The Circulating miR-107 as a Potential Biomarker Up-Regulated in Castration-Resistant Prostate Cancer
Abstract
:1. Introduction
2. Results
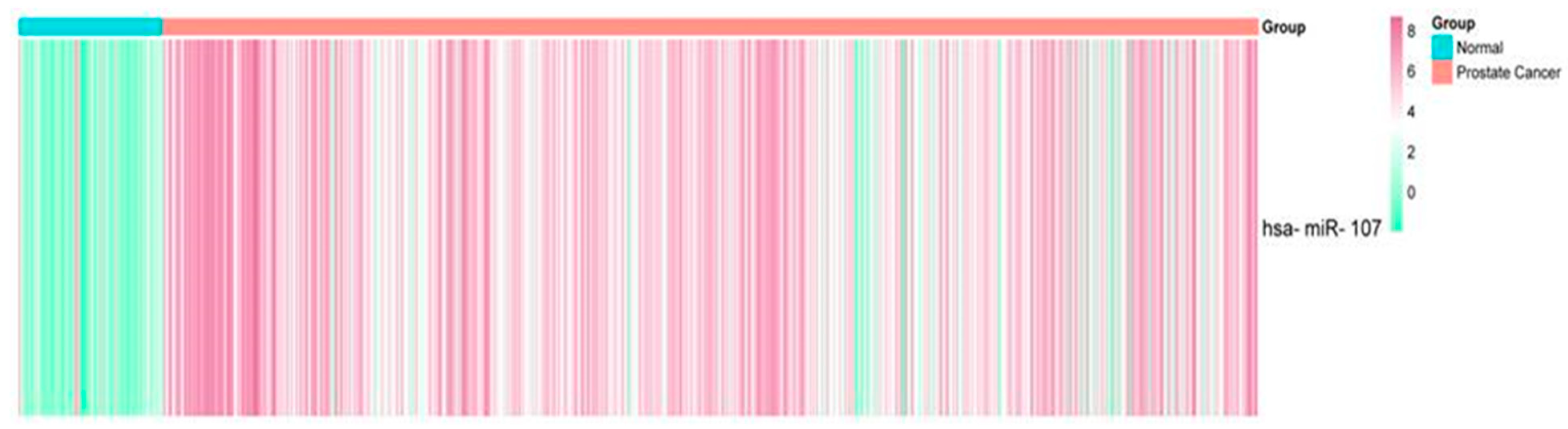
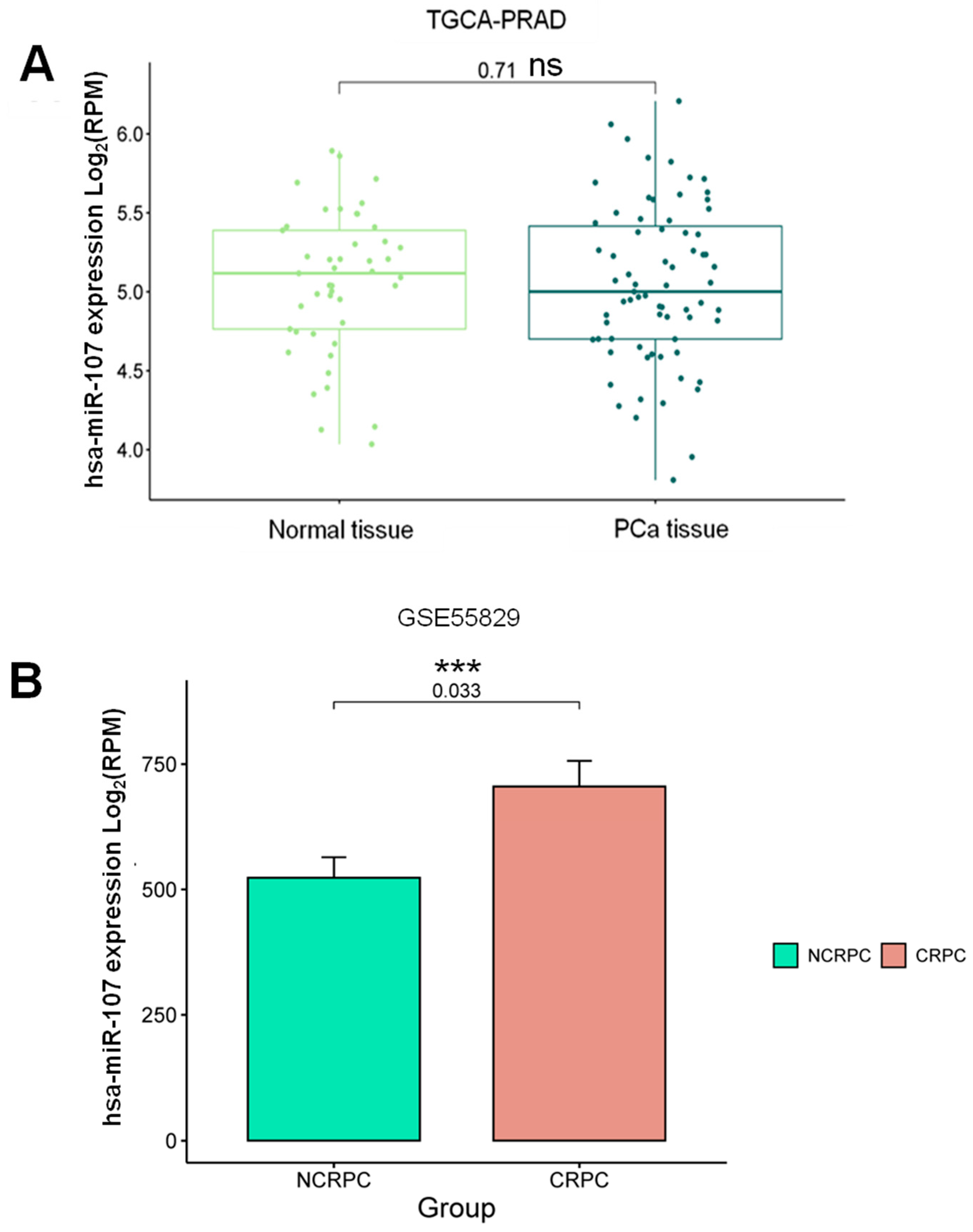
2.1. Expression Profile of miR-107 in the Prostate Cancer Liquid Biopsies Dataset
2.2. The miR-107 Expression Levels Are Higher in Liquid Biopsies from Cancer Patients with Androgen Deprivation Therapy
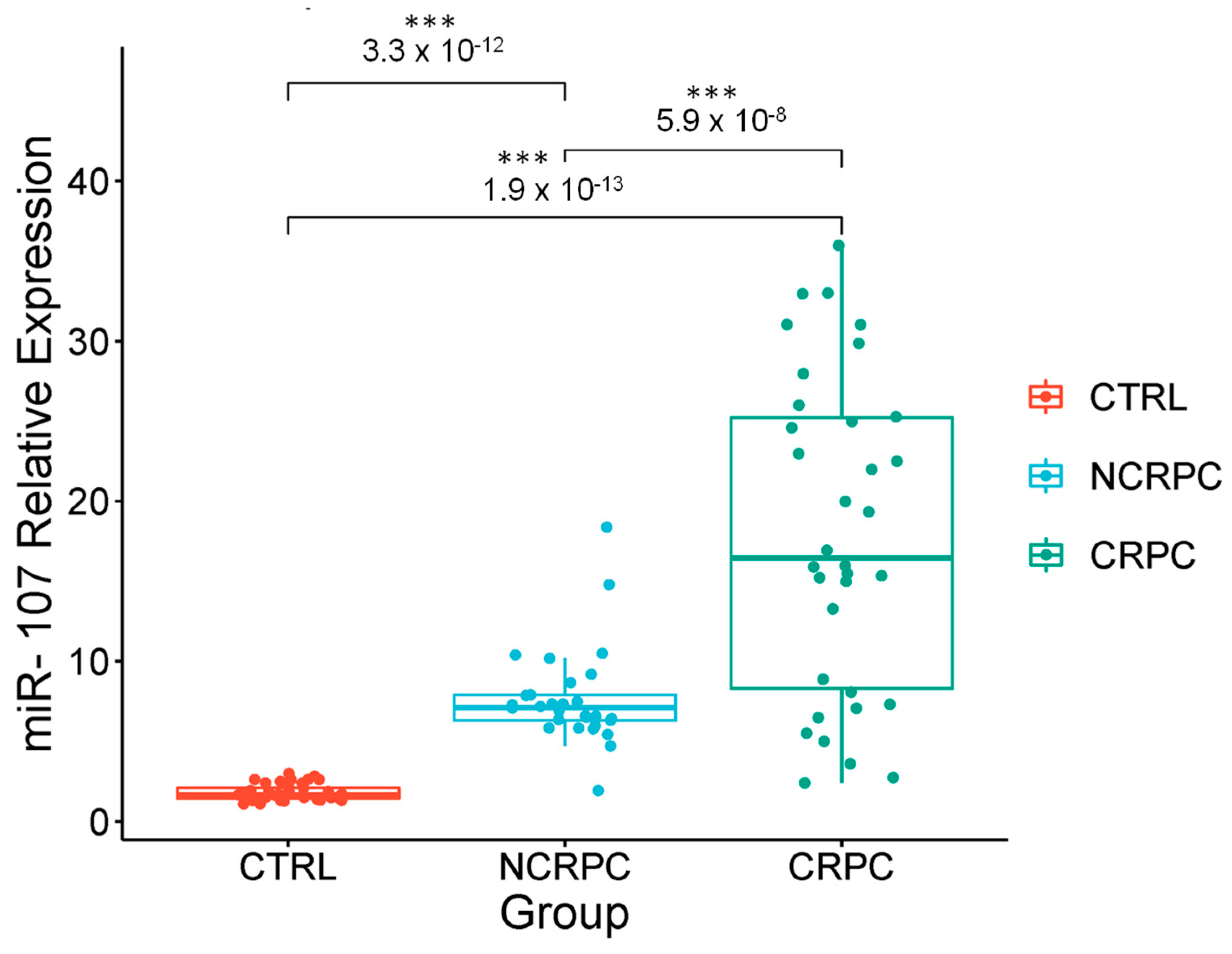
2.3. Stratification of Androgen Privation Resistance Prostate Cancer Cases in Mexican Patients
2.4. Correlation of the Expression of miR-107 with the Clinical Stages of Androgen Privation Therapy Prostate Cancer and Liquid Biopsies
2.5. Circulating miR-107 as a Potential Biomarker for Castration-Resistant Prostate Cancer
2.6. Evaluation of miR-107 as a Potential CRPC Diagnostic Biomarker
3. Discussion
4. Materials and Methods
4.1. Data Acquisition of PCa and CRPC from Liquid Biopsies and Tissue
4.2. Liquid Biopsies from NCRPC, CRPC Patients, and Controls
4.3. RNA Isolation from Liquid Biopsies
4.4. cDNA Synthesis and qRT-PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (version 1.1). Global Cancer Observatory 2024. Available online: https://gco.iarc.who.int/today (accessed on 17 May 2024).
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, L.E.; Hernández-Pérez, J.G.; Escamilla-Nuñez, C.; Rodríguez-Covarrubias, F.; Manzanilla-García, H.; Mohar, A.; Morales-Carmona, E.; Espin-Arellano, L.I.; Hernández-Ávila, J.E.; Lajous, M. Disparities on prostate cancer survival in Mexico: A retrospective cohort study. Salud Publica Mex. 2023, 65, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; O’Neil, M.E.; Richards, T.B.; Dowling, N.F.; Weir, H.K. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity—United States, 2001–2017. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- SEER. Prostate Cancer Statistics. SEER Cancer Statistics 2024. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 11 July 2024).
- Schoen, M.W.; Montgomery, R.B.; Owens, L.; Khan, S.; Sanfilippo, K.M.; Etzioni, R.B. Survival in Patients with De Novo Metastatic Prostate Cancer. JAMA Netw. Open 2024, 7, e241970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loriot, Y.; Massard, C.; Fizazi, K. Recent developments in treatments targeting castration-resistant prostate cancer bone metastases. Ann. Oncol. 2012, 23, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Velonas, V.M.; Woo, H.H.; Remedios, C.G.d.; Assinder, S.J. Current Status of Biomarkers for Prostate Cancer. Int. J. Mol. Sci. 2013, 14, 11034–11060. [Google Scholar] [CrossRef] [PubMed]
- Karrich, J.J.; Jachimowski, L.C.; Libouban, M.; Iyer, A.; Brandwijk, K.; Taanman-Kueter, E.W.; Nagasawa, M.; de Jong, E.C.; Uittenbogaart, C.H.; Blom, B. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood 2013, 122, 3001–3009. [Google Scholar] [CrossRef]
- Xu, X.H.; Li, D.W.; Feng, H.; Chen, H.M.; Song, Y.Q. MiR-300 Regulates the Malignancy of Breast Cancer by Targeting p53. Int. J. Clin. Exp. Med. 2015, 8, 6957–6966. [Google Scholar]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New Regulators of Immune Cell Development and Function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are Correlated with Tumor Progression in Prostate Cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531, Erratum in Nat. Rev. Genet. 2004, 5, 631. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA Signatures in Human Cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gayosso-Gómez, L.V.; Ortiz-Quintero, B. Circulating MicroRNAs in Blood and Other Body Fluids as Biomarkers for Diagnosis, Prognosis, and Therapy Response in Lung Cancer. Diagnostics 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating MicroRNA in Body Fluid: A New Potential Biomarker for Cancer Diagnosis and Prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasselmann, D.O.; Rappl, G.; Tilgen, W.; Reinhold, U. Extracellular Tyrosinase mRNA Within Apoptotic Bodies Is Protected from Degradation in Human Serum. Clin. Chem. 2001, 47, 1488–1489. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tsujiura, M.; Komatsu, S.; Ichikawa, D.; Shiozaki, A.; Konishi, H.; Takeshita, H.; Moriumura, R.; Nagata, H.; Kawaguchi, T.; Hirajima, S.; et al. Circulating miR-18a in Plasma Contributes to Cancer Detection and Monitoring in Patients with Gastric Cancer. Gastric Cancer 2015, 18, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Komatsu, S.; Ichikawa, D.; Tsujiura, M.; Takeshita, H.; Hirajima, S.; Miyamae, M.; Okajima, W.; Ohashi, T.; Imamura, T.; et al. Circulating MicroRNAs: A Next-Generation Clinical Biomarker for Digestive System Cancers. Int. J. Mol. Sci. 2016, 17, 1459. [Google Scholar] [CrossRef]
- Zedan, A.H.; Hansen, T.F.; Assenholt, J.; Madsen, J.S.; Osther, P.J.S. Circulating miRNAs in Localized/Locally Advanced Prostate Cancer Patients After Radical Prostatectomy and Radiotherapy. Prostate 2019, 79, 425–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kachris, S.; Papadaki, C.; Rounis, K.; Tsitoura, E.; Kokkinaki, C.; Nikolaou, C.; Sourvinos, G.; Mavroudis, D. Circulating miRNAs as Potential Biomarkers in Prostate Cancer Patients Undergoing Radiotherapy. Cancer Manag. Res. 2021, 13, 8257–8271, Erratum in Cancer Manag. Res. 2022, 14, 409–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrero-Aguayo, V.; Sáez-Martínez, P.; Jiménez-Vacas, J.M.; Moreno-Montilla, M.T.; Montero-Hidalgo, A.J.; Pérez-Gómez, J.M.; López-Canovas, J.L.; Porcel-Pastrana, F.; Carrasco-Valiente, J.; Anglada, F.J.; et al. Dysregulation of the miRNome Unveils a Crosstalk Between Obesity and Prostate Cancer: miR-107 as a Personalized Diagnostic and Therapeutic Tool. Mol. Ther. Nucleic Acids 2022, 27, 1164–1178. [Google Scholar] [CrossRef]
- Sapre, N.; Selth, L.A. Circulating MicroRNAs as Biomarkers of Prostate Cancer: The State of Play. Prostate Cancer 2013, 2013, 539680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catto, J.W.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larré, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct MicroRNA Alterations Characterize High- and Low-Grade Bladder Cancer. Cancer Res. 2009, 69, 8472–8481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.X.; Zhang, X.J.; Li, Q.; Wang, K.; Wang, Y.; Jiao, J.Q.; Feng, C.; Teng, S.; Zhou, L.Y.; Gong, Y.; et al. MicroRNA-103/107 Regulate Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury Through Targeting FADD. Circ Res. 2015, 117, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kobeissi, A.; Dong, Y.; Kaplan, N.; Yang, W.; He, C.; Zeng, K.; Peng, H. MicroRNAs-103/107 Regulate Autophagy in the Epidermis. J. Investig. Dermatol. 2018, 138, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Wu, S.; Muhammad, S.; Ren, Q.; Sun, C. miR-103/107 promote ER stress-mediated apoptosis via targeting the Wnt3a/β-catenin/ATF6 pathway in preadipocytes. J. Lipid Res. 2018, 59, 843–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer—An Emerging Concept. EBioMedicine 2016, 12, 34–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.Y.; Lin, Y.M.; Chung, H.C.; Lang, Y.D.; Lin, C.J.; Huang, J.; Wang, W.C.; Lin, F.M.; Chen, Z.; Huang, H.D.; et al. miR-103/107 Promote Metastasis of Colorectal Cancer by Targeting the Metastasis Suppressors DAPK and KLF4. Cancer Res. 2012, 72, 3631–3641, Erratum in Cancer Res. 2017, 77, 6788. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Qiao, Y.; Zhang, L.; Lu, S. miR-103 Promotes Proliferation and Metastasis by Targeting KLF4 in Gastric Cancer. Int. J. Mol. Sci. 2017, 18, 910. [Google Scholar] [CrossRef]
- Xiong, B.; Lei, X.; Zhang, L.; Fu, J. miR-103 Regulates Triple Negative Breast Cancer Cells Migration and Invasion Through Targeting Olfactomedin 4. Biomed. Pharmacother. 2017, 89, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 are Associated with Treatment Outcome in Metastatic Castration Resistant Prostate Cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.C.; Xie, W.; Yang, M.; Hsieh, C.L.; Drouin, S.; Lee, G.S.; Kantoff, P.W. Expression Differences of Circulating MicroRNAs in Metastatic Castration Resistant Prostate Cancer and Low-Risk, Localized Prostate Cancer. Prostate 2013, 73, 346–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, J.; Wang, D.; Wei, A.; Lu, H.; Tan, C.; Li, A.; Tang, J.; Wang, Y.; He, S.; Liu, X.; et al. Deregulated Expression of miR-107 Inhibits Metastasis of PDAC Through Inhibition PI3K/Akt Signaling Via Caveolin-1 and PTEN. Exp. Cell Res. 2017, 361, 316–323. [Google Scholar] [CrossRef] [PubMed]
- DeVere White, R.W.; Vinall, R.L.; Tepper, C.G.; Shi, X.B. MicroRNAs and Their Potential for Translation in Prostate Cancer. Urol. Oncol. 2009, 27, 307–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.B.; Xue, L.; Yang, J.; Ma, A.H.; Zhao, J.; Xu, M.; Tepper, C.G.; Evans, C.P.; Kung, H.J.; deVere White, R.W. An Androgen-Regulated miRNA Suppresses Bak1 Expression and Induces Androgen-Independent Growth of Prostate Cancer Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 19983–19988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sartor, O.; de Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Gupta, K.; Kyprianou, N. Epigenetic Mechanisms Underlying Subtype Heterogeneity and Tumor Recurrence in Prostate Cancer. Nat. Commun. 2023, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Hudson, M.A.; Scardino, P.T.; Richie, J.P.; Ahmann, F.R.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Selection of Optimal Prostate Specific Antigen Cutoffs for Early Detection of Prostate Cancer: Receiver Operating Characteristic Curves. J. Urol. 1994, 152 Pt 1, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- El Farhaoui, H.; Jdaini, A.; Elabbassi, O.; Bounouar, O.; Elmoudane, A.; Barki, A. Management of a Localized Prostatic Adenocarcinoma Despite the Very High Rate of PSA and the Large Tumor Mass: Does PSA Level Indicate the Stage of Prostate Cancer? Radiol. Case Rep. 2023, 18, 3501–3503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Y.; Zhu, D.; Hou, L.; Wang, Y.; Huang, X.; Zhou, C.; Zhu, L.; Wang, Y.; Li, L.; Gu, Y.; et al. MiR-107 Confers Chemoresistance to Colorectal Cancer by Targeting Calcium-Binding Protein 39. Br. J. Cancer 2020, 122, 705–714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mihelich, B.L.; Maranville, J.C.; Nolley, R.; Peehl, D.M.; Nonn, L. Elevated Serum MicroRNA Levels Associate with Absence of High-Grade Prostate Cancer in a Retrospective Cohort. PLoS ONE 2015, 10, e0124245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Jin, K.; Luo, J.D.; Liu, B.; Xie, L.P. MicroRNA-107 Inhibits Proliferation of Prostate Cancer Cells by Targeting Cyclin E1. Neoplasma 2019, 66, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Hansen, T.F.; Assenholt, J.; Pleckaitis, M.; Madsen, J.S.; Osther, P.J.S. MicroRNA Expression in Tumour Tissue and Plasma in Patients with Newly Diagnosed Metastatic Prostate Cancer. Tumour Biol. 2018, 40, 1010428318775864. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Jung, M.; Miller, K.; Lein, M.; Kristiansen, G.; Erbersdobler, A.; Jung, K. Suitable reference genes for relative quantification of miRNA expression in prostate cancer. Exp. Mol. Med. 2010, 42, 749–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, W.; Fei, X.; Wang, X.; Chen, F.; Song, Y. Circulating miRNAs as Biomarkers for Prostate Cancer Diagnosis in Subjects with Benign Prostatic Hyperplasia. J Immunol Res. 2020, 2020, 5873056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilyazova, I.; Ivanova, E.; Gupta, H.; Mustafin, A.; Ishemgulov, R.; Izmailov, A.; Gilyazova, G.; Pudova, E.; Pavlov, V.; Khusnutdinova, E. miRNA Expression Patterns in Early- and Late-Stage Prostate Cancer Patients: High-Throughput Analysis. Biomedicines 2023, 11, 3073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Clinical Stage | CS Cases (n) | Cases (n) | Gleason Score 1 |
|---|---|---|---|
| CSI | 13 | 6 | 6 (3 + 3) |
| 4 | 7 (4 + 3) | ||
| 3 | 8 (4 + 4) | ||
| CSII | 10 | 1 | 6 (4 + 2) |
| 6 | 7 (4 + 3) | ||
| 1 | 8 (4 + 4) | ||
| 2 | 9 (5 + 4) | ||
| CSIII | 20 | 12 | 7 (4 + 3) |
| 5 | 6 (4 + 2) | ||
| 3 | 8 (4 + 4) | ||
| CSIV | 20 | 8 | 9 |
| 2 | 8 | ||
| 8 | 10 | ||
| 1 | 7 | ||
| 1 | 6 |
| Clinical Stage | Number of Patients (n) | Gleason Score (n) |
|---|---|---|
| CSI | 10 | 6 (6), 7 (4) |
| CSII | 2 | 9 (2) |
| CSIII | 3 | 8 (3) |
| CSIV | 18. | 9 (8), 10 (8), 7 (2) |
| Total | 33 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente-Rivera, J.; De la Rosa Pérez, D.A.; Olvera, S.I.N.; Figueroa-Angulo, E.E.; Saucedo, J.G.C.; Hernández-León, O.; Alvarez-Sánchez, M.E. The Circulating miR-107 as a Potential Biomarker Up-Regulated in Castration-Resistant Prostate Cancer. Non-Coding RNA 2024, 10, 47. https://doi.org/10.3390/ncrna10050047
Puente-Rivera J, De la Rosa Pérez DA, Olvera SIN, Figueroa-Angulo EE, Saucedo JGC, Hernández-León O, Alvarez-Sánchez ME. The Circulating miR-107 as a Potential Biomarker Up-Regulated in Castration-Resistant Prostate Cancer. Non-Coding RNA. 2024; 10(5):47. https://doi.org/10.3390/ncrna10050047
Chicago/Turabian StylePuente-Rivera, Jonathan, David Alejandro De la Rosa Pérez, Stephanie I. Nuñez Olvera, Elisa Elvira Figueroa-Angulo, José Gadú Campos Saucedo, Omar Hernández-León, and María Elizbeth Alvarez-Sánchez. 2024. "The Circulating miR-107 as a Potential Biomarker Up-Regulated in Castration-Resistant Prostate Cancer" Non-Coding RNA 10, no. 5: 47. https://doi.org/10.3390/ncrna10050047







