Circulating miRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of the Patient Groups
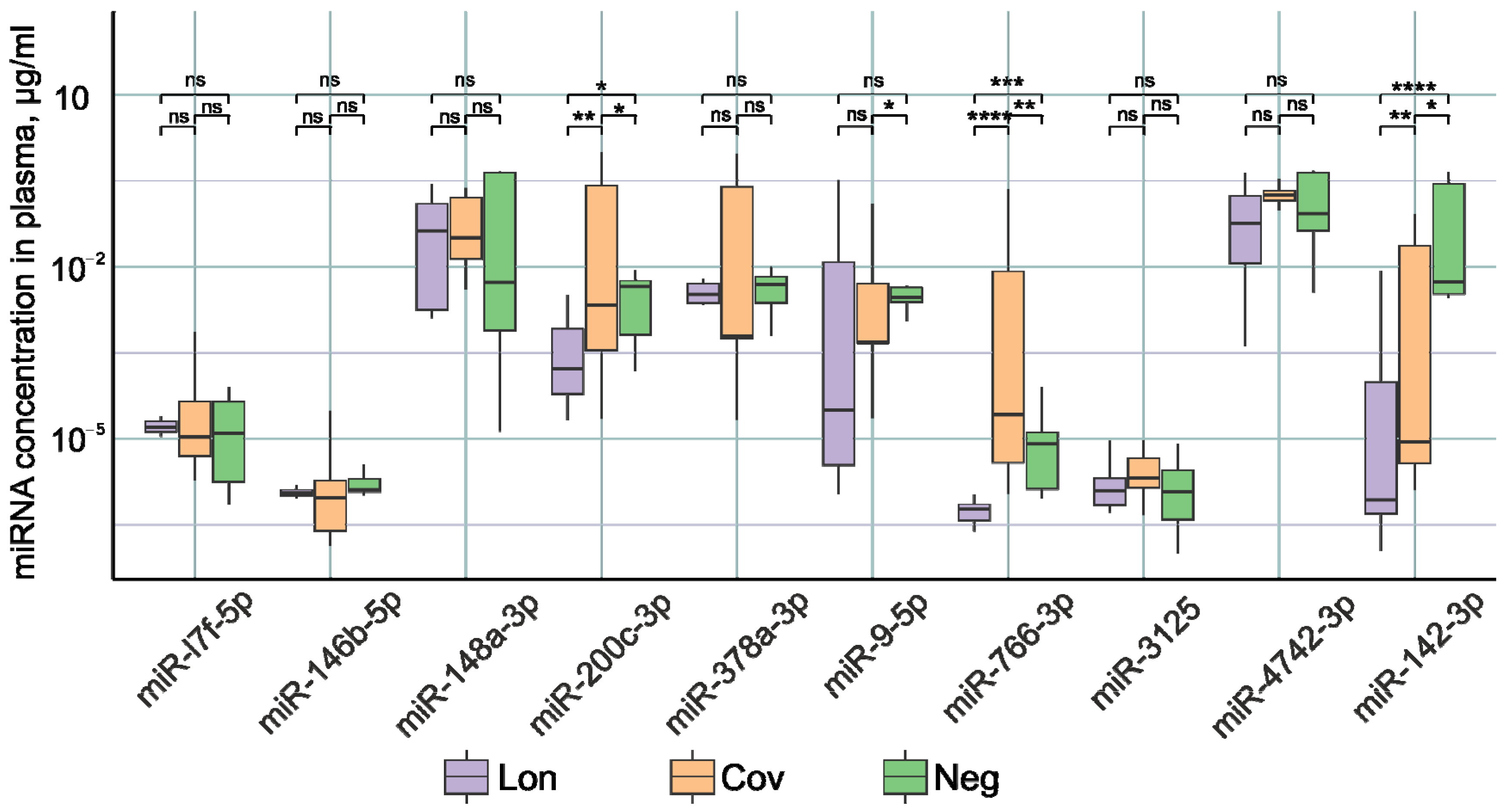
2.2. Analysis of miRNA Concentration in the Plasma of Patients
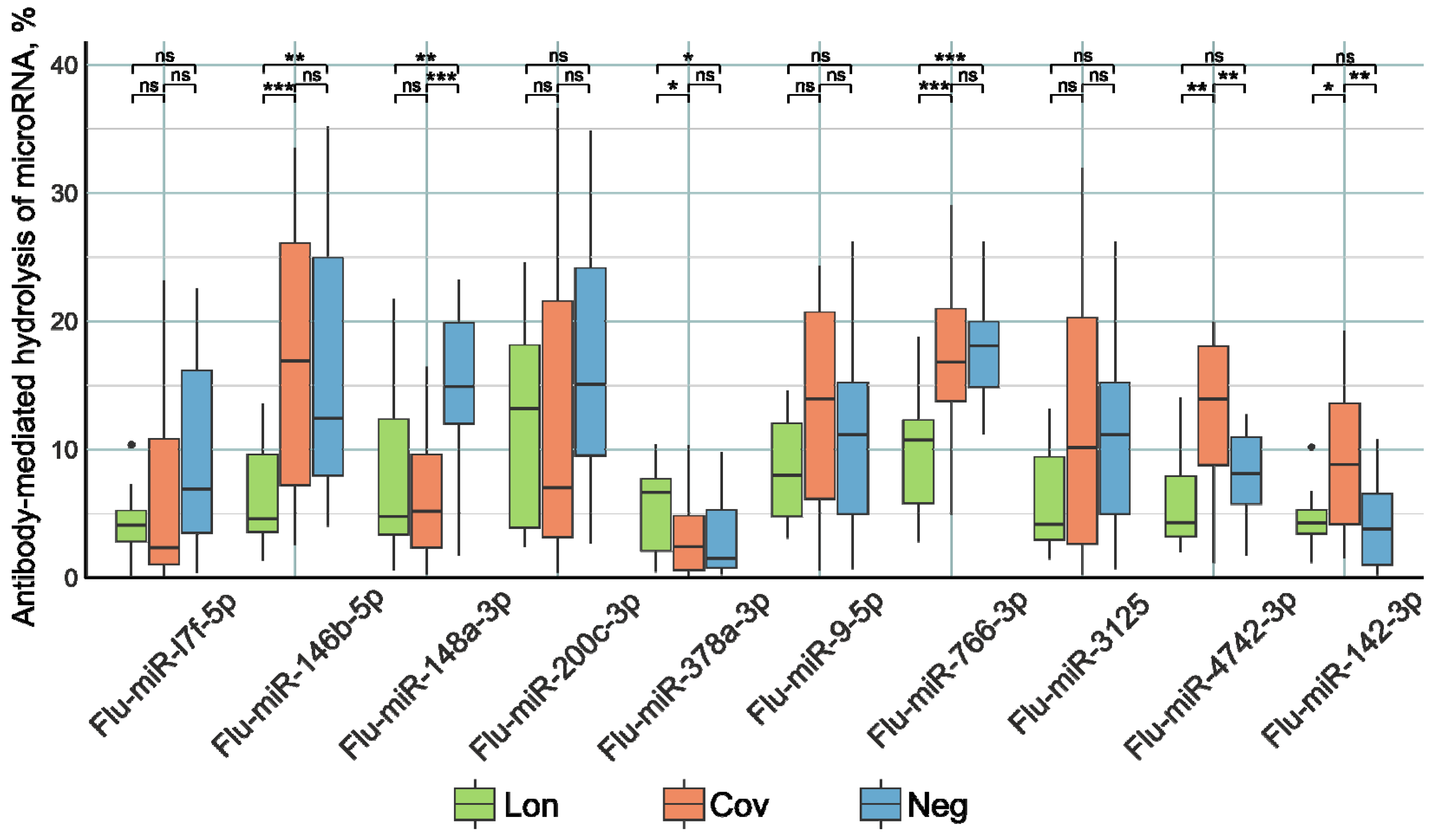
2.3. Analysis of the Catalytic Activity of Antibodies in the Hydrolysis of miRNAs
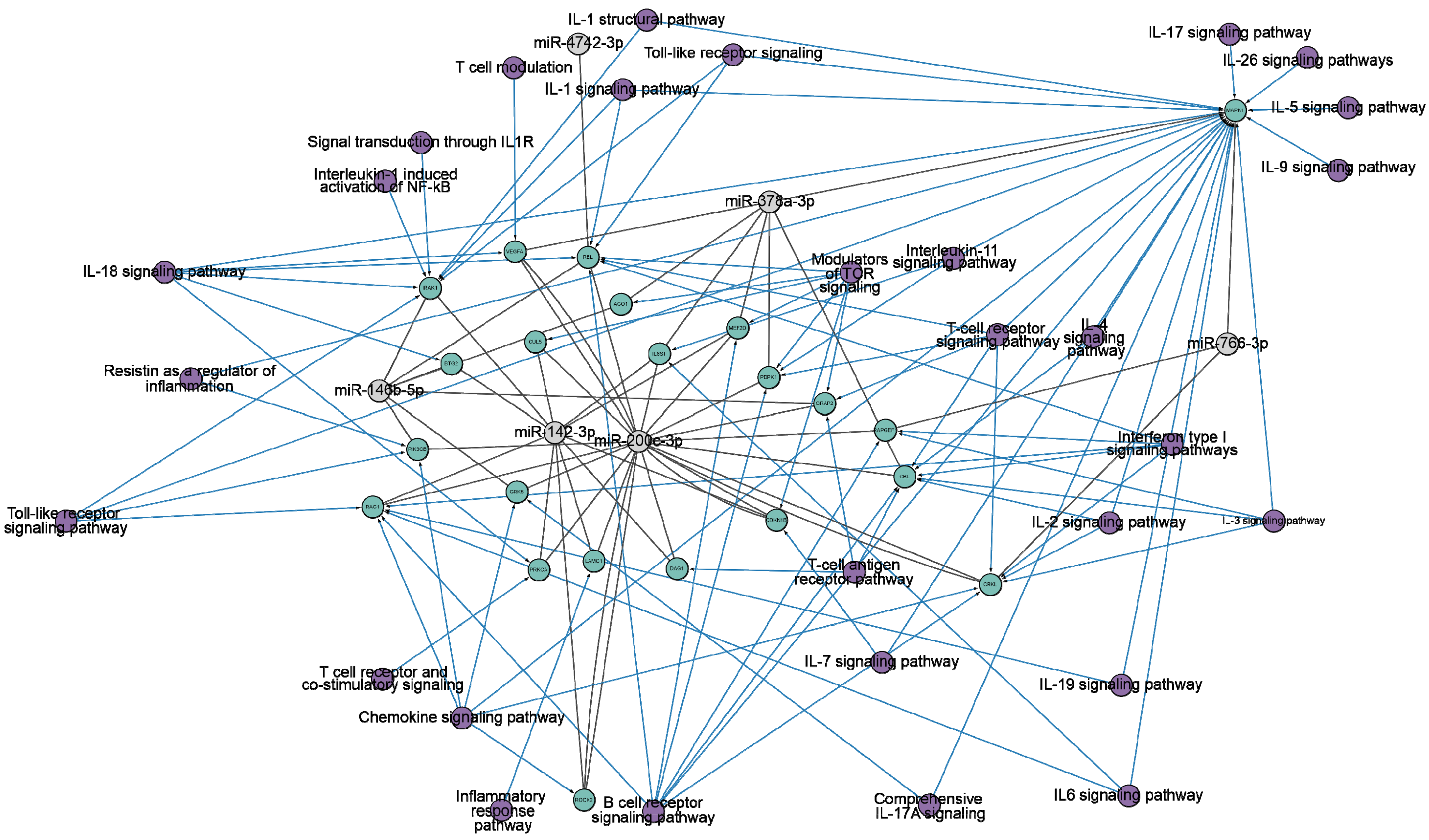
3. Discussion
4. Materials and Methods
4.1. Donors and Patients
4.2. Isolation of miRNAs
4.3. Reverse Transcription Using SL-Primers
4.4. Real-Time PCR
4.5. Identification of IgG Activity in the miRNA Hydrolysis Reaction
4.6. Statistical Analysis
4.7. Target Predictions of the miRNAs Analyzed
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kincaid, R.P.; Sullivan, C.S. Virus-Encoded MicroRNAs: An Overview and a Look to the Future. PLoS Pathog. 2012, 8, e1003018. [Google Scholar] [CrossRef] [PubMed]
- Intartaglia, D.; Giamundo, G.; Conte, I. The Impact of MiRNAs in Health and Disease of Retinal Pigment Epithelium. Front. Cell Dev. Biol. 2021, 8, 589985. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Galvão-Lima, L.J.; Morais, A.H.F.; Valentim, R.A.M.; Barreto, E.J.S.S. MiRNAs as Biomarkers for Early Cancer Detection and Their Application in the Development of New Diagnostic Tools. Biomed. Eng. Online 2021, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Dash, C.; Pandhare, J. Therapeutic Significance of MicroRNA-Mediated Regulation of PARP-1 in SARS-CoV-2 Infection. Non-Coding RNA 2021, 7, 60. [Google Scholar] [CrossRef]
- Belmonte, T.; Perez-Pons, M.; Benítez, I.D.; Molinero, M.; García-Hidalgo, M.C.; Rodríguez-Muñoz, C.; Gort-Paniello, C.; Moncusí-Moix, A.; Madè, A.; Devaux, Y.; et al. Addressing the Unsolved Challenges in MicroRNA-Based Biomarker Development: Suitable Endogenous Reference MicroRNAs for SARS-CoV-2 Infection Severity. Int. J. Biol. Macromol. 2024, 269, 131926. [Google Scholar] [CrossRef]
- Wicik, Z.; Eyileten, C.; Nowak, A.; Keshwani, D.; Simões, S.N.; Martins, D.C.; Klos, K.; Wlodarczyk, W.; Assinger, A.; Soldacki, D.; et al. Alteration of Circulating ACE2-Network Related MicroRNAs in Patients with COVID-19. Sci. Rep. 2024, 14, 13573. [Google Scholar] [CrossRef]
- Garcia-Giralt, N.; Du, J.; Marin-Corral, J.; Bódalo-Torruella, M.; Blasco-Hernando, F.; Muñoz-Bermúdez, R.; Clarós, M.; Nonell, L.; Perera-Bel, J.; Fernandez-González, M.; et al. Circulating MicroRNA Profiling Is Altered in the Acute Respiratory Distress Syndrome Related to SARS-CoV-2 Infection. Sci. Rep. 2022, 12, 6929. [Google Scholar] [CrossRef] [PubMed]
- Togami, Y.; Matsumoto, H.; Yoshimura, J.; Matsubara, T.; Ebihara, T.; Matsuura, H.; Mitsuyama, Y.; Kojima, T.; Ishikawa, M.; Sugihara, F.; et al. Significance of Interferon Signaling Based on MRNA-MicroRNA Integration and Plasma Protein Analyses in Critically Ill COVID-19 Patients. Mol. Ther.-Nucleic Acids 2022, 29, 343–353. [Google Scholar] [CrossRef]
- Gao, L.; Kyubwa, E.M.; Starbird, M.A.; Diaz de Leon, J.; Nguyen, M.; Rogers, C.J.; Menon, N. Circulating MiRNA Profiles in COVID-19 Patients and Meta-Analysis: Implications for Disease Progression and Prognosis. Sci. Rep. 2023, 13, 21656. [Google Scholar] [CrossRef]
- de Souza Nicoletti, A.; Berlofa Visacri, M.; Regina da Silva Correa da Ronda, C.; Tiemi Siguemoto, J.; Motta Neri, C.; Nogueira de Souza, R.; de Souza Ventura, D.; Eguti, A.; Ferreira de Souza Silva, L.; Wesley Perroud Junior, M.; et al. Increased Expression of MiR-320b in Blood Plasma of Patients in Response to SARS-CoV-2 Infection. Sci. Rep. 2024, 14, 13702. [Google Scholar] [CrossRef] [PubMed]
- Giannella, A.; Riccetti, S.; Sinigaglia, A.; Piubelli, C.; Razzaboni, E.; Di Battista, P.; Agostini, M.; Dal Molin, E.; Manganelli, R.; Gobbi, F.; et al. Circulating MicroRNA Signatures Associated with Disease Severity and Outcome in COVID-19 Patients. Front. Immunol. 2022, 13, 968991. [Google Scholar] [CrossRef] [PubMed]
- Duecker, R.P.; Adam, E.H.; Wirtz, S.; Gronau, L.; Khodamoradi, Y.; Eberhardt, F.J.; Donath, H.; Gutmann, D.; Vehreschild, M.J.G.T.; Zacharowski, K.; et al. The MiR-320 Family Is Strongly Downregulated in Patients with COVID-19 Induced Severe Respiratory Failure. Int. J. Mol. Sci. 2021, 22, 10351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, X.; Li, L.; Li, J. Differential MicroRNA Expression in the Peripheral Blood from Human Patients with COVID-19. J. Clin. Lab. Anal. 2020, 34, e23590. [Google Scholar] [CrossRef] [PubMed]
- Meidert, A.S.; Hermann, S.; Brandes, F.; Kirchner, B.; Buschmann, D.; Billaud, J.-N.; Klein, M.; Lindemann, A.; Aue, E.; Schelling, G.; et al. Extracellular Vesicle Associated MiRNAs Regulate Signaling Pathways Involved in COVID-19 Pneumonia and the Progression to Severe Acute Respiratory Corona Virus-2 Syndrome. Front. Immunol. 2021, 12, 784028. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Ma, J.; Hu, F.; Wang, X.; Qin, H.; Li, M.; Huang, S.; Yang, Y.; Li, Y.; et al. Distinct MiRNAs Associated with Various Clinical Presentations of SARS-CoV-2 Infection. iScience 2022, 25, 104309. [Google Scholar] [CrossRef]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating Cardiovascular microRNAs in Critically Ill COVID-19 Patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Nicoletti, A.d.S.; Visacri, M.B.; da Ronda, C.R.d.S.C.; Vasconcelos, P.E.d.N.S.; Quintanilha, J.C.F.; de Souza, R.N.; Ventura, D.d.S.; Eguti, A.; Silva, L.F.d.S.; Perroud Junior, M.W.; et al. Differentially Expressed Plasmatic MicroRNAs in Brazilian Patients with Coronavirus Disease 2019 (COVID-19): Preliminary Results. Mol. Biol. Rep. 2022, 49, 6931–6943. [Google Scholar] [CrossRef]
- Najafipour, R.; Mohammadi, D.; Estaki, Z.; Zarabadi, K.; Jalilvand, M.; Moghbelinejad, S. Screening for Differentially Expressed MicroRNAs in BALF and Blood Samples of Infected COVID-19 ARDS Patients by Small RNA Deep Sequencing. J. Clin. Lab. Anal. 2022, 36, e24672. [Google Scholar] [CrossRef]
- tenOever, B.R. RNA Viruses and the Host MicroRNA Machinery. Nat. Rev. Microbiol. 2013, 11, 169–180. [Google Scholar] [CrossRef]
- Rad, S.M.A.H.; Wannigama, D.L.; Hirankarn, N.; McLellan, A.D. The Impact of Non-Synonymous Mutations on MiRNA Binding Sites within the SARS-CoV-2 NSP3 and NSP4 Genes. Sci. Rep. 2023, 13, 16945. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Rad SM, A.; McLellan, A.D. Implications of SARS-CoV-2 Mutations for Genomic RNA Structure and Host MicroRNA Targeting. Int. J. Mol. Sci. 2020, 21, 4807. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Furuyama, W.; Lin, Z. RNA Virus-Encoded MiRNAs: Current Insights and Future Challenges. Front. Microbiol. 2021, 12, 679210. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Integrating MicroRNA Annotation and Deep-Sequencing Data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef]
- Grundhoff, A.; Sullivan, C.S. Virus-Encoded MicroRNAs. Virology 2011, 411, 325–343. [Google Scholar] [CrossRef]
- Cullen, B.R. Five Questions about Viruses and MicroRNAs. PLoS Pathog. 2010, 6, e1000787. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Lorefice, E.; Carissimi, C.; Laudadio, I.; Ciccosanti, F.; Di Rienzo, M.; Colavita, F.; Meschi, S.; Maggi, F.; Fimia, G.M.; et al. A MicroRNA Arising from the Negative Strand of SARS-CoV-2 Genome Targets FOS to Reduce AP-1 Activity. Non-Coding RNA 2023, 9, 33. [Google Scholar] [CrossRef]
- Morales, L.; Oliveros, J.C.; Fernandez-Delgado, R.; TenOever, B.R.; Enjuanes, L.; Sola, I. SARS-CoV-Encoded Small RNAs Contribute to Infection-Associated Lung Pathology. Cell Host Microbe 2017, 21, 344–355. [Google Scholar] [CrossRef]
- Meng, F.; Siu, G.K.-H.; Mok, B.W.-Y.; Sun, J.; Fung, K.S.C.; Lam, J.Y.-W.; Wong, N.K.; Gedefaw, L.; Luo, S.; Lee, T.M.H.; et al. Viral MicroRNAs Encoded by Nucleocapsid Gene of SARS-CoV-2 Are Detected during Infection, and Targeting Metabolic Pathways in Host Cells. Cells 2021, 10, 1762. [Google Scholar] [CrossRef]
- Gracia-Ramos, A.E.; Martin-Nares, E.; Hernández-Molina, G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021, 10, 3592. [Google Scholar] [CrossRef]
- Talotta, R.; Robertson, E. Autoimmunity as the Comet Tail of COVID-19 Pandemic. World J. Clin. Cases 2020, 8, 3621–3644. [Google Scholar] [CrossRef]
- Gollapudi, S.; Chimurkar, V. Comprehensive Insights Into the Multi-Faceted Manifestations of COVID-19: A Narrative Review. Cureus 2024, 16, e63493. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Ono, H.; Haraguchi, S.; Igarashi, S.; Hebisawa, A.; Tsuboi, E. Post-coronavirus Disease 2019 Organizing Pneumonia Confirmed Pathologically by Video-assisted Thoracoscopic Surgery. Respirol. Case Rep. 2021, 9, e0871. [Google Scholar] [CrossRef] [PubMed]
- Bazdyrev, E.; Rusina, P.; Panova, M.; Novikov, F.; Grishagin, I.; Nebolsin, V. Lung Fibrosis after COVID-19: Treatment Prospects. Pharmaceuticals 2021, 14, 807. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain–Barré Syndrome Spectrum Associated with COVID-19: An up-to-Date Systematic Review of 73 Cases. J. Neurol. 2021, 268, 1133–1170. [Google Scholar] [CrossRef]
- Kozłowski, P.; Leszczyńska, A.; Ciepiela, O. Long COVID Definition, Symptoms, Risk Factors, Epidemiology and Autoimmunity: A Narrative Review. Am. J. Med. Open 2024, 11, 100068. [Google Scholar] [CrossRef]
- Roghani, S.A.; Dastbaz, M.; Lotfi, R.; Shamsi, A.; Abdan, Z.; Rostampour, R.; Soleymani, B.; Zamanian, M.H.; Soufivand, P.; Pournazari, M.; et al. The Development of Anticyclic Citrullinated Peptide (Anti-CCP) Antibody Following Severe COVID-19. Immun. Inflamm. Dis. 2024, 12, e1276. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M.; et al. Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef]
- Migliorini, F.; Bell, A.; Vaishya, R.; Eschweiler, J.; Hildebrand, F.; Maffulli, N. Reactive Arthritis Following COVID-19 Current Evidence, Diagnosis, and Management Strategies. J. Orthop. Surg. Res. 2023, 18, 205. [Google Scholar] [CrossRef]
- Reyes-Long, S.; Cortés-Altamirano, J.L.; Bandala, C.; Avendaño-Ortiz, K.; Bonilla-Jaime, H.; Bueno-Nava, A.; Ávila-Luna, A.; Sánchez-Aparicio, P.; Clavijo-Cornejo, D.; Dotor-LLerena, A.L.; et al. Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review. Int. J. Mol. Sci. 2023, 24, 3574. [Google Scholar] [CrossRef]
- Mone, P.; Jankauskas, S.S.; Manzi, M.V.; Gambardella, J.; Coppola, A.; Kansakar, U.; Izzo, R.; Fiorentino, G.; Lombardi, A.; Varzideh, F.; et al. Endothelial Extracellular Vesicles Enriched in MicroRNA-34a Predict New-Onset Diabetes in Coronavirus Disease 2019 (COVID-19) Patients: Novel Insights for Long COVID Metabolic Sequelae. J. Pharmacol. Exp. Ther. 2024, 389, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Visco, V.; Gambardella, J.; Ferruzzi, G.J.; Rispoli, A.; Rusciano, M.R.; Toni, A.L.; Virtuoso, N.; Carrizzo, A.; Di Pietro, P.; et al. Cardiovascular Implications of MicroRNAs in Coronavirus Disease 2019. J. Pharmacol. Exp. Ther. 2023, 384, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Santulli, G. What Is Linking COVID-19 and Endothelial Dysfunction? Updates on Nanomedicine and Bioengineering from the 2020 AHA Scientific Sessions. Eur. Hear. J.-Cardiovasc. Pharmacother. 2021, 7, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Fayyad-Kazan, M.; Makki, R.; Skafi, N.; El Homsi, M.; Hamade, A.; El Majzoub, R.; Hamade, E.; Fayyad-Kazan, H.; Badran, B. Circulating MiRNAs: Potential Diagnostic Role for Coronavirus Disease 2019 (COVID-19). Infect. Genet. Evol. 2021, 94, 105020. [Google Scholar] [CrossRef]
- Gambardella, J.; Kansakar, U.; Sardu, C.; Messina, V.; Jankauskas, S.S.; Marfella, R.; Maggi, P.; Wang, X.; Mone, P.; Paolisso, G.; et al. Exosomal MiR-145 and MiR-885 Regulate Thrombosis in COVID-19. J. Pharmacol. Exp. Ther. 2023, 384, 109–115. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The Pathogenesis and Treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front. Immunol. 2020, 10, 3081. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Abdelkafy, A.E.; Khairy, R.M.M.; Abdelraheim, S.R.; Kamel, B.A.; Marey, H. MicroRNAs and Cytokines as Potential Predictive Biomarkers for COVID-19 Disease Progression. Sci. Rep. 2023, 13, 3531. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, H.; Guo, Q. MicroRNA-20a Promotes Inflammation via the Nuclear Factor-κB Signaling Pathway in Pediatric Pneumonia. Mol. Med. Rep. 2017, 17, 612–617. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N. Natural Catalytic Antibodies in Norm, Autoimmune, Viral, and Bacterial Diseases. Sci. World J. 2010, 10, 1203–1233. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Onvumere, M.K.; Sedykh, S.E.; Timofeeva, A.M.; Nevinsky, G.A. Catalase Activity of IgGs of Patients Infected with SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 10081. [Google Scholar] [CrossRef] [PubMed]
- McConnell, S.A.; Sachithanandham, J.; Mudrak, N.J.; Zhu, X.; Farhang, P.A.; Cordero, R.J.B.; Wear, M.P.; Shapiro, J.R.; Park, H.-S.; Klein, S.L.; et al. Spike-Protein Proteolytic Antibodies in COVID-19 Convalescent Plasma Contribute to SARS-CoV-2 Neutralization. Cell Chem. Biol. 2023, 30, 726–738.e4. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Shayakhmetova, L.S.; Nikitin, A.O.; Sedykh, T.A.; Matveev, A.L.; Shanshin, D.V.; Volosnikova, E.A.; Merkuleva, I.A.; Shcherbakov, D.N.; Tikunova, N.V.; et al. Natural Antibodies Produced in Vaccinated Patients and COVID-19 Convalescents Hydrolyze Recombinant RBD and Nucleocapsid (N) Proteins. Biomedicines 2024, 12, 1007. [Google Scholar] [CrossRef]
- Lee, G.; Budhathoki, S.; Lee, G.-Y.; Oh, K.; Ham, Y.; Kim, Y.-J.; Lim, Y.; Hoang, P.; Lee, Y.; Lim, S.-W.; et al. Broad-Spectrum Antiviral Activity of 3D8, a Nucleic Acid-Hydrolyzing Single-Chain Variable Fragment (ScFv), Targeting SARS-CoV-2 and Multiple Coronaviruses In Vitro. Viruses 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Luong, Q.X.T.; Hoang, P.T.; Lee, Y.; Ayun, R.Q.; Na, K.; Park, S.; Lin, C.; Ho, P.T.; Lee, T.-K.; Lee, S. An RNA-Hydrolyzing Recombinant Minibody Prevents Both Influenza A Virus and Coronavirus in Co-Infection Models. Sci. Rep. 2024, 14, 8472. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.T.; Luong, Q.X.T.; Ayun, R.Q.; Lee, Y.; Oh, K.-J.; Kim, T.; Lee, T.-K.; Lee, S. A Synergistic Therapy against Influenza Virus A/H1N1/PR8 by a HA1 Specific Neutralizing Single-Domain VL and an RNA Hydrolyzing ScFv. Front. Microbiol. 2024, 15, 1355599. [Google Scholar] [CrossRef]
- Lee, Y.; Hoang, P.; Kim, D.; Ayun, R.; Luong, Q.; Na, K.; Kim, T.; Oh, Y.; Kim, W.-K.; Lee, S. A Therapeutically Active Minibody Exhibits an Antiviral Activity in Oseltamivir-Resistant Influenza-Infected Mice via Direct Hydrolysis of Viral RNAs. Viruses 2022, 14, 1105. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Kabirova, E.M.; Sizikov, A.E.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the Sera of Patients with Systemic Lupus Erythematosus Hydrolyzed MiRNAs. J. Inflamm. Res. 2020, 13, 681–699. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Kabirova, E.M.; Buneva, V.N.; Nevinsky, G.A. IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze MiRNAs. Int. J. Mol. Sci. 2021, 22, 2812. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Urusov, A.E.; Aulova, K.S.; Ermakov, E.A. Experimental Autoimmune Encephalomyelitis of Mice: IgGs from the Sera of Mice Hydrolyze MiRNAs. Int. J. Mol. Sci. 2023, 24, 4433. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Ivanova, S.A.; Buneva, V.N.; Nevinsky, G.A. Hydrolysis by Catalytic IgGs of MicroRNA Specific for Patients with Schizophrenia. IUBMB Life 2018, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Tesch, F.; Ehm, F.; Vivirito, A.; Wende, D.; Batram, M.; Loser, F.; Menzer, S.; Jacob, J.; Roessler, M.; Seifert, M.; et al. Incident Autoimmune Diseases in Association with SARS-CoV-2 Infection: A Matched Cohort Study. Clin. Rheumatol. 2023, 42, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-Dimensional Characterization of Post-Acute Sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-Month Neurological and Psychiatric Outcomes in 236 379 Survivors of COVID-19: A Retrospective Cohort Study Using Electronic Health Records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Al-Aly, Z. Risks of Mental Health Outcomes in People with COVID-19: Cohort Study. BMJ 2022, 376, e068993. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-Term Neurologic Outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415. [Google Scholar] [CrossRef]
- Roessler, M.; Tesch, F.; Batram, M.; Jacob, J.; Loser, F.; Weidinger, O.; Wende, D.; Vivirito, A.; Toepfner, N.; Ehm, F.; et al. Post-COVID-19-Associated Morbidity in Children, Adolescents, and Adults: A Matched Cohort Study Including More than 157,000 Individuals with COVID-19 in Germany. PLOS Med. 2022, 19, e1004122. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Rezaei, N. Autoimmune Complications of COVID-19. J. Med. Virol. 2022, 94, 54–62. [Google Scholar] [CrossRef]
- Chang, R.; Yen-Ting Chen, T.; Wang, S.-I.; Hung, Y.-M.; Chen, H.-Y.; Wei, C.-C.J. Risk of Autoimmune Diseases in Patients with COVID-19: A Retrospective Cohort Study. eClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef] [PubMed]
- Klink, G.V.; Safina, K.R.; Garushyants, S.K.; Moldovan, M.; Nabieva, E.; Komissarov, A.B.; Lioznov, D.; Bazykin, G.A. Spread of Endemic SARS-CoV-2 Lineages in Russia before April 2021. PLoS ONE 2022, 17, e0270717. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The Noncoding and Coding Transcriptional Landscape of the Peripheral Immune Response in Patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. MicroRNA Regulation of Inflammatory Responses. Annu. Rev. Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J. Turning 21: Induction of MiR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 Represses Endothelial Activation by Inhibiting Pro-inflammatory Pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef]
- Pimenta, R.; Viana, N.I.; Dos Santos, G.A.; Candido, P.; Guimarães, V.R.; Romão, P.; Silva, I.A.; de Camargo, J.A.; Hatanaka, D.M.; Queiroz, P.G.S.; et al. MiR-200c-3p Expression May Be Associated with Worsening of the Clinical Course of Patients with COVID-19. Mol. Biol. Res. Commun. 2021, 10, 141–147. [Google Scholar] [CrossRef]
- Shaker, O.; El Amir, M.; Elfatah, Y.A.; Elwi, H.M. Expression Patterns of LncRNA MALAT-1 in SARS-CoV-2 Infection and Its Potential Effect on Disease Severity via MiR-200c-3p and SIRT1. Biochem. Biophys. Rep. 2023, 36, 101562. [Google Scholar] [CrossRef]
- Roustai Geraylow, K.; Hemmati, R.; Kadkhoda, S.; Ghafouri-Fard, S. MiRNA Expression in COVID-19. Gene Rep. 2022, 28, 101641. [Google Scholar] [CrossRef]
- Nersisyan, S.; Shkurnikov, M.; Turchinovich, A.; Knyazev, E.; Tonevitsky, A. Integrative Analysis of MiRNA and MRNA Sequencing Data Reveals Potential Regulatory Mechanisms of ACE2 and TMPRSS2. PLoS ONE 2020, 15, e0235987. [Google Scholar] [CrossRef]
- Onabajo, O.O.; Banday, A.R.; Stanifer, M.L.; Yan, W.; Obajemu, A.; Santer, D.M.; Florez-Vargas, O.; Piontkivska, H.; Vargas, J.M.; Ring, T.J.; et al. Interferons and Viruses Induce a Novel Truncated ACE2 Isoform and Not the Full-Length SARS-CoV-2 Receptor. Nat. Genet. 2020, 52, 1283–1293. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sensi, S.; Goebel, A. Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification. Bio-Protocol 2022, 12, e4562. [Google Scholar] [CrossRef]
- Paul, S.; Li, L.; Kalaga, R.; Wilkins-Stevens, P.; Stevens, F.J.; Solomon, A. Natural Catalytic Antibodies: Peptide-Hydrolyzing Activities of Bence Jones Proteins and VL Fragment. J. Biol. Chem. 1995, 270, 15257–15261. [Google Scholar] [CrossRef] [PubMed]
- Roush, S.; Slack, F.J. The Let-7 Family of MicroRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Shi, R.; Chiang, V.L. Facile Means for Quantifying Microrna Expression by Real-Time PCR. Biotechniques 2005, 39, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Mei, Q.; Li, X.; Meng, Y.; Wu, Z.; Guo, M.; Zhao, Y.; Fu, X.; Han, W. A Facile and Specific Assay for Quantifying MicroRNA by an Optimized RT-QPCR Approach. PLoS ONE 2012, 7, e46890. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Nevinsky, G.A.; Buneva, V.N. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. Int. J. Mol. Sci. 2020, 21, 5392. [Google Scholar] [CrossRef]
- Papadopoulos, G.L.; Reczko, M.; Simossis, V.A.; Sethupathy, P.; Hatzigeorgiou, A.G. The Database of Experimentally Supported Targets: A Functional Update of TarBase. Nucleic Acids Res. 2009, 37, D155–D158. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Balcı, H.; Hanspers, K.; Coort, S.L.; Martens, M.; Slenter, D.N.; Ehrhart, F.; Digles, D.; Waagmeester, A.; Wassink, I.; et al. WikiPathways 2024: Next Generation Pathway Database. Nucleic Acids Res. 2024, 52, D679–D689. [Google Scholar] [CrossRef] [PubMed]
- Pico, A.R.; Kelder, T.; van Iersel, M.P.; Hanspers, K.; Conklin, B.R.; Evelo, C. WikiPathways: Pathway Editing for the People. PLoS Biol. 2008, 6, e184. [Google Scholar] [CrossRef]
- Buggele, W.A.; Johnson, K.E.; Horvath, C.M. Influenza A Virus Infection of Human Respiratory Cells Induces Primary MicroRNA Expression. J. Biol. Chem. 2012, 287, 31027–31040. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.d.F.; Freitas, D.F.d.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), Adipokines and Inflammation. Metabolism 2019, 95, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, C.-H.; Li, W.; Li, T.; Luo, W.; Huang, S.; Wu, P.-S.; Cai, S.-X.; Li, X. Angiotensin-Converting Enzyme 2/Angiotensin-(1-7)/Mas Axis Protects against Lung Fibrosis by Inhibiting the MAPK/NF-ΚB Pathway. Am. J. Respir. Cell Mol. Biol. 2014, 50, 723–736. [Google Scholar] [CrossRef]
- Dalan, R.; Bornstein, S.R.; El-Armouche, A.; Rodionov, R.N.; Markov, A.; Wielockx, B.; Beuschlein, F.; Boehm, B.O. The ACE-2 in COVID-19: Foe or Friend? Horm. Metab. Res. 2020, 52, 257–263. [Google Scholar] [CrossRef]
- Uhal, B.D.; Dang, M.; Dang, V.; Llatos, R.; Cano, E.; Abdul-Hafez, A.; Markey, J.; Piasecki, C.C.; Molina-Molina, M. Cell Cycle Dependence of ACE-2 Explains Downregulation in Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2013, 42, 198–210. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Nemeth, Z.; Kiss, E.; Takacs, I. The Role of Epigenetic Regulator SIRT1 in Balancing the Homeostasis and Preventing the Formation of Specific “Soil” of Metabolic Disorders and Related Cancers. Front. Biosci. 2022, 27, 253. [Google Scholar] [CrossRef]
- Rossi, G.A.; Sacco, O.; Capizzi, A.; Mastromarino, P. Can Resveratrol-Inhaled Formulations Be Considered Potential Adjunct Treatments for COVID-19? Front. Immunol. 2021, 12, 670955. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting Enzyme 2 (ACE2), SARS-CoV-2 and the Pathophysiology of Coronavirus Disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Sun, Y.; Varambally, S.; Maher, C.A.; Cao, Q.; Chockley, P.; Toubai, T.; Malter, C.; Nieves, E.; Tawara, I.; Wang, Y.; et al. Targeting of MicroRNA-142-3p in Dendritic Cells Regulates Endotoxin-Induced Mortality. Blood 2011, 117, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Neilson, J.R.; Kumar, P.; Manocha, M.; Shankar, P.; Sharp, P.A.; Manjunath, N. MiRNA Profiling of Naïve, Effector and Memory CD8 T Cells. PLoS ONE 2007, 2, e1020. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA Profiling of Multiple Sclerosis Lesions Identifies Modulators of the Regulatory Protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Expression, Regulation and Function of MicroRNAs in Multiple Sclerosis. Int. J. Med. Sci. 2014, 11, 810–818. [Google Scholar] [CrossRef]
- Mandolesi, G.; De Vito, F.; Musella, A.; Gentile, A.; Bullitta, S.; Fresegna, D.; Sepman, H.; Di Sanza, C.; Haji, N.; Mori, F.; et al. MiR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation. J. Neurosci. 2017, 37, 546–561. [Google Scholar] [CrossRef] [PubMed]
- De Vito, F.; Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; Stampanoni Bassi, M.; Gilio, L.; Buttari, F.; Procaccini, C.; Colamatteo, A.; et al. MiR-142-3p Regulates Synaptopathy-driven Disease Progression in Multiple Sclerosis. Neuropathol. Appl. Neurobiol. 2022, 48, e12765. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Hoiland, R.L.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Confronting the Controversy: Interleukin-6 and the COVID-19 Cytokine Storm Syndrome. Eur. Respir. J. 2020, 56, 2003006. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G. Immune Response and Blood–Brain Barrier Dysfunction during Viral Neuroinvasion. Innate Immun. 2021, 27, 109–117. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Liang, X.; Liu, X.; Zhao, Y.; Ma, C.; Gao, L. Tim-4 in Health and Disease: Friend or Foe? Front. Immunol. 2020, 11, 537. [Google Scholar] [CrossRef]
- Santiago, C.; Ballesteros, A.; Martínez-Muñoz, L.; Mellado, M.; Kaplan, G.G.; Freeman, G.J.; Casasnovas, J.M. Structures of T Cell Immunoglobulin Mucin Protein 4 Show a Metal-Ion-Dependent Ligand Binding Site Where Phosphatidylserine Binds. Immunity 2007, 27, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, Endothelial Injury and Complement-Induced Coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.; Raju, S.; Wu, R.; Ching, C.; Veitch, S.; Rathnakumar, K.; Boudreau, E.; Howe, K.L.; Fish, J.E. Overcoming Barriers. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1818–1829. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 Trans-Signaling Induces Plasminogen Activator Inhibitor-1 from Vascular Endothelial Cells in Cytokine Release Syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism Risk of COVID-19 Is High and Associated with a Higher Risk of Mortality: A Systematic Review and Meta-Analysis. eClinicalMedicine 2020, 29–30, 100639. [Google Scholar] [CrossRef]
- Piazza, G.; Morrow, D.A. Diagnosis, Management, and Pathophysiology of Arterial and Venous Thrombosis in COVID-19. JAMA 2020, 324, 2548. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Quintili, A.L.; Karamchandani, K.; Bose, S. Thromboembolic Disease in COVID-19 Patients: A Brief Narrative Review. J. Intensive Care 2020, 8, 70. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA 2020, 324, 799. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients with COVID-19 in Wuhan, China. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Gjorgjieva, M.; Sobolewski, C.; Dolicka, D.; Correia de Sousa, M.; Foti, M. MiRNAs and NAFLD: From Pathophysiology to Therapy. Gut 2019, 68, 2065–2079. [Google Scholar] [CrossRef]
- Mosca, N.; Pezzullo, M.; De Leo, I.; Truda, A.; Marchese, G.; Russo, A.; Potenza, N. A Novel CeRNET Relying on the LncRNA JPX, MiR-378a-3p, and Its MRNA Targets in Lung Cancer. Cancers 2024, 16, 1526. [Google Scholar] [CrossRef] [PubMed]
- Moatar, A.I.; Chis, A.R.; Marian, C.; Sirbu, I.-O. Gene Network Analysis of the Transcriptome Impact of SARS-CoV-2 Interacting MicroRNAs in COVID-19 Disease. Int. J. Mol. Sci. 2022, 23, 9239. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Nayeem, M.; Vanderven, H.A.; Sarker, S. Role of MiRNA in Highly Pathogenic H5 Avian Influenza Virus Infection: An Emphasis on Cellular and Chicken Models. Viruses 2024, 16, 1102. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Sedykh, T.A.; Nevinsky, G.A. Natural Antibodies Produced in Vaccinated Patients and COVID-19 Convalescents Recognize and Hydrolyze Oligopeptides Corresponding to the S-Protein of SARS-CoV-2. Vaccines 2023, 11, 1494. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Sedykh, S.E.; Ermakov, E.A.; Matveev, A.L.; Odegova, E.I.; Sedykh, T.A.; Shcherbakov, D.N.; Merkuleva, I.A.; Volosnikova, E.A.; Nesmeyanova, V.S.; et al. Natural IgG against S-Protein and RBD of SARS-CoV-2 Do Not Bind and Hydrolyze DNA and Are Not Autoimmune. Int. J. Mol. Sci. 2022, 23, 13681. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Kutmon, M.; Ehrhart, F.; Willighagen, E.L.; Evelo, C.T.; Coort, S.L. CyTargetLinker App Update: A Flexible Solution for Network Extension in Cytoscape. F1000Research 2018, 7, 743. [Google Scholar] [CrossRef]
| Name | Nucleotide Sequence |
|---|---|
| let–7f–5p SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAACTAT–3′ |
| 146b–5p SL | 5′–GTTGGCTCTGGTGCGGGTCCGAGGTATTGCACCAAGAGCCAACCAGCCT–3′ |
| 148a–3p SL | 5′–GTTGGCTCTGGTGCGGGTCCGAGGTATTGCACCAAGAGCCAACACAAAG–3′ |
| 200c–3p SL | 5′–GTTGGCTCTGGTGCGGGTCCGAGGTATTGCACCAAGAGCCAACCTCCATC–3′ |
| 378a–3p SL | 5′–GTTGGCTCTGGTGCGGGTCCGAGGTATTGCACCAAGAGCCAACGCCTTC–3′ |
| 9–5p SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTCATAC–3′ |
| 766–3p SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACGCTGAG–3′ |
| 3125 SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTCTCTC–3′ |
| 4742–3p SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACCTGCAG–3′ |
| 142–3p SL | 5′–GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACTCCATA–3′ |
| Name | Nucleotide Sequence | Length in Nucleotides | Melting Point (°C) |
|---|---|---|---|
| let–7f–5p for | 5′–GTTTGGTGAGGTAGTAGATTGT–3′ | 22 | 51 |
| 146b–5p for | 5′–GTTTTTCGTGAGAACTGAATTCCAT–3′ | 25 | 52.8 |
| 148a–3p for | 5′–GTTTTGGTCAGTGCACTACAGAA–3′ | 23 | 53.5 |
| 200c–3p for | 5′–GTTTGGTAATACTGCCGGGTAAT–3′ | 23 | 53.5 |
| 378a–3p for | 5′–GTTTTTGACTGGACTTGGAGTCA–3′ | 23 | 53.5 |
| 9–5p for | 5′–GUGGAAGACUUCGAGGCCUUG–3′ | 22 | 56.3 |
| 766–3p for | 5′–GAGCUUGGGAUAGAGGGCUUA–3′ | 22 | 54.4 |
| 3125 for | 5′–GCCAGCUGGAAGUUGAGGAAG–3′ | 22 | 56.3 |
| 4742–3p for | 5′–GCUUAGCUCGUGGUCCCGGAC–3′ | 21 | 60.2 |
| 142–3p for | 5′–UGGAGGAAGAGGUGGAGGAAG–3′ | 21 | 56.3 |
| Un rev primer | 5′–GTGCAGGGTCCGAGGT–3′ | 16 | 51.1 |
| Flu-miR | Nucleotide Sequence | Length in Nucleotides |
|---|---|---|
| Flu-let–7f–5p | 5′–Flu–UGAGGUAGUAGAUUGUAUAGUU | 22 |
| Flu-146b–5p | 5′–Flu–UGAGAACUGAAUUCCAUAGCCUG | 23 |
| Flu-148a–3p | 5′–Flu–UCAGUGCACUACAGAACUUUGU | 22 |
| Flu-200c–3p | 5′–Flu–UAAUACUGCCGGGUAAUGAUGGA | 23 |
| Flu-378a–3p | 5′–Flu–ACUGGACUUGGAGUCAGAAGGC | 22 |
| Flu-9–5p | 5′–Flu–UCUUUGGUUAUCUAGCUGUAUGA | 23 |
| Flu-766–3p | 5′–Flu–ACUCCAGCCCCACAGCCUCAGC | 22 |
| Flu-3125 | 5′–Flu–UAGAGGAAGCUGUGGAGAGA | 20 |
| Flu-4742–3p | 5′–Flu–UCUGUAUUCUCCUUUGCCUGCAG | 23 |
| Flu-142–3p | 5′–Flu–UGUAGUGUUUCCUACUUUAUGGA | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.M.; Nikitin, A.O.; Nevinsky, G.A. Circulating miRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways. Non-Coding RNA 2024, 10, 48. https://doi.org/10.3390/ncrna10050048
Timofeeva AM, Nikitin AO, Nevinsky GA. Circulating miRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways. Non-Coding RNA. 2024; 10(5):48. https://doi.org/10.3390/ncrna10050048
Chicago/Turabian StyleTimofeeva, Anna M., Artem O. Nikitin, and Georgy A. Nevinsky. 2024. "Circulating miRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways" Non-Coding RNA 10, no. 5: 48. https://doi.org/10.3390/ncrna10050048








