MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study
Abstract
1. Introduction
2. Results
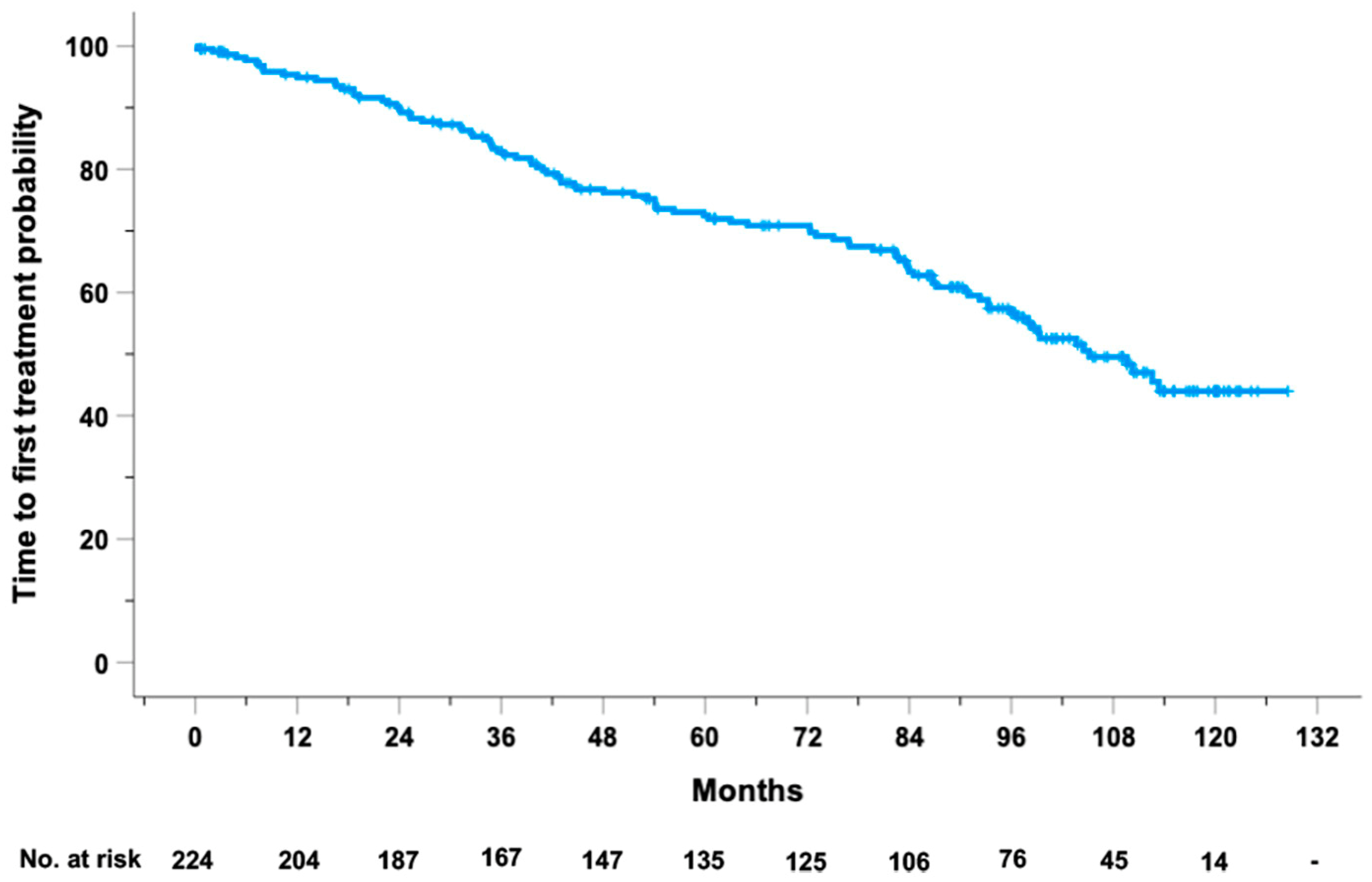
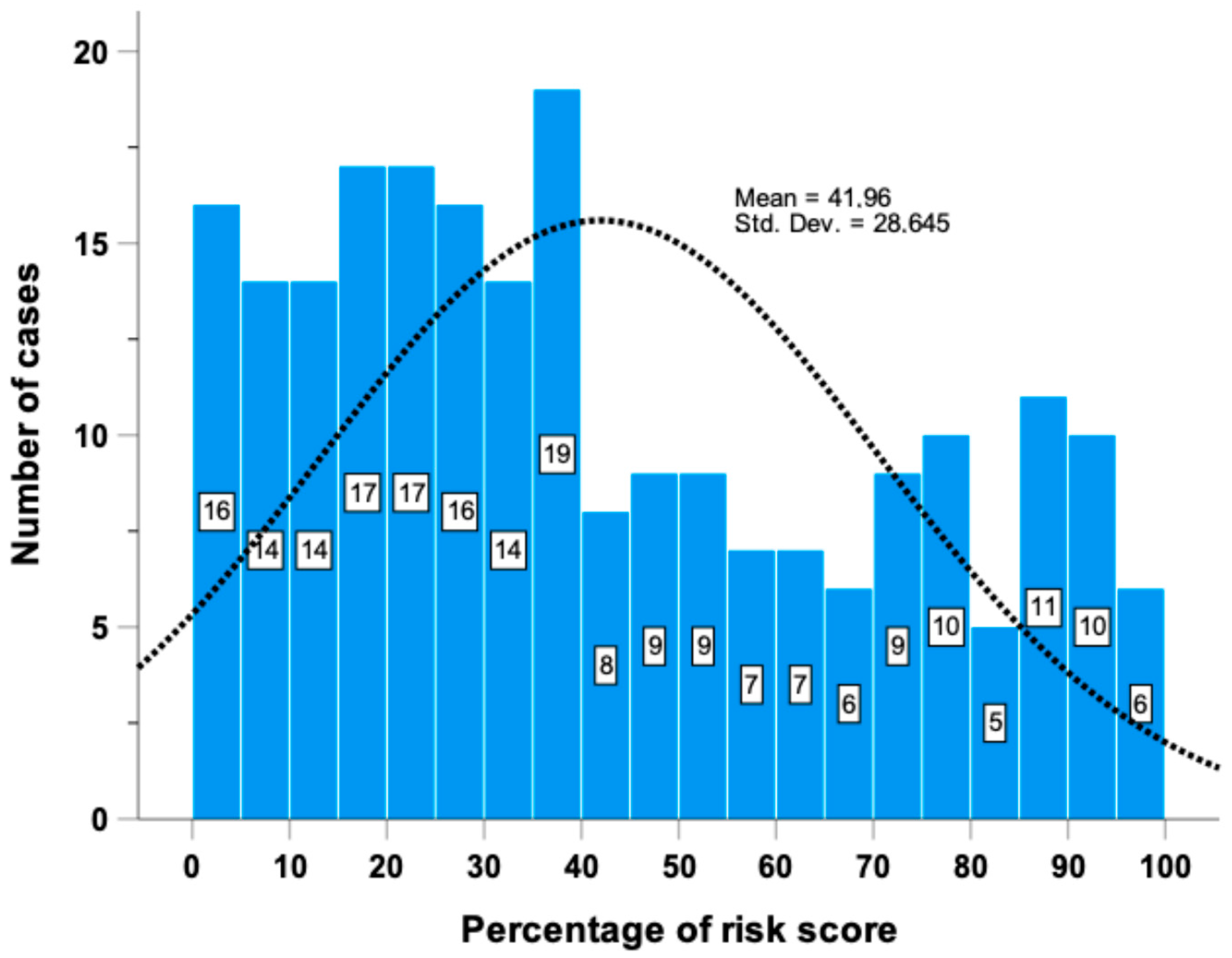
2.1. TTFT Prediction by the Basic Model
2.2. TTFT Prediction by miRNAs
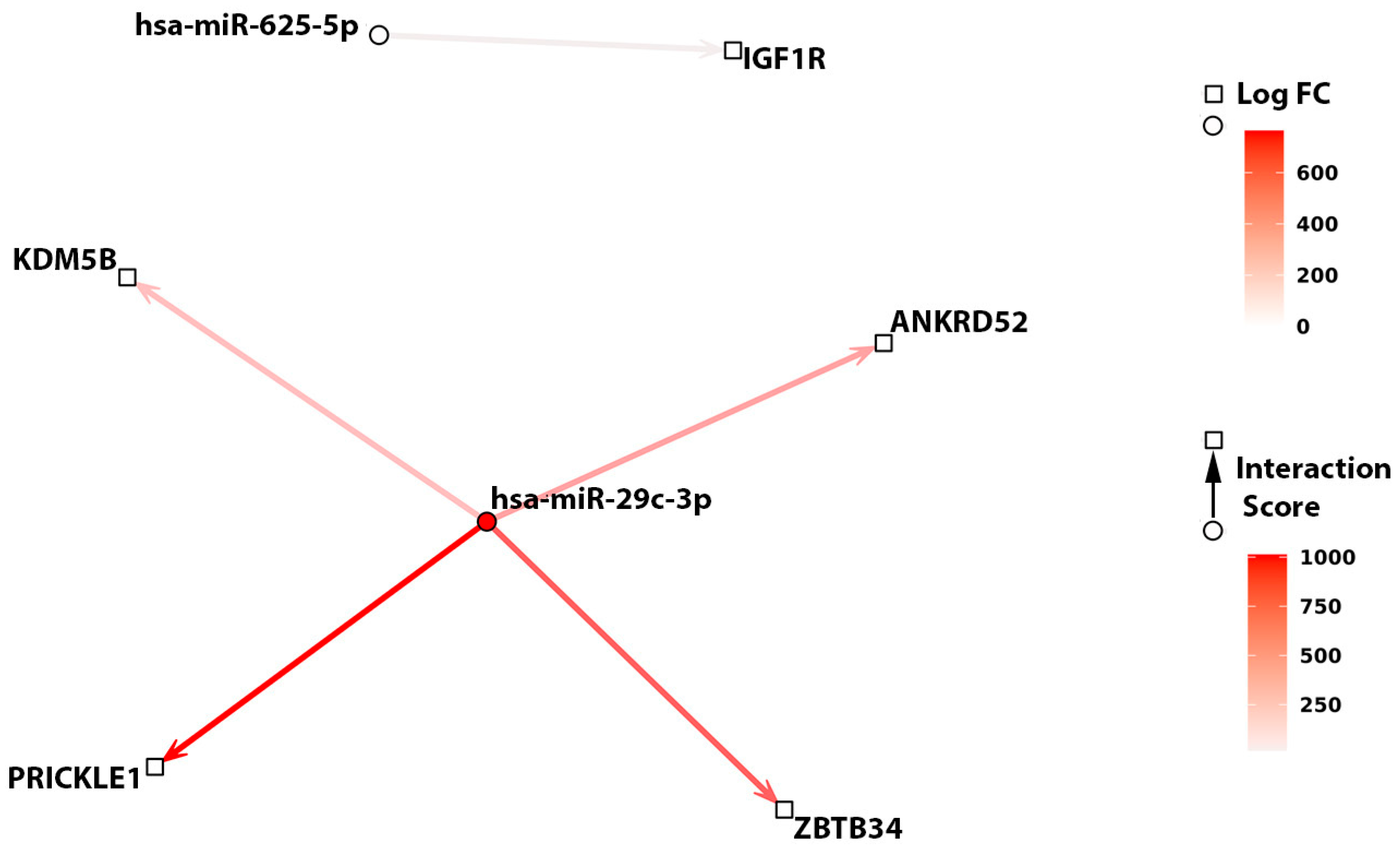
2.3. Correlation and Interaction Analysis between miRNAs and Genes Found to Be Related to TTFT by an AI Model
2.4. Pathways Regulation by the 16 miRNAs Linked to TTFT
3. Discussion
4. Materials and Methods
4.1. Patient Population and Study Design
4.2. Assessment of Biological Markers
4.3. miRNAs Analysis
4.4. miRNA–mRNA Correlation, Interaction, and Enrichment Analyses
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef] [PubMed]
- Gaidano, G.; Rossi, D. The mutational landscape of chronic lymphocytic leukemia and its impact on prognosis and treatment. Hematol. Am. Soc. Hematol. Educ. Program. 2017, 2017, 329–337. [Google Scholar] [CrossRef]
- Guieze, R.; Wu, C.J. Genomic and epigenomic heterogeneity in chronic lymphocytic leukemia. Blood 2015, 126, 445–453. [Google Scholar] [CrossRef]
- Dohner, H.; Stilgenbauer, S.; Benner, A.; Leupolt, E.; Krober, A.; Bullinger, L.; Dohner, K.; Bentz, M.; Lichter, P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000, 343, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Bottcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- Puente, X.S.; Bea, S.; Valdes-Mas, R.; Villamor, N.; Gutierrez-Abril, J.; Martin-Subero, J.I.; Munar, M.; Rubio-Perez, C.; Jares, P.; Aymerich, M.; et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef]
- Puente, X.S.; Pinyol, M.; Quesada, V.; Conde, L.; Ordonez, G.R.; Villamor, N.; Escaramis, G.; Jares, P.; Bea, S.; Gonzalez-Diaz, M.; et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011, 475, 101–105. [Google Scholar] [CrossRef]
- Baliakas, P.; Mattsson, M.; Stamatopoulos, K.; Rosenquist, R. Prognostic indices in chronic lymphocytic leukaemia: Where do we stand how do we proceed? J. Intern. Med. 2016, 279, 347–357. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gascon, Y.M.I.; Munoz-Novas, C.; Rodriguez-Vicente, A.E.; Quijada-Alamo, M.; Hernandez-Sanchez, M.; Perez-Carretero, C.; Ramos-Ascanio, V.; Hernandez-Rivas, J.A. From Biomarkers to Models in the Changing Landscape of Chronic Lymphocytic Leukemia: Evolve or Become Extinct. Cancers 2021, 13, 1782. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Shanafelt, T.D.; Mauro, F.R.; Reda, G.; Rossi, D.; Laurenti, L.; Del Principe, M.I.; Cutrona, G.; Angeletti, I.; Coscia, M.; et al. Predictive value of the CLL-IPI in CLL patients receiving chemo-immunotherapy as first-line treatment. Eur. J. Haematol. 2018, 101, 703–706. [Google Scholar] [CrossRef]
- International, C.L.L.I.P.I.w.g. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): A meta-analysis of individual patient data. Lancet Oncol. 2016, 17, 779–790. [Google Scholar] [CrossRef]
- Condoluci, A.; Terzi di Bergamo, L.; Langerbeins, P.; Hoechstetter, M.A.; Herling, C.D.; De Paoli, L.; Delgado, J.; Rabe, K.G.; Gentile, M.; Doubek, M.; et al. International prognostic score for asymptomatic early-stage chronic lymphocytic leukemia. Blood 2020, 135, 1859–1869. [Google Scholar] [CrossRef]
- Smolej, L.; Turcsanyi, P.; Kubova, Z.; Zuchnicka, J.; Mihalyova, J.; Simkovic, M.; Vodarek, P.; Krcmeryova, M.; Mocikova, H.; Brejcha, M.; et al. External validation of International Prognostic Score for asymptomatic early stage chronic lymphocytic leukaemia and proposal of an alternative score. Br. J. Haematol. 2021, 193, 133–137. [Google Scholar] [CrossRef]
- Morabito, F.; Tripepi, G.; Vigna, E.; Bossio, S.; D’Arrigo, G.; Martino, E.A.; Storino, F.; Recchia, A.G.; Fronza, G.; Di Raimondo, F.; et al. Validation of the Alternative International Prognostic Score-E (AIPS-E): Analysis of Binet stage A chronic lymphocytic leukemia patients enrolled into the O-CLL1-GISL protocol. Eur. J. Haematol. 2021, 106, 831–835. [Google Scholar] [CrossRef]
- Mansouri, L.; Thorvaldsdottir, B.; Sutton, L.A.; Karakatsoulis, G.; Meggendorfer, M.; Parker, H.; Nadeu, F.; Brieghel, C.; Laidou, S.; Moia, R.; et al. Different prognostic impact of recurrent gene mutations in chronic lymphocytic leukemia depending on IGHV gene somatic hypermutation status: A study by ERIC in HARMONY. Leukemia 2023, 37, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Fontana, B.; Calin, G.A.; Nemeth, K. miRNA Biology in Chronic Lymphocytic Leukemia. Semin. Hematol. 2024, 61, 181–193. [Google Scholar] [CrossRef]
- Balatti, V.; Croce, C.M. MicroRNA dysregulation and multi-targeted therapy for cancer treatment. Adv. Biol. Regul. 2020, 75, 100669. [Google Scholar] [CrossRef]
- Autore, F.; Ramassone, A.; Stirparo, L.; Pagotto, S.; Fresa, A.; Innocenti, I.; Visone, R.; Laurenti, L. Role of microRNAs in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2023, 24, 12471. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, B.; Meuleman, N.; Haibe-Kains, B.; Saussoy, P.; Van Den Neste, E.; Michaux, L.; Heimann, P.; Martiat, P.; Bron, D.; Lagneaux, L. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood 2009, 113, 5237–5245. [Google Scholar] [CrossRef]
- Rossi, S.; Shimizu, M.; Barbarotto, E.; Nicoloso, M.S.; Dimitri, F.; Sampath, D.; Fabbri, M.; Lerner, S.; Barron, L.L.; Rassenti, L.Z.; et al. microRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood 2010, 116, 945–952. [Google Scholar] [CrossRef]
- Fulci, V.; Chiaretti, S.; Goldoni, M.; Azzalin, G.; Carucci, N.; Tavolaro, S.; Castellano, L.; Magrelli, A.; Citarella, F.; Messina, M.; et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007, 109, 4944–4951. [Google Scholar] [CrossRef]
- Mraz, M.; Malinova, K.; Kotaskova, J.; Pavlova, S.; Tichy, B.; Malcikova, J.; Stano Kozubik, K.; Smardova, J.; Brychtova, Y.; Doubek, M.; et al. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia 2009, 23, 1159–1163. [Google Scholar] [CrossRef]
- Mraz, M.; Dolezalova, D.; Plevova, K.; Stano Kozubik, K.; Mayerova, V.; Cerna, K.; Musilova, K.; Tichy, B.; Pavlova, S.; Borsky, M.; et al. MicroRNA-650 expression is influenced by immunoglobulin gene rearrangement and affects the biology of chronic lymphocytic leukemia. Blood 2012, 119, 2110–2113. [Google Scholar] [CrossRef]
- Negrini, M.; Cutrona, G.; Bassi, C.; Fabris, S.; Zagatti, B.; Colombo, M.; Ferracin, M.; D’Abundo, L.; Saccenti, E.; Matis, S.; et al. microRNAome expression in chronic lymphocytic leukemia: Comparison with normal B-cell subsets and correlations with prognostic and clinical parameters. Clin. Cancer Res. 2014, 20, 4141–4153. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Klein, U.; Lia, M.; Crespo, M.; Siegel, R.; Shen, Q.; Mo, T.; Ambesi-Impiombato, A.; Califano, A.; Migliazza, A.; Bhagat, G.; et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 2010, 17, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Cui, B.; Chen, L.; Zhang, S.; Mraz, M.; Fecteau, J.F.; Yu, J.; Ghia, E.M.; Zhang, L.; Bao, L.; Rassenti, L.Z.; et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood 2014, 124, 546–554. [Google Scholar] [CrossRef]
- Mraz, M.; Chen, L.; Rassenti, L.Z.; Ghia, E.M.; Li, H.; Jepsen, K.; Smith, E.N.; Messer, K.; Frazer, K.A.; Kipps, T.J. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood 2014, 124, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Palacios, F.; Abreu, C.; Prieto, D.; Morande, P.; Ruiz, S.; Fernandez-Calero, T.; Naya, H.; Libisch, G.; Robello, C.; Landoni, A.I.; et al. Activation of the PI3K/AKT pathway by microRNA-22 results in CLL B-cell proliferation. Leukemia 2015, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Luo, Z.; Volinia, S.; Rassenti, L.Z.; Kipps, T.J.; Croce, C.M. The down-regulation of miR-125b in chronic lymphocytic leukemias leads to metabolic adaptation of cells to a transformed state. Blood 2012, 120, 2631–2638. [Google Scholar] [CrossRef]
- Cerna, K.; Oppelt, J.; Chochola, V.; Musilova, K.; Seda, V.; Pavlasova, G.; Radova, L.; Arigoni, M.; Calogero, R.A.; Benes, V.; et al. MicroRNA miR-34a downregulates FOXP1 during DNA damage response to limit BCR signalling in chronic lymphocytic leukaemia B cells. Leukemia 2019, 33, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Guinn, D.; Ruppert, A.S.; Maddocks, K.; Jaglowski, S.; Gordon, A.; Lin, T.S.; Larson, R.; Marcucci, G.; Hertlein, E.; Woyach, J.; et al. miR-155 expression is associated with chemoimmunotherapy outcome and is modulated by Bruton’s tyrosine kinase inhibition with Ibrutinib. Leukemia 2015, 29, 1210–1213. [Google Scholar] [CrossRef]
- Guinn, D.; Lehman, A.; Fabian, C.; Yu, L.; Maddocks, K.; Andritsos, L.A.; Jones, J.A.; Flynn, J.M.; Jaglowski, S.M.; Woyach, J.A.; et al. The regulation of tumor-suppressive microRNA, miR-126, in chronic lymphocytic leukemia. Cancer Med. 2017, 6, 778–787. [Google Scholar] [CrossRef]
- Visone, R.; Rassenti, L.Z.; Veronese, A.; Taccioli, C.; Costinean, S.; Aguda, B.D.; Volinia, S.; Ferracin, M.; Palatini, J.; Balatti, V.; et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood 2009, 114, 3872–3879. [Google Scholar] [CrossRef]
- Van Roosbroeck, K.; Calin, G.A. MicroRNAs in chronic lymphocytic leukemia: miRacle or miRage for prognosis and targeted therapies? Semin. Oncol. 2016, 43, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, N.; Ntoufa, S.; Chartomatsidou, E.; Papadopoulos, G.; Hatzigeorgiou, A.; Anagnostopoulos, A.; Chlichlia, K.; Ghia, P.; Muzio, M.; Belessi, C.; et al. Differential microRNA profiles and their functional implications in different immunogenetic subsets of chronic lymphocytic leukemia. Mol. Med. 2013, 19, 115–123. [Google Scholar] [CrossRef]
- Van Roosbroeck, K.; Bayraktar, R.; Calin, S.; Bloehdorn, J.; Dragomir, M.P.; Okubo, K.; Bertilaccio, M.T.S.; Zupo, S.; You, M.J.; Gaidano, G.; et al. The involvement of microRNA in the pathogenesis of Richter syndrome. Haematologica 2019, 104, 1004–1015. [Google Scholar] [CrossRef]
- Balatti, V.; Tomasello, L.; Rassenti, L.Z.; Veneziano, D.; Nigita, G.; Wang, H.Y.; Thorson, J.A.; Kipps, T.J.; Pekarsky, Y.; Croce, C.M. miR-125a and miR-34a expression predicts Richter syndrome in chronic lymphocytic leukemia patients. Blood 2018, 132, 2179–2182. [Google Scholar] [CrossRef]
- Scandurra, M.; Rossi, D.; Deambrogi, C.; Rancoita, P.M.; Chigrinova, E.; Mian, M.; Cerri, M.; Rasi, S.; Sozzi, E.; Forconi, F.; et al. Genomic profiling of Richter’s syndrome: Recurrent lesions and differences with de novo diffuse large B-cell lymphomas. Hematol. Oncol. 2010, 28, 62–67. [Google Scholar] [CrossRef]
- Condoluci, A.; Rossi, D. Richter Syndrome. Curr. Oncol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Cimmino, A.; Fabbri, M.; Ferracin, M.; Wojcik, S.E.; Shimizu, M.; Taccioli, C.; Zanesi, N.; Garzon, R.; Aqeilan, R.I.; et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 5166–5171. [Google Scholar] [CrossRef]
- Cutrona, G.; Matis, S.; Colombo, M.; Massucco, C.; Baio, G.; Valdora, F.; Emionite, L.; Fabris, S.; Recchia, A.G.; Gentile, M.; et al. Effects of miRNA-15 and miRNA-16 expression replacement in chronic lymphocytic leukemia: Implication for therapy. Leukemia 2017, 31, 1894–1904. [Google Scholar] [CrossRef]
- Matis, S.; Grazia Recchia, A.; Colombo, M.; Cardillo, M.; Fabbi, M.; Todoerti, K.; Bossio, S.; Fabris, S.; Cancila, V.; Massara, R.; et al. MiR-146b-5p regulates IL-23 receptor complex expression in chronic lymphocytic leukemia cells. Blood Adv. 2022, 6, 5593–5612. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, M.; Barbieri, M.; Favasuli, V.; Taiana, E.; Fabris, S.; Favoino, C.; Ciceri, G.; Matis, S.; Colombo, M.; Massara, R.; et al. Frequency and clinical relevance of coding and noncoding NOTCH1 mutations in early stage Binet A chronic lymphocytic leukemia patients. Hematol. Oncol. 2020, 38, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Lionetti, M.; De Luca, G.; Menichini, P.; Recchia, A.G.; Matis, S.; Colombo, M.; Fabris, S.; Speciale, A.; Barbieri, M.; et al. Time to first treatment and P53 dysfunction in chronic lymphocytic leukaemia: Results of the O-CLL1 study in early stage patients. Sci. Rep. 2020, 10, 18427. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Tripepi, G.; Moia, R.; Recchia, A.G.; Boggione, P.; Mauro, F.R.; Bossio, S.; D’Arrigo, G.; Martino, E.A.; Vigna, E.; et al. Lymphocyte Doubling Time As A Key Prognostic Factor To Predict Time To First Treatment In Early-Stage Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 11, 684621. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Adornetto, C.; Monti, P.; Amaro, A.; Reggiani, F.; Colombo, M.; Rodriguez-Aldana, Y.; Tripepi, G.; D’Arrigo, G.; Vener, C.; et al. Genes selection using deep learning and explainable artificial intelligence for chronic lymphocytic leukemia predicting the need and time to therapy. Front. Oncol. 2023, 13, 1198992. [Google Scholar] [CrossRef]
- Herling, C.D.; Cymbalista, F.; Gross-Ophoff-Muller, C.; Bahlo, J.; Robrecht, S.; Langerbeins, P.; Fink, A.M.; Al-Sawaf, O.; Busch, R.; Porcher, R.; et al. Early treatment with FCR versus watch and wait in patients with stage Binet A high-risk chronic lymphocytic leukemia (CLL): A randomized phase 3 trial. Leukemia 2020, 34, 2038–2050. [Google Scholar] [CrossRef]
- Muchtar, E.; Kay, N.E.; Parikh, S.A. Early intervention in asymptomatic chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2021, 19, 92–103. [Google Scholar]
- Gentile, M.; Shanafelt, T.D.; Mauro, F.R.; Laurenti, L.; Rossi, D.; Molica, S.; Vincelli, I.; Cutrona, G.; Uccello, G.; Pepe, S.; et al. Comparison between the CLL-IPI and the Barcelona-Brno prognostic model: Analysis of 1299 newly diagnosed cases. Am. J. Hematol. 2018, 93, E35–E37. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Shanafelt, T.D.; Rossi, D.; Laurenti, L.; Mauro, F.R.; Molica, S.; Cutrona, G.; Uccello, G.; Campanelli, M.; Vigna, E.; et al. Validation of the CLL-IPI and comparison with the MDACC prognostic index in newly diagnosed patients. Blood 2016, 128, 2093–2095. [Google Scholar] [CrossRef]
- Gentile, M.; Shanafelt, T.D.; Cutrona, G.; Molica, S.; Tripepi, G.; Alvarez, I.; Mauro, F.R.; Di Renzo, N.; Di Raimondo, F.; Vincelli, I.; et al. A progression-risk score to predict treatment-free survival for early stage chronic lymphocytic leukemia patients. Leukemia 2016, 30, 1440–1443. [Google Scholar] [CrossRef]
- Fabbri, M.; Bottoni, A.; Shimizu, M.; Spizzo, R.; Nicoloso, M.S.; Rossi, S.; Barbarotto, E.; Cimmino, A.; Adair, B.; Wojcik, S.E.; et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA 2011, 305, 59–67. [Google Scholar] [CrossRef]
- Sharma, S.; Pavlasova, G.M.; Seda, V.; Cerna, K.A.; Vojackova, E.; Filip, D.; Ondrisova, L.; Sandova, V.; Kostalova, L.; Zeni, P.F.; et al. miR-29 modulates CD40 signaling in chronic lymphocytic leukemia by targeting TRAF4: An axis affected by BCR inhibitors. Blood 2021, 137, 2481–2494. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, K.; Lin, W.; Liu, J.; Qi, Q.; Shen, G.; Chen, W.; He, W. Long noncoding RNA LINC01291 promotes the aggressive properties of melanoma by functioning as a competing endogenous RNA for microRNA-625-5p and subsequently increasing IGF-1R expression. Cancer Gene Ther. 2022, 29, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Roy, U.; Desai, S.; Nilavar, N.M.; Van Nieuwenhuijze, A.; Paranjape, A.; Radha, G.; Bawa, P.; Srivastava, M.; Nambiar, M.; et al. MicroRNA miR-29c regulates RAG1 expression and modulates V(D)J recombination during B cell development. Cell Rep. 2021, 36, 109390. [Google Scholar] [CrossRef]
- Li, L.; Shou, H.; Wang, Q.; Liu, S. Investigation of the potential theranostic role of KDM5B/miR-29c signaling axis in paclitaxel resistant endometrial carcinoma. Gene 2019, 694, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cutrona, G.; Tripodo, C.; Matis, S.; Recchia, A.G.; Massucco, C.; Fabbi, M.; Colombo, M.; Emionite, L.; Sangaletti, S.; Gulino, A.; et al. Microenvironmental regulation of the IL-23R/IL-23 axis overrides chronic lymphocytic leukemia indolence. Sci. Transl. Med. 2018, 10, eaal1571. [Google Scholar] [CrossRef]
- Vila-Casadesus, M.; Gironella, M.; Lozano, J.J. MiRComb: An R Package to Analyse miRNA-mRNA Interactions. Examples across Five Digestive Cancers. PLoS ONE 2016, 11, e0151127. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, Y.C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]

| miRNA-ID | Units of Increase 1 | HR | 95%CI Lower Limit | 95% CI Upper Limit | p-Value |
|---|---|---|---|---|---|
| 1-3p | 1 | 1.166 | 1.006 | 1.352 | 0.041 |
| 103a-3p | 1 | 0.998 | 0.996 | 1.000 | 0.05 |
| 106a-5p | 1 | 1.438 | 1.003 | 2.062 | 0.048 |
| 10b-3p | 1 | 1.239 | 1.055 | 1.456 | 0.009 |
| 1224-5p | 1 | 1.064 | 1.024 | 1.105 | 0.002 |
| 1225-5p | 100 | 1.062 | 1.001 | 1.126 | 0.046 |
| 124-3p | 1 | 1.458 | 1.178 | 1.805 | <0.001 |
| 125b-5p | 1 | 0.744 | 0.56 | 0.989 | 0.042 |
| 138-5p | 1 | 0.599 | 0.4 | 0.896 | 0.013 |
| 140-3p | 1 | 0.991 | 0.986 | 0.996 | 0.001 |
| 144-3p | 1 | 1.004 | 1.002 | 1.006 | 0.002 |
| 144-5p | 1 | 1.024 | 1.008 | 1.041 | 0.003 |
| 146b-5p | 1 | 0.991 | 0.983 | 0.998 | 0.019 |
| 148a-3p | 1 | 1.006 | 1.002 | 1.010 | 0.007 |
| 150-5p | 1000 | 0.925 | 0.858 | 0.997 | 0.041 |
| 150-3p | 1 | 1.035 | 1.009 | 1.062 | 0.008 |
| 151-3p | 1 | 0.971 | 0.946 | 0.996 | 0.026 |
| 151-5p | 1 | 0.996 | 0.993 | 0.998 | 0.001 |
| 155-5p | 100 | 1.058 | 1.014 | 1.104 | 0.009 |
| 15a-5p | 100 | 1.074 | 1.034 | 1.117 | <0.001 |
| 184 | 1 | 1.472 | 1.126 | 1.925 | 0.005 |
| 193a-3p | 1 | 1.132 | 1.026 | 1.250 | 0.014 |
| 20a-3p | 1 | 1.049 | 1.007 | 1.093 | 0.022 |
| 21-5p | 1000 | 1.152 | 1.056 | 1.257 | 0.001 |
| 222-3p | 1 | 0.946 | 0.907 | 0.988 | 0.012 |
| 223-5p | 1 | 0.819 | 0.712 | 0.943 | 0.006 |
| 24-1-5p | 1 | 1.312 | 1.013 | 1.698 | 0.04 |
| 26a-5p | 100 | 0.901 | 0.813 | 0.999 | 0.047 |
| 28-5p | 1 | 1.005 | 1.001 | 1.009 | 0.012 |
| 296-3p | 1 | 0.570 | 0.348 | 0.934 | 0.026 |
| 298 | 1 | 1.307 | 1.022 | 1.670 | 0.033 |
| 29c-3p | 100 | 0.951 | 0.923 | 0.980 | <0.001 |
| 29c-5p | 1 | 0.952 | 0.924 | 0.982 | 0.002 |
| 301a-3p | 1 | 1.038 | 1.005 | 1.073 | 0.024 |
| 30c-5p | 100 | 0.621 | 0.400 | 0.962 | 0.033 |
| 323-3p | 1 | 0.646 | 0.426 | 0.979 | 0.04 |
| 338-5p | 1 | 0.785 | 0.643 | 0.960 | 0.018 |
| 339-3p | 1 | 0.689 | 0.521 | 0.910 | 0.009 |
| 33a-3p | 1 | 0.375 | 0.220 | 0.639 | <0.001 |
| 361-3p | 1 | 0.989 | 0.978 | 0.999 | 0.034 |
| 370 | 1 | 1.058 | 1.028 | 1.088 | <0.001 |
| 371-5p | 1 | 1.082 | 1.016 | 1.152 | 0.014 |
| 373-5p | 1 | 1.071 | 1.006 | 1.14 | 0.031 |
| 376b-3p | 1 | 1.523 | 1.046 | 2.218 | 0.028 |
| 491-3p | 1 | 1.766 | 1.331 | 2.343 | <0.001 |
| 500-3p | 1 | 0.817 | 0.717 | 0.931 | 0.002 |
| 502-3p | 1 | 0.872 | 0.796 | 0.955 | 0.003 |
| 502-5p | 1 | 0.673 | 0.504 | 0.898 | 0.007 |
| 513a-5p | 1 | 1.007 | 1.002 | 1.012 | 0.008 |
| 518c-5p | 1 | 1.148 | 1.033 | 1.276 | 0.01 |
| 520b | 1 | 1.234 | 1.067 | 1.426 | 0.005 |
| 532-3p | 1 | 0.898 | 0.841 | 0.959 | 0.001 |
| 532-5p | 1 | 0.939 | 0.891 | 0.989 | 0.018 |
| 552 | 1 | 1.556 | 1.014 | 2.388 | 0.043 |
| 557 | 1 | 1.197 | 1.099 | 1.303 | <0.001 |
| 566 | 1 | 1.616 | 1.188 | 2.197 | 0.002 |
| 574-3p | 1 | 1.030 | 1.006 | 1.055 | 0.015 |
| 582-3p | 1 | 0.465 | 0.274 | 0.789 | 0.005 |
| 584-5p | 1 | 1.162 | 1.022 | 1.32 | 0.022 |
| 596 | 1 | 0.597 | 0.391 | 0.913 | 0.017 |
| 601 | 1 | 1.069 | 1.01 | 1.131 | 0.022 |
| 603 | 1 | 1.552 | 1.023 | 2.356 | 0.039 |
| 625-5p | 1 | 0.960 | 0.940 | 0.981 | <0.001 |
| 628-3p | 1 | 0.630 | 0.417 | 0.952 | 0.028 |
| 631 | 1 | 1.180 | 1.006 | 1.385 | 0.042 |
| 645 | 1 | 1.604 | 1.091 | 2.358 | 0.016 |
| 659-3p | 1 | 1.114 | 1.01 | 1.228 | 0.03 |
| 661 | 1 | 0.579 | 0.342 | 0.981 | 0.042 |
| 665 | 1 | 1.145 | 1.008 | 1.300 | 0.037 |
| 671-5p | 1 | 1.046 | 1.014 | 1.079 | 0.004 |
| 877-5p | 1 | 1.245 | 1.031 | 1.503 | 0.023 |
| 9-3p | 1 | 1.086 | 1.015 | 1.163 | 0.017 |
| 99a-5p | 1 | 0.615 | 0.421 | 0.898 | 0.012 |
| miRNA-ID | Units of Increase 1 | HR | 95% CI Lower Limit | 95% CI Upper Limit | p-Value |
|---|---|---|---|---|---|
| 582-3p | 1 | 0.278 | 0.145 | 0.535 | <0.001 |
| 33a-3p | 1 | 0.334 | 0.16 | 0.697 | 0.003 |
| 516a-5p | 1 | 0.490 | 0.297 | 0.810 | 0.005 |
| 99a-5p | 1 | 0.512 | 0.341 | 0.769 | 0.001 |
| 296-3p | 1 | 0.539 | 0.301 | 0.967 | 0.038 |
| 502-5p | 1 | 0.623 | 0.43 | 0.905 | 0.013 |
| 625-5p | 1 | 0.958 | 0.937 | 0.98 | <0.001 |
| 29c-3p | 100 | 0.936 | 0.903 | 0.970 | <0.001 |
| 150-5p | 1000 | 1.112 | 1.005 | 1.231 | 0.039 |
| 148a-3p | 1 | 1.009 | 1.004 | 1.014 | <0.001 |
| 28-5p | 1 | 1.01 | 1.005 | 1.014 | <0.001 |
| 144-5p | 1 | 1.049 | 1.026 | 1.072 | <0.001 |
| 671-5p | 1 | 1.075 | 1.027 | 1.125 | 0.002 |
| 1-3p | 1 | 1.261 | 1.047 | 1.517 | 0.014 |
| 193a-3p | 1 | 1.343 | 1.186 | 1.52 | <0.001 |
| 124-3p | 1 | 1.536 | 1.233 | 1.913 | <0.001 |
| Basic Model | Expanded Model | |
|---|---|---|
| Harrell’s C-index | 75.0% | 81.1% |
| Explained variation in TTFT | 45.4% | 63.3% |
| IDI 1 | - | 14.9%, p < 0.001 |
| NRI 2 | - | 44.2%, p < 0.001 |
| Enrichment | ID | Term Specification | Associated miRNA Genes | Adjusted p-Value | q-Value |
|---|---|---|---|---|---|
| KEGG ORA summary | hsa05206 | MicroRNAs in cancer | MIR1-2, MIR28, MIR29C, MIR99A, MIR124-3, MIR150, MIR625 | 1.41 × 10−10 | 7.41 × 10−10 |
| WikiPathways ORA summary | WP299 | Nuclear receptors in lipid metabolism and toxicity | MIR33A | 0.03 | 0.009 |
| WP430 | Statin inhibition of cholesterol production | MIR33A | 0.03 | 0.009 | |
| WP1545 | miRNAs involved in DNA damage response | MIR29C | 0.04 | 0.01 | |
| WP1601 | Fluoropyrimidine activity | MIR29C | 0.03 | 0.009 | |
| WP2023 | Cell differentiation expanded index | MIR150 | 0.04 | 0.01 | |
| WP2249 | Metastatic brain tumor | MIR29C | 0.03 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nano, E.; Reggiani, F.; Amaro, A.A.; Monti, P.; Colombo, M.; Bertola, N.; Ferrero, F.; Fais, F.; Bruzzese, A.; Martino, E.A.; et al. MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study. Non-Coding RNA 2024, 10, 46. https://doi.org/10.3390/ncrna10050046
Nano E, Reggiani F, Amaro AA, Monti P, Colombo M, Bertola N, Ferrero F, Fais F, Bruzzese A, Martino EA, et al. MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study. Non-Coding RNA. 2024; 10(5):46. https://doi.org/10.3390/ncrna10050046
Chicago/Turabian StyleNano, Ennio, Francesco Reggiani, Adriana Agnese Amaro, Paola Monti, Monica Colombo, Nadia Bertola, Fabiana Ferrero, Franco Fais, Antonella Bruzzese, Enrica Antonia Martino, and et al. 2024. "MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study" Non-Coding RNA 10, no. 5: 46. https://doi.org/10.3390/ncrna10050046
APA StyleNano, E., Reggiani, F., Amaro, A. A., Monti, P., Colombo, M., Bertola, N., Ferrero, F., Fais, F., Bruzzese, A., Martino, E. A., Vigna, E., Puccio, N., Pistoni, M., Torricelli, F., D’Arrigo, G., Greco, G., Tripepi, G., Adornetto, C., Gentile, M., ... Cutrona, G. (2024). MicroRNA Profiling as a Predictive Indicator for Time to First Treatment in Chronic Lymphocytic Leukemia: Insights from the O-CLL1 Prospective Study. Non-Coding RNA, 10(5), 46. https://doi.org/10.3390/ncrna10050046










