavior of malachite green onto natural red clay. Environ. Prog. Sustain. Energy 2021, 40, e13471. [Google Scholar] [CrossRef]
- Aksu, Z. Biosorption of reactive dyes by dried activated sludge: Equilibrium and kinetic modelling. Biochem. Eng. J. 2001, 7, 79–84. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Khattri, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Thennarasu, G.; Saravanan, S. Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J. Hazard. Mater. 2010, 181, 271–280. [Google Scholar] [CrossRef]
- Sekhar, C.P.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. Bio-polymer adsorbent for the removal of malachite green from aqueous solution. Chemosphere 2009, 77, 842–847. [Google Scholar] [CrossRef]
- Mulinari, D.R.; Baptista, C.A.R.P.; Souza, J.V.C.; Voorwald, H.J.C. Mechanical properties of coconut fibers reinforced polyester composites. Procedia Eng. 2011, 10, 2074–2079. [Google Scholar] [CrossRef]
- Albunia, A.R.; Musto, P.; Guerra, G. FTIR spectra of pure helical crystalline phases of syndiotactic polystyrene. Polymer 2006, 47, 234–242. [Google Scholar] [CrossRef]








| Isotherm | Equation | Parameter |
|---|---|---|
| Freundlich | ||
| Langmuir | ||
| Pseudo-first-order (PFO) | ||
| Pseudo-second-order (PSO) |
| Ci (M) | Qe (exp) | Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | ||||
|---|---|---|---|---|---|---|---|
| K1 | R2 | Qe | K2 | R2 | Qe | ||
| 10−5 | 12 | 3.09 | 0.71 | 11.795 | 0.004 | 0.99 | 15.16 |
| 5 × 10−5 | 22 | 4.92 | 0.90 | 21.708 | 0.006 | 0.99 | 24.72 |
| 10−4 | 42 | 8.48 | 0.97 | 41.28 | 0.008 | 0.99 | 43.89 |
| Temperature (°C) | Modeled of Langmuir | Modeled of Frendlich | ||||
|---|---|---|---|---|---|---|
| KL | Qm | R2 | KF | N | R2 | |
| 30 | 0.003 | 173 | 0.99 | 2.45 | 1.64 | 0.96 |
| 40 | 0.005 | 176 | 0.99 | 5.37 | 1.96 | 0.97 |
| 50 | 0.008 | 201 | 0.98 | 7.53 | 2.17 | 0.97 |
| T (°C) | ΔH° (kJ/mol) | ΔS° (J/Kmol) | ΔG° (kJ/mol) |
|---|---|---|---|
| 30 | 121.085 | 415.5 | −4.81 |
| 40 | −8.966 | ||
| 50 | −13.121 |
| Adsorbent | Qm (mg/g) | Isotherm Model | Reference |
|---|---|---|---|
| Ntural red clay | 84 | Langumir | [35] |
| Activated sludge | 250 | Freundlich | [36] |
| Rambutan peel-based activated carbon | 388 | Langmuir | [37] |
| Neem sawdust | 4.3 | Langmuir | [38] |
| Activated carbon derived from Borassus aethiopum flower biomass | 20.46 | Langmuir | [39] |
| Bio-polymer | 0.4768 | Langmuir | [40] |
| Acorn pericarp | 200 | Langmuir | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabri, M.; Dehmani, Y.; Loulidi, I.; Kali, A.; Amar, A.; Lgaz, H.; Hadey, C.; Boukhlifi, F. Valorization of Lignocellulosic Wastes Material for Efficient Adsorption of a Cationic Azo Dye and Sludge Recycling as a Reinforcement of Thermoplastic Composite. Fluids 2023, 8, 37. https://doi.org/10.3390/fluids8020037
Jabri M, Dehmani Y, Loulidi I, Kali A, Amar A, Lgaz H, Hadey C, Boukhlifi F. Valorization of Lignocellulosic Wastes Material for Efficient Adsorption of a Cationic Azo Dye and Sludge Recycling as a Reinforcement of Thermoplastic Composite. Fluids. 2023; 8(2):37. https://doi.org/10.3390/fluids8020037
Chicago/Turabian StyleJabri, Maria, Younes Dehmani, Ilyasse Loulidi, Abderahim Kali, Abdelouahed Amar, Hassane Lgaz, Chaimaa Hadey, and Fatima Boukhlifi. 2023. "Valorization of Lignocellulosic Wastes Material for Efficient Adsorption of a Cationic Azo Dye and Sludge Recycling as a Reinforcement of Thermoplastic Composite" Fluids 8, no. 2: 37. https://doi.org/10.3390/fluids8020037
APA StyleJabri, M., Dehmani, Y., Loulidi, I., Kali, A., Amar, A., Lgaz, H., Hadey, C., & Boukhlifi, F. (2023). Valorization of Lignocellulosic Wastes Material for Efficient Adsorption of a Cationic Azo Dye and Sludge Recycling as a Reinforcement of Thermoplastic Composite. Fluids, 8(2), 37. https://doi.org/10.3390/fluids8020037








