The Role of Long Non-Coding RNA in the Pathogenesis of Psoriasis
Abstract
1. Introduction
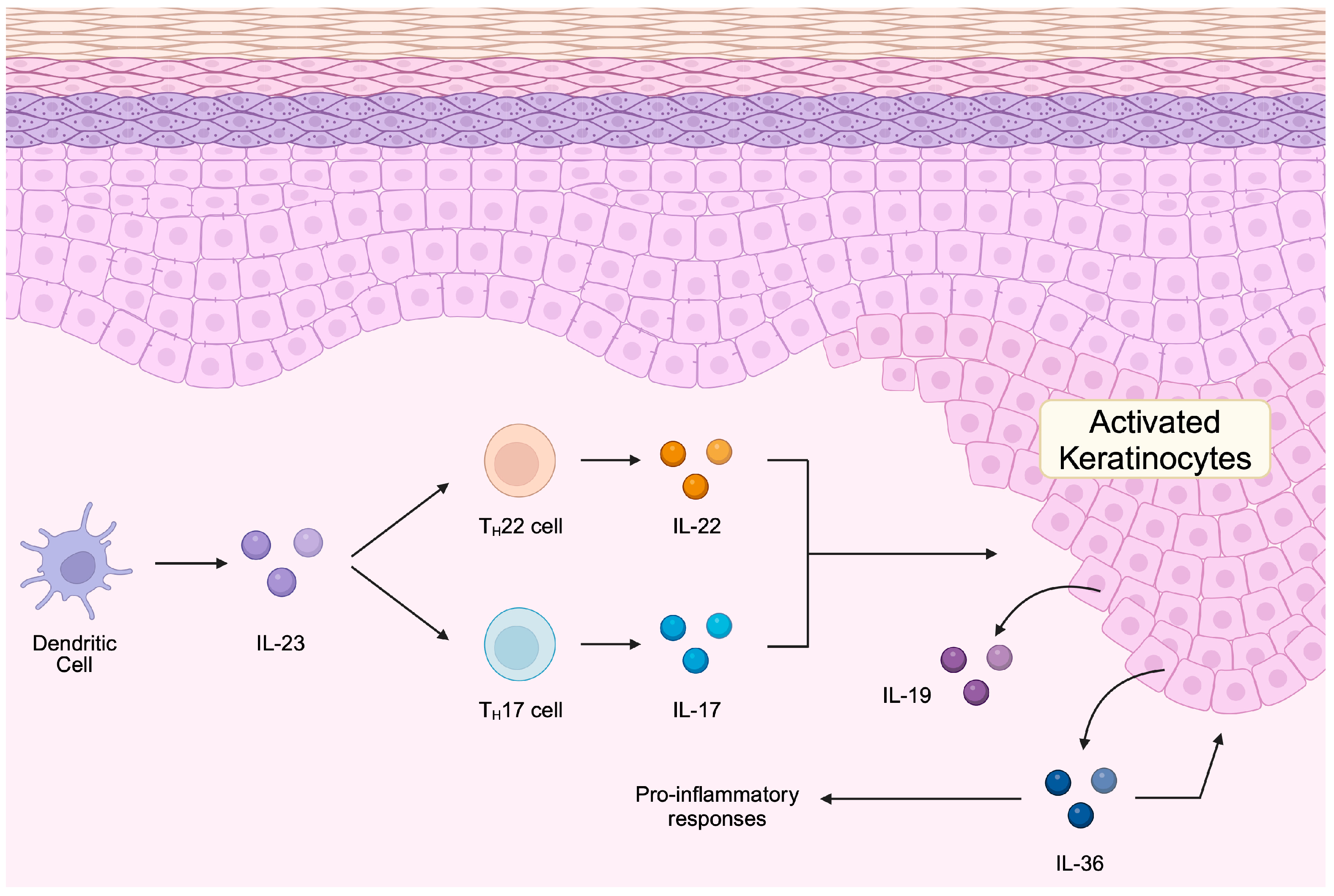
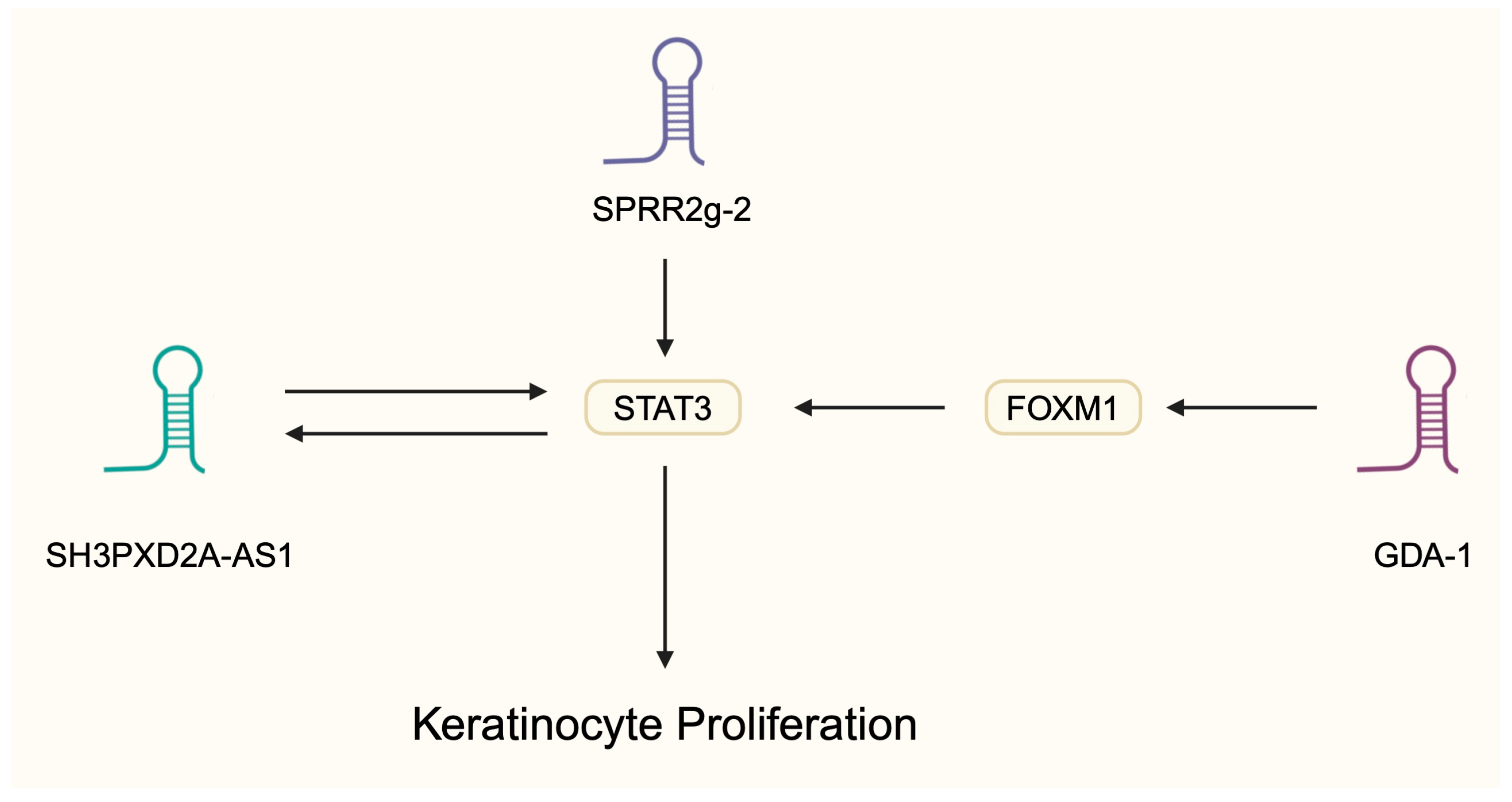
2. Long Non-Coding RNAs in the Pathogenesis of Psoriasis
3. Benefits and Limitations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The Role of Epigenetic Factors in Psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [Google Scholar] [CrossRef]
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M.; Atlas, G.P. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Rastogi, S.; Lin, J. Effect of Prior Biologic Use on Cost-Effectiveness of Brodalumab vs. Ustekinumab for Treatment of Moderate-to-Severe Psoriasis in the United States. Dermatol. Ther. 2018, 8, 441–453. [Google Scholar] [CrossRef]
- Ho, D.; Koo, E.; Mamalis, A.; Jagdeo, J. A Systematic Review of Light Emitting Diode (LED) Phototherapy for Treatment of Psoriasis: An Emerging Therapeutic Modality. J. Drugs Dermatol. 2017, 16, 482–488. [Google Scholar] [PubMed]
- Lee, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef] [PubMed]
- Koks, S.; Keermann, M.; Reimann, E.; Prans, E.; Abram, K.; Silm, H.; Koks, G.; Kingo, K. Psoriasis-Specific RNA Isoforms Identified by RNA-Seq Analysis of 173,446 Transcripts. Front. Med. 2016, 3, 46. [Google Scholar] [CrossRef]
- Song, J.K.; Yin, S.Y.; Li, W.; Li, X.D.; Luo, Y.; Xing, M.; Li, B.; Kuai, L. An update on the role of long non-coding RNAs in psoriasis. Chin. Med. J. 2020, 134, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, Q.; Gong, Y.; Liu, Z.; Xu, H.; Wang, Y.; Shi, Y. Construction of a lncRNA-miRNA-mRNA network to determine the regulatory roles of lncRNAs in psoriasis. Exp. Ther. Med. 2019, 18, 4011–4021. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018, 36, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Billi, A.C.; Gudjonsson, J.E.; Voorhees, J.J. Psoriasis: Past, Present, and Future. J. Investig. Dermatol. 2019, 139, e133–e142. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019, 33, 13241–13253. [Google Scholar] [CrossRef]
- Xu, X.; Prens, E.; Florencia, E.; Leenen, P.; Boon, L.; Asmawidjaja, P.; Mus, A.M.; Lubberts, E. Interleukin-17A Drives IL-19 and IL-24 Expression in Skin Stromal Cells Regulating Keratinocyte Proliferation. Front. Immunol. 2021, 12, 719562. [Google Scholar] [CrossRef] [PubMed]
- Sachen, K.L.; Arnold Greving, C.N.; Towne, J.E. Role of IL-36 cytokines in psoriasis and other inflammatory skin conditions. Cytokine 2022, 156, 155897. [Google Scholar] [CrossRef]
- Amin, M.; Lee, E.B.; Tsai, T.F.; Wu, J.J. Psoriasis and Co-morbidity. Acta Derm. Venereol. 2020, 100, adv00033. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Miao, X.; Wang, H.; Wang, Y.; Li, F.; Yang, Q.; Cui, R.; Li, B. Association of Serum Uric Acid Levels in Psoriasis: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e3676. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Guttman, M.; Rinn, J.L. Modular regulatory principles of large non-coding RNAs. Nature 2012, 482, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Szczerkowska-Dobosz, A.; Stawczyk-Macieja, M.; Owczarczyk-Saczonek, A.; Reich, A.; Bartosinska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; et al. Pathogenesis of psoriasis in the “omic” era. Part II. Genetic, genomic and epigenetic changes in psoriasis. Postepy Dermatol. Alergol. 2020, 37, 283–298. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Duan, Y.; Luo, Y.; Tang, S.; Wang, J. LncRNA MALAT-1 regulates the growth of interleukin-22-stimulated keratinocytes via the miR-330-5p/S100A7 axis. Autoimmunity 2022, 55, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Danis, J.; Göblös, A.; Bata-Csörgő, Z.; Kemény, L.; Széll, M. PRINS Non-Coding RNA Regulates Nucleic Acid-Induced Innate Immune Responses of Human Keratinocytes. Front. Immunol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Pan, H.F.; Leng, R.X.; Wang, D.G.; Li, X.P.; Li, X.M.; Ye, D.Q. Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun. Rev. 2015, 14, 798–805. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Iyer, M.K.; Stuart, P.E.; Swindell, W.R.; Gudjonsson, J.E.; Tejasvi, T.; Sarkar, M.K.; Li, B.; Ding, J.; Voorhees, J.J.; et al. Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol. 2015, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Stacey, V.M.; Koks, S. Genome-Wide Differential Transcription of Long Noncoding RNAs in Psoriatic Skin. Int. J. Mol. Sci. 2023, 24, 16344. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Luo, Y.; Hara, T.; Kawashima, A.; Ishido, Y.; Suzuki, S.; Ishii, N.; Kambara, T.; Suzuki, K. Pathological role of excessive DNA as a trigger of keratinocyte proliferation in psoriasis. Clin. Exp. Immunol. 2020, 202, 1–10. [Google Scholar] [CrossRef]
- Kong, S.M.; Sun, X.Y.; Cui, W.Y.; Cao, Y.C. Chemerin Exacerbates Psoriasis by Stimulating Keratinocyte Proliferation and Cytokine Production. Curr. Med. Sci. 2023, 43, 399–408. [Google Scholar] [CrossRef]
- Qiao, P.; Guo, W.; Ke, Y.; Fang, H.; Zhuang, Y.; Jiang, M.; Zhang, J.; Shen, S.; Qiao, H.; Dang, E.; et al. Mechanical Stretch Exacerbates Psoriasis by Stimulating Keratinocyte Proliferation and Cytokine Production. J. Investig. Dermatol. 2019, 139, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.C.; Aubert, A.; Turner, C.T.; Nabai, L.; Hiroyasu, S.; Pawluk, M.A.; Cederberg, R.A.; Zhao, H.; Jung, K.; Burleigh, A.; et al. Granzyme K mediates IL-23-dependent inflammation and keratinocyte proliferation in psoriasis. Front. Immunol. 2024, 15, 1398120. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, M.; Sun, Y.; Xie, M.; Le, K.; Xu, M.; Huang, C. The potential of Diosgenin in treating psoriasis: Studies from HaCaT keratinocytes and imiquimod-induced murine model. Life Sci. 2020, 241, 117115. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Fongsodsri, K.; Tuentam, K.; Reamtong, O.; Thiangtrongjit, T.; Kanjanapruthipong, T.; Yadavalli, V.K.; Ampawong, S. Sericin coated thin polymeric films reduce keratinocyte proliferation via the mTOR pathway and epidermal inflammation through IL17 signaling in psoriasis rat model. Sci. Rep. 2023, 13, 12133. [Google Scholar] [CrossRef]
- Tian, S.; Wang, C. An ensemble of the iCluster method to analyze longitudinal lncRNA expression data for psoriasis patients. Hum. Genomics 2021, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.Y.; Tawfik, N.Z.; Soliman, N.H.; Eldeen, L.A.T. The lncRNA PRINS-miRNA-mRNA Axis Gene Expression Profile as a Circulating Biomarker Panel in Psoriasis. Mol. Diagn. Ther. 2022, 26, 451–465. [Google Scholar] [CrossRef]
- Szegedi, K.; Göblös, A.; Bacsa, S.; Antal, M.; Németh, I.B.; Bata-Csörgő, Z.; Kemény, L.; Dobozy, A.; Széll, M. Expression and functional studies on the noncoding RNA, PRINS. Int. J. Mol. Sci. 2012, 14, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Bata-Csorgo, Z.; Pivarcsi, A.; Polyanka, H.; Kenderessy-Szabo, A.; Molnar, G.; Szentpali, K.; Bari, L.; Megyeri, K.; Mandi, Y.; et al. Identification and characterization of a novel, psoriasis susceptibility-related noncoding RNA gene, PRINS. J. Biol. Chem. 2005, 280, 24159–24167. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, K.; Sonkoly, E.; Nagy, N.; Nemeth, I.B.; Bata-Csorgo, Z.; Kemeny, L.; Dobozy, A.; Szell, M. The anti-apoptotic protein G1P3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS. Exp. Dermatol. 2010, 19, 269–278. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Beji, S.; Sileno, S.; Lulli, D.; Mercurio, L.; Madonna, S.; Cirielli, C.; Pallotta, S.; Albanesi, C.; Capogrossi, M.C.; et al. Extracellular Nucleophosmin Is Increased in Psoriasis and Correlates with the Determinants of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 867813. [Google Scholar] [CrossRef]
- Sun, D.S.; Guan, C.H.; Wang, W.N.; Hu, Z.T.; Zhao, Y.Q.; Jiang, X.M. LncRNA NORAD promotes proliferation, migration and angiogenesis of hepatocellular carcinoma cells through targeting miR-211-5p/FOXD1/VEGF-A axis. Microvasc. Res. 2021, 134, 104120. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Wang, J.; Zhu, X.; Chen, J.; Zhang, W.; Wang, C.; Jiang, L. LncRNA NORAD Mediates the Proliferation and Apoptosis of Diffuse Large-B-Cell Lymphoma via Regulation of miR-345-3p/TRAF6 Axis. Arch. Med. Res. 2022, 53, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Qiao, X.; Zheng, J. LncRNA NORAD regulates the mechanism of the miR-532-3p/Nectin-4 axis in pancreatic cancer cell proliferation and angiogenesis. Toxicol. Res. 2023, 12, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tian, H.; Gao, X. NORAD regulates proliferation and apoptosis in cardiomyocytes under high-glucose treatment through miRNA-150-5p/ZEB1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11259–11265. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, X.; Zhang, N.; Cao, R.; Zhao, L.; Li, X.; Zhang, J.; Yu, J. LncRNA NORAD engages in psoriasis by binding to miR-26a to regulate keratinocyte proliferation. Autoimmunity 2021, 54, 129–137. [Google Scholar] [CrossRef]
- Li, J.; Pang, D.; Zhou, L.; Ouyang, H.; Tian, Y.; Yu, H. miR-26a-5p inhibits the proliferation of psoriasis-like keratinocytes in vitro and in vivo by dual interference with the CDC6/CCNE1 axis. Aging 2024, 16, 4631–4653. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Kielbowski, K.; Rusinska, K.; Bakinowska, E.; Gromowska, E.; Pawlik, A. Targeting cyclin-dependent kinases in rheumatoid arthritis and psoriasis—A review of current evidence. Expert. Opin. Ther. Targets 2023, 27, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Karner, J.; Wawrzyniak, M.; Tankov, S.; Runnel, T.; Aints, A.; Kisand, K.; Altraja, A.; Kingo, K.; Akdis, C.A.; Akdis, M.; et al. Increased microRNA-323-3p in IL-22/IL-17-producing T cells and asthma: A role in the regulation of the TGF-beta pathway and IL-22 production. Allergy 2017, 72, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kielbowski, K.; Ptaszynski, K.; Wojcik, J.; Wojtys, M.E. The role of selected non-coding RNAs in the biology of non-small cell lung cancer. Adv. Med. Sci. 2023, 68, 121–137. [Google Scholar] [CrossRef]
- Elamir, A.M.; Shaker, O.G.; El-Komy, M.H.; Mahmoud Sharabi, M.; Aboraia, N.M. The role of LncRNA MALAT-1 and MiRNA-9 in Psoriasis. Biochem. Biophys. Rep. 2021, 26, 101030. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Aggarwal, D.; Spector, D.L. Long Non-Coding RNA: Functional Implications. Noncoding RNA 2020, 6, 22. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Gholipour, M.; Abak, A.; Hussen, B.M.; Kholghi Oskooei, V.; Taheri, M.; Rakhshan, A. Association analysis of MALAT1 polymorphisms and risk of psoriasis among Iranian patients. Int. J. Immunogenet. 2022, 49, 83–87. [Google Scholar] [CrossRef]
- Masoumi, F.; Ghorbani, S.; Talebi, F.; Branton, W.G.; Rajaei, S.; Power, C.; Noorbakhsh, F. Malat1 long noncoding RNA regulates inflammation and leukocyte differentiation in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2019, 328, 50–59. [Google Scholar] [CrossRef]
- Xue, Y.; Ke, J.; Zhou, X.; Chen, Q.; Chen, M.; Huang, T.; Lin, F.; Chen, F. Knockdown of LncRNA MALAT1 Alleviates Coxsackievirus B3-Induced Acute Viral Myocarditis in Mice via Inhibiting Th17 Cells Differentiation. Inflammation 2022, 45, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Z.; Wang, C.; Huang, P.; Luo, M.; Zhou, R. STAT3/SH3PXD2A-AS1/miR-125b/STAT3 positive feedback loop affects psoriasis pathogenesis via regulating human keratinocyte proliferation. Cytokine 2021, 144, 155535. [Google Scholar] [CrossRef]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, F.; Ju, J.; Yin, X.; Yang, Z.; Li, Z.; Sun, Q. Long Non-Coding RNA-GDA-1 Promotes Keratinocyte Proliferation and Psoriasis Inflammation by Regulating the STAT3/NF-kappaB Signaling Pathway via Forkhead Box M1. Inflammation 2023, 46, 1209–1220. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Sun, T.; Zhang, Z.; Liu, C. FOXM1: Functional Roles of FOXM1 in Non-Malignant Diseases. Biomolecules 2023, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Li, X.; Huang, S.; Wang, R.; Yang, L.; Huang, Y.; Yan, J.; Ju, J.; Wen, H.; Sun, Q. LncRNA lnc-SPRR2G-2 contributes to keratinocyte hyperproliferation and inflammation in psoriasis by activating the STAT3 pathway and downregulating KHSRP. Mol. Cell Probes 2024, 76, 101967. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, L.; Lu, X.; Du, J.; Xu, J. LncRNA AGXT2L1-2:2 facilitates keratinocytes proliferation and inhibits apoptosis by interacting with estrogen-related receptor alpha in psoriasis. Mol. Cell Probes 2022, 62, 101803. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zeng, L.; Ou, J.; Wang, T.; Chen, Y.; Nandakumar, K.S. Estrogen Acts Through Estrogen Receptor-beta to Promote Mannan-Induced Psoriasis-Like Skin Inflammation. Front. Immunol. 2022, 13, 818173. [Google Scholar] [CrossRef]
- Luo, L.; Pasquali, L.; Srivastava, A.; Freisenhausen, J.C.; Pivarcsi, A.; Sonkoly, E. The Long Noncoding RNA LINC00958 Is Induced in Psoriasis Epidermis and Modulates Epidermal Proliferation. J. Investig. Dermatol. 2023, 143, 999–1010. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, L.; Jiang, L.; Zeng, H.; Zhang, Y.; Fu, C.; Guo, H.; Dong, Y.; Ouyang, Y.; Zhang, X.; et al. LncRNA UCA1 promotes keratinocyte-driven inflammation via suppressing METTL14 and activating the HIF-1alpha/NF-kappaB axis in psoriasis. Cell Death Dis. 2023, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Zhang, K.; Lu, W.J.; Xu, G.W.; Zhang, J.F.; Tang, Z.L. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Mol. Cell Biol. 2019, 20, 46. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, K.; Lv, S.C.; Xu, G.W.; Zhang, J.F.; Jia, H.Y. LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway to enhance autophagy and inhibit inflammation in TNF-alpha-treated keratinocytes and psoriatic mice. Cytokine 2021, 148, 155657. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Banang-Mbeumi, S.; Boateng, S.T.; Ruiz, E.M.; Chamcheu, R.N.; Kang, L.; King, J.A.; Walker, A.L.; Nagalo, B.M.; Kousoulas, K.G.; et al. Dual targeting of mTOR/IL-17A and autophagy by fisetin alleviates psoriasis-like skin inflammation. Front. Immunol. 2022, 13, 1075804. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Kuma, A.; Kobayashi, Y.; Yamamoto, A.; Matsubae, M.; Takao, T.; Natsume, T.; Ohsumi, Y.; Yoshimori, T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J. Cell Sci. 2003, 116, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zhang, K. The link between autophagy and psoriasis. Acta Histochem. 2024, 126, 152166. [Google Scholar] [CrossRef]
- Douroudis, K.; Kingo, K.; Traks, T.; Reimann, E.; Raud, K.; Rätsep, R.; Mössner, R.; Silm, H.; Vasar, E.; Kõks, S. Polymorphisms in the ATG16L1 gene are associated with psoriasis vulgaris. Acta Derm. Venereol. 2012, 92, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, D.; Subtil, A. ATG16L1 functions in cell homeostasis beyond autophagy. FEBS J. 2022, 289, 1779–1800. [Google Scholar] [CrossRef]
- He, R.; Wu, S.; Gao, R.; Chen, J.; Peng, Q.; Hu, H.; Zhu, L.; Du, Y.; Sun, W.; Ma, X.; et al. Identification of a Long Noncoding RNA TRAF3IP2-AS1 as Key Regulator of IL-17 Signaling through the SRSF10-IRF1-Act1 Axis in Autoimmune Diseases. J. Immunol. 2021, 206, 2353–2365. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, H.; Mai, C.; Lin, H.; Shen, J.; Zhang, X.; Gao, Y.; Mao, Y.; Xie, X. LncRNA-MIAT-Mediated miR-214-3p Silencing Is Responsible for IL-17 Production and Cardiac Fibrosis in Diabetic Cardiomyopathy. Front. Cell Dev. Biol. 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, J.; Zhou, Y.; Zhao, Y.; Hang, D.; Cao, Y. LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci. Rep. 2019, 39, BSR20182454. [Google Scholar] [CrossRef]
- He, H.; Qiu, X.; Qi, M.; Bajinka, O.; Qin, L.; Tan, Y. lncRNA STAT4-AS1 Inhibited TH17 Cell Differentiation by Targeting RORgammat Protein. J. Immunol. Res. 2022, 2022, 8307280. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, J.; Sun, W.; Zhu, J.; Lu, S.; Feng, Y.; Song, Z.; Yang, Y.; Wu, X. The LINC01176-miR-218-5p-IL-36G Network is Responsible for the Pathogenesis of Psoriasis by Promoting Inflammation. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Na, M.; Yao, X.; Li, C.; Li, L.; Yang, G.; Li, Y.; Hu, Y. Integrative single-cell transcriptomic investigation unveils long non-coding RNAs associated with localized cellular inflammation in psoriasis. Front. Immunol. 2023, 14, 1265517. [Google Scholar] [CrossRef] [PubMed]
- Fierro, C.; Gatti, V.; La Banca, V.; De Domenico, S.; Scalera, S.; Corleone, G.; Fanciulli, M.; De Nicola, F.; Mauriello, A.; Montanaro, M.; et al. The long non-coding RNA NEAT1 is a ΔNp63 target gene modulating epidermal differentiation. Nat. Commun. 2023, 14, 3795. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, S.; Zou, G.; Ding, X. Paeoniflorin inhibits proliferation and migration of psoriatic keratinocytes via the lncRNA NEAT1/miR-3194-5p/Galectin-7 axis. Anticancer. Drugs 2022, 33, e423–e433. [Google Scholar] [CrossRef]
- Chen, H.L.; Lo, C.H.; Huang, C.C.; Lu, M.P.; Hu, P.Y.; Chen, C.S.; Chueh, D.Y.; Chen, P.; Lin, T.N.; Lo, Y.H.; et al. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J. Clin. Investig. 2021, 131, e130740. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, A.; Zarrinpour, N.; Moradi, A.; Ahadi, M.; Omrani, M.D.; Ghafouri-Fard, S.; Taheri, M. Genetic variants within ANRIL (antisense non coding RNA in the INK4 locus) are associated with risk of psoriasis. Int. Immunopharmacol. 2020, 78, 106053. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hao, S.; Xue, T.; Zhou, K.; Zhang, Y.; Li, H. Association of HOTAIR Polymorphisms with Susceptibility to Psoriasis in a Chinese Han Population. Biomed. Res. Int. 2021, 2021, 5522075. [Google Scholar] [CrossRef]
- Ziegler, C.; Graf, J.; Faderl, S.; Schedlbauer, J.; Strieder, N.; Förstl, B.; Spang, R.; Bruckmann, A.; Merkl, R.; Hombach, S.; et al. The long non-coding RNA LINC00941 and SPRR5 are novel regulators of human epidermal homeostasis. EMBO Rep. 2019, 20, e46612. [Google Scholar] [CrossRef]
- Yin, X.; Yang, Z.; Zhu, M.; Chen, C.; Huang, S.; Li, X.; Zhong, H.; Wen, H.; Sun, Q.; Yu, X.; et al. ILF2 Contributes to Hyperproliferation of Keratinocytes and Skin Inflammation in a KLHDC7B-DT-Dependent Manner in Psoriasis. Front. Genet. 2022, 13, 890624. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, F.; Hua, M.; Guo, J.; Nong, Y.; Tang, Q.; Zhong, F.; Qin, L. Knockdown of lncRNA MIR31HG inhibits cell proliferation in human HaCaT keratinocytes. Biol. Res. 2018, 51, 30. [Google Scholar] [CrossRef]
- He, Y.; Yin, X.; Yan, J.; Li, X.; Sun, Q. The lncRNA H19/miR-766-3p/S1PR3 Axis Contributes to the Hyperproliferation of Keratinocytes and Skin Inflammation in Psoriasis via the AKT/mTOR Pathway. Mediators Inflamm. 2021, 2021, 9991175. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Shehata, W.; Maraee, A.; Abd El Monem Ellaithy, M.; Tayel, N.; Abo-Ghazala, A.; Mohammed El-Hefnawy, S. Circulating long noncoding RNA growth arrest-specific transcript 5 as a diagnostic marker and indicator of degree of severity in plaque psoriasis. Int. J. Dermatol. 2021, 60, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Wen, G.D.; Yu, C.; Zhao, Z.; Gao, N.; Liu, Z.Y. LncRNA UCA1 negatively regulates NF-kB activity in psoriatic keratinocytes through the miR125a-A20 axis. Kaohsiung J. Med. Sci. 2021, 37, 172–180. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, G.; Wang, M.; Chen, C.; Zhang, M.; Liu, M.; Shao, Y.; Zheng, Y. LncRNA RP6-65G23.1 accelerates proliferation and inhibits apoptosis via p-ERK1/2/p-AKT signaling pathway on keratinocytes. J. Cell Biochem. 2020, 121, 4580–4589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Piipponen, M.; Liu, Z.; Li, D.; Bian, X.; Niu, G.; Geara, J.; Toma, M.A.; Sommar, P.; Xu Landen, N. Human skin specific long noncoding RNA HOXC13-AS regulates epidermal differentiation by interfering with Golgi-ER retrograde transport. Cell Death Differ. 2023, 30, 1334–1348. [Google Scholar] [CrossRef]
- Camina-Conforto, G.; Mateu-Arrom, L.; Lopez-Ferrer, A.; Puig, L. Bimekizumab in the Treatment of Plaque Psoriasis: Focus on Patient Selection and Perspectives. Patient Prefer. Adherence 2023, 17, 1541–1549. [Google Scholar] [CrossRef]
- Hoy, S.M. Deucravacitinib: First Approval. Drugs 2022, 82, 1671–1679. [Google Scholar] [CrossRef]
- Bernardo, D.; Thaci, D.; Torres, T. Spesolimab for the Treatment of Generalized Pustular Psoriasis. Drugs 2024, 84, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Dolcino, M.; Pelosi, A.; Fiore, P.F.; Patuzzo, G.; Tinazzi, E.; Lunardi, C.; Puccetti, A. Long Non-Coding RNAs Play a Role in the Pathogenesis of Psoriatic Arthritis by Regulating MicroRNAs and Genes Involved in Inflammation and Metabolic Syndrome. Front. Immunol. 2018, 9, 1533. [Google Scholar] [CrossRef] [PubMed]

| Material | lncRNA | Expression/Levels | Reference |
|---|---|---|---|
| Blood, skin biopsy | PRINS | Blood: downregulated Skin: upregulated | [34] |
| Blood | ANRIL | Up | [79] |
| Blood, skin biopsy | HOTAIR | Up | [80] |
| Skin biopsy | LINC00941 | Down | [81] |
| Skin biopsy | LINC00958 | Up | [61] |
| Skin biopsy | MEG3 | Down | [63] |
| Skin biopsy | KLHDC7B-DT | Up | [82] |
| Skin biopsy | MALAT-1 | Up | [49] |
| Skin biopsy | MIR31HG | Up | [83] |
| Skin biopsy | H19 | Down | [84] |
| Skin biopsy | GAS5 | Up | [85] |
| Skin biopsy | UCA1 | Down | [86] |
| Skin biopsy | RP6-65G23.1 | Up | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbowski, K.; Jędrasiak, A.; Bakinowska, E.; Pawlik, A. The Role of Long Non-Coding RNA in the Pathogenesis of Psoriasis. Non-Coding RNA 2025, 11, 7. https://doi.org/10.3390/ncrna11010007
Kiełbowski K, Jędrasiak A, Bakinowska E, Pawlik A. The Role of Long Non-Coding RNA in the Pathogenesis of Psoriasis. Non-Coding RNA. 2025; 11(1):7. https://doi.org/10.3390/ncrna11010007
Chicago/Turabian StyleKiełbowski, Kajetan, Anna Jędrasiak, Estera Bakinowska, and Andrzej Pawlik. 2025. "The Role of Long Non-Coding RNA in the Pathogenesis of Psoriasis" Non-Coding RNA 11, no. 1: 7. https://doi.org/10.3390/ncrna11010007
APA StyleKiełbowski, K., Jędrasiak, A., Bakinowska, E., & Pawlik, A. (2025). The Role of Long Non-Coding RNA in the Pathogenesis of Psoriasis. Non-Coding RNA, 11(1), 7. https://doi.org/10.3390/ncrna11010007






