RNA Surveillance by the Nuclear RNA Exosome: Mechanisms and Significance
Abstract
:1. Introduction
2. The Nuclear RNA Exosome: Structure and RNA Degradation Mechanisms
3. Molecular Apparatus for RNA Targeting of the Exosome in Yeasts and Humans
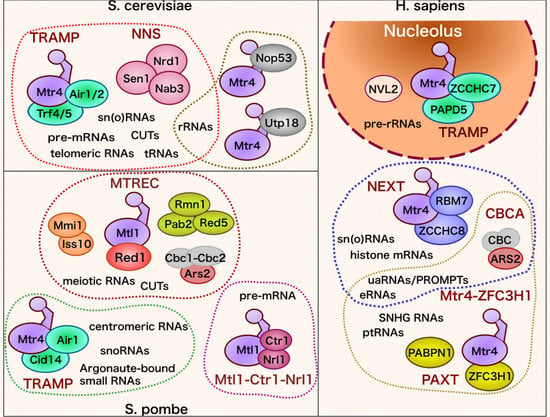
3.1. Saccharomyces cerevisiae
3.2. Schizosaccharomyces pombe
3.3. Homo sapiens
4. Significance of the Nuclear RNA Exosome in Mammalian Biological Processes
4.1. DNA Damage Response
4.2. R-Loop Resolution and Genomic Integrity
4.3. RNA Export and Translation
4.4. Stem Cell Self-Renewal and Differentiation
4.5. Influenza A Virus (IAV) Ribogenesis and Infectivity
5. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Mitchell, P. Exosome substrate targeting: The long and short of it. Biochem. Soc. Trans. 2014, 42, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Kilchert, C.; Wittmann, S.; Vasiljeva, L. The regulation and functions of the nuclear RNA exosome complex. Nat. Rev. Mol. Cell Biol. 2016, 17, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zinder, J.C.; Lima, C.D. Targeting RNA for processing or destruction by the eukaryotic RNA exosome and its cofactors. Genes Dev. 2017, 31, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hilleren, P.; McCarthy, T.; Rosbash, M.; Parker, R.; Jensen, T.H. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 2001, 413, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Milligan, L.; Torchet, C.; Allmang, C.; Shipman, T.; Tollervey, D. A nuclear surveillance pathway for mRNAs with defective polyadenylation. Mol. Cell. Biol. 2005, 25, 9996–10004. [Google Scholar] [CrossRef] [PubMed]
- Torchet, C.; Bousquet-Antonelli, C.; Milligan, L.; Thompson, E.; Kufel, J.; Tollervey, D. Processing of 3′-extended read-through transcripts by the exosome can generate functional mRNAs. Mol. Cell 2002, 9, 1285–1296. [Google Scholar] [CrossRef]
- Kazerouninia, A.; Ngo, B.; Martinson, H.G. Poly(A) signal-dependent degradation of unprocessed nascent transcripts accompanies poly(A) signal-dependent transcriptional pausing in vitro. RNA 2010, 16, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Di Giammartino, D.C.; Li, W.; Ogami, K.; Yashinskie, J.J.; Hoque, M.; Tian, B.; Manley, J.L. RBBP6 isoforms regulate the human polyadenylation machinery and modulate expression of mRNAs with AU-rich 3′ UTRs. Genes Dev. 2014, 28, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, C.; Marguerat, S.; Lafontaine, J.; Barbezier, N.; Bahler, J.; Bachand, F. A pre-mRNA degradation pathway that selectively targets intron-containing genes requires the nuclear poly(A)-binding protein. Mol. Cell 2011, 44, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bousquet-Antonelli, C.; Presutti, C.; Tollervey, D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell 2000, 102, 765–775. [Google Scholar] [CrossRef]
- Gudipati, R.K.; Xu, Z.; Lebreton, A.; Seraphin, B.; Steinmetz, L.M.; Jacquier, A.; Libri, D. Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol. Cell 2012, 48, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Kudla, G.; Wlotzka, W.; Tuck, A.; Tollervey, D. Transcriptome-wide analysis of exosome targets. Mol. Cell 2012, 48, 422–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, S.; Gromak, N.; Norbury, C.J.; Proudfoot, N.J. Adenylation and exosome-mediated degradation of cotranscriptionally cleaved pre-messenger RNA in human cells. Mol. Cell 2006, 21, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wyers, F.; Rougemaille, M.; Badis, G.; Rousselle, J.C.; Dufour, M.E.; Boulay, J.; Regnault, B.; Devaux, F.; Namane, A.; Seraphin, B.; et al. Cryptic Pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 2005, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Neil, H.; Malabat, C.; d’Aubenton-Carafa, Y.; Xu, Z.; Steinmetz, L.M.; Jacquier, A. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 2009, 457, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wei, W.; Gagneur, J.; Perocchi, F.; Clauder-Munster, S.; Camblong, J.; Guffanti, E.; Stutz, F.; Huber, W.; Steinmetz, L.M. Bidirectional promoters generate pervasive transcription in yeast. Nature 2009, 457, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Preker, P.; Nielsen, J.; Kammler, S.; Lykke-Andersen, S.; Christensen, M.S.; Mapendano, C.K.; Schierup, M.H.; Jensen, T.H. RNA exosome depletion reveals transcription upstream of active human promoters. Science 2008, 322, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Almada, A.E.; Zamudio, J.R.; Sharp, P.A. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc. Natl. Acad. Sci. USA 2011, 108, 10460–10465. [Google Scholar] [CrossRef] [PubMed]
- Szczepinska, T.; Kalisiak, K.; Tomecki, R.; Labno, A.; Borowski, L.S.; Kulinski, T.M.; Adamska, D.; Kosinska, J.; Dziembowski, A. DIS3 shapes the RNA polymerase II transcriptome in humans by degrading a variety of unwanted transcripts. Genome Res. 2015, 25, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Ogami, K.; Richard, P.; Chen, Y.; Hoque, M.; Li, W.; Moresco, J.J.; Yates, J.R., 3rd; Tian, B.; Manley, J.L. An Mtr4/ZFC3H1 complex facilitates turnover of unstable nuclear RNAs to prevent their cytoplasmic transport and global translational repression. Genes Dev. 2017, 31, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Henriques, T.; Gilchrist, D.A.; Nechaev, S.; Bern, M.; Muse, G.W.; Burkholder, A.; Fargo, D.C.; Adelman, K. Stable pausing by RNA polymerase II provides an opportunity to target and integrate regulatory signals. Mol. Cell 2013, 52, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Gebhard, C.; Miguel-Escalada, I.; Hoof, I.; Bornholdt, J.; Boyd, M.; Chen, Y.; Zhao, X.; Schmidl, C.; Suzuki, T.; et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Schlackow, M.; Nojima, T.; Gomes, T.; Dhir, A.; Carmo-Fonseca, M.; Proudfoot, N.J. Distinctive patterns of transcription and RNA processing for human lincRNAs. Mol. Cell 2017, 65, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Schwalb, B.; Michel, M.; Zacher, B.; Fruhauf, K.; Demel, C.; Tresch, A.; Gagneur, J.; Cramer, P. TT-seq maps the human transient transcriptome. Science 2016, 352, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Makino, D.L.; Conti, E. Structure determination of an 11-subunit exosome in complex with RNA by molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Januszyk, K.; Lima, C.D. Structure of an Rrp6-RNA exosome complex bound to poly(A) RNA. Nature 2014, 511, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Makino, D.L.; Schuch, B.; Stegmann, E.; Baumgartner, M.; Basquin, C.; Conti, E. RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 2015, 524, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, A.; Tomecki, R.; Dziembowski, A.; Seraphin, B. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 2008, 456, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.; Tsanova, B.; Barbas, A.; Reis, F.P.; Dastidar, E.G.; Sanchez-Rotunno, M.; Arraiano, C.M.; van Hoof, A. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat. Struct. Mol. Biol. 2009, 16, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Leung, E.; Brown, J.; Tollervey, D. The N-terminal pin domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res. 2009, 37, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Lima, C.D. The Rrp6 C-terminal domain binds RNA and activates the nuclear RNA exosome. Nucleic Acids Res. 2017, 45, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Allmang, C.; Petfalski, E.; Podtelejnikov, A.; Mann, M.; Tollervey, D.; Mitchell, P. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev. 1999, 13, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Tomecki, R.; Kristiansen, M.S.; Lykke-Andersen, S.; Chlebowski, A.; Larsen, K.M.; Szczesny, R.J.; Drazkowska, K.; Pastula, A.; Andersen, J.S.; Stepien, P.P.; et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 2010, 29, 2342–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staals, R.H.; Bronkhorst, A.W.; Schilders, G.; Slomovic, S.; Schuster, G.; Heck, A.J.; Raijmakers, R.; Pruijn, G.J. Dis3-like 1: A novel exoribonuclease associated with the human exosome. EMBO J. 2010, 29, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Fukushima, K.; Suzuki, N.; Nakashima, N.; Noguchi, E.; Nishimoto, T. Human Dis3p, which binds to either GTP- or GDP-Ran, complements Saccharomyces cerevisiae dis3. J. Biochem. 1998, 123, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Schuch, B.; Feigenbutz, M.; Makino, D.L.; Falk, S.; Basquin, C.; Mitchell, P.; Conti, E. The exosome-binding factors Rrp6 and Rrp47 form a composite surface for recruiting the Mtr4 helicase. EMBO J. 2014, 33, 2829–2846. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Bonneau, F.; Ebert, J.; Kogel, A.; Conti, E. Mpp6 incorporation in the nuclear exosome contributes to RNA channeling through the Mtr4 helicase. Cell Rep. 2017, 20, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Zinder, J.C.; Zattas, D.; Das, M.; Lima, C.D. Structure and reconstitution of yeast Mpp6-nuclear exosome complexes reveals that Mpp6 stimulates RNA decay and recruits the Mtr4 helicase. eLife 2017, 6, e29062. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Tollervey, D. Threading the barrel of the RNA exosome. Trends Biochem. Sci. 2013, 38, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Lima, C.D. Structure and activities of the eukaryotic RNA exosome. Enzymes 2012, 31, 53–75. [Google Scholar] [PubMed]
- Zinder, J.C.; Wasmuth, E.V.; Lima, C.D. Nuclear RNA exosome at 3.1 Å reveals substrate specificities, RNA paths, and allosteric inhibition of Rrp44/Dis3. Mol. Cell 2016, 64, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Bratkowski, M.A.; Liu, X.; Niu, C.Y.; Ke, A.; Wang, H.W. Visualization of distinct substrate-recruitment pathways in the yeast exosome by electron microscopy. Nat. Struct. Mol. Biol. 2014, 21, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, F.; Basquin, J.; Ebert, J.; Lorentzen, E.; Conti, E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell 2009, 139, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Malet, H.; Topf, M.; Clare, D.K.; Ebert, J.; Bonneau, F.; Basquin, J.; Drazkowska, K.; Tomecki, R.; Dziembowski, A.; Conti, E.; et al. RNA channelling by the eukaryotic exosome. EMBO Rep. 2010, 11, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Wang, J.; Ding, F.; Callahan, K.; Bratkowski, M.A.; Butler, J.S.; Nogales, E.; Ke, A. Architecture of the yeast Rrp44 exosome complex suggests routes of RNA recruitment for 3′ end processing. Proc. Natl. Acad. Sci. USA 2007, 104, 16844–16849. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; van Hoof, A. The RNA exosome channeling and direct access conformations have distinct in vivo functions. Cell Rep. 2016, 16, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Delan-Forino, C.; Schneider, C.; Tollervey, D. Transcriptome-wide analysis of alternative routes for RNA substrates into the exosome complex. PLoS Genet. 2017, 13, e1006699. [Google Scholar] [CrossRef] [PubMed]
- Delan-Forino, C.; Schneider, C.; Tollervey, D. RNA substrate length as an indicator of exosome interactions in vivo. Wellcome Open Res. 2017, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Meola, N.; Domanski, M.; Karadoulama, E.; Chen, Y.; Gentil, C.; Pultz, D.; Vitting-Seerup, K.; Lykke-Andersen, S.; Andersen, J.S.; Sandelin, A.; et al. Identification of a nuclear exosome decay pathway for processed transcripts. Mol. Cell 2016, 64, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, S.; Krueger, A.; Trice, T.; Krecic, A.M.; Hinnebusch, A.G.; Anderson, J. Nuclear surveillance and degradation of hypomodified initiator tRNAmet in S. cerevisiae. Genes Dev. 2004, 18, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- LaCava, J.; Houseley, J.; Saveanu, C.; Petfalski, E.; Thompson, E.; Jacquier, A.; Tollervey, D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 2005, 121, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Vanacova, S.; Wolf, J.; Martin, G.; Blank, D.; Dettwiler, S.; Friedlein, A.; Langen, H.; Keith, G.; Keller, W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005, 3, e189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houseley, J.; Tollervey, D. Yeast Trf5p is a nuclear poly(A) polymerase. EMBO Rep. 2006, 7, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hamill, S.; Wolin, S.L.; Reinisch, K.M. Structure and function of the polymerase core of TRAMP, a RNA surveillance complex. Proc. Natl. Acad. Sci. USA 2010, 107, 15045–15050. [Google Scholar] [CrossRef] [PubMed]
- Holub, P.; Lalakova, J.; Cerna, H.; Pasulka, J.; Sarazova, M.; Hrazdilova, K.; Arce, M.S.; Hobor, F.; Stefl, R.; Vanacova, S. Air2p is critical for the assembly and RNA-binding of the TRAMP complex and the KOW domain of Mtr4p is crucial for exosome activation. Nucleic Acids Res. 2012, 40, 5679–5693. [Google Scholar] [CrossRef] [PubMed]
- Fasken, M.B.; Leung, S.W.; Banerjee, A.; Kodani, M.O.; Chavez, R.; Bowman, E.A.; Purohit, M.K.; Rubinson, M.E.; Rubinson, E.H.; Corbett, A.H. Air1 zinc knuckles 4 and 5 and a conserved IWRXY motif are critical for the function and integrity of the Trf4/5-Air1/2-Mtr4 polyadenylation (TRAMP) RNA quality control complex. J. Biol. Chem. 2011, 286, 37429–37445. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, H.; Jankowsky, E.; Anderson, J.T. Degradation of hypomodified tRNAiMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA 2008, 14, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Anderson, J.T.; Tollervey, D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol. Cell 2007, 27, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wlotzka, W.; Kudla, G.; Granneman, S.; Tollervey, D. The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J. 2011, 30, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, S.; Wang, X.; Anderson, J.T. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 2006, 12, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Dez, C.; Houseley, J.; Tollervey, D. Surveillance of nuclear-restricted pre-ribosomes within a subnucleolar region of Saccharomyces cerevisiae. EMBO J. 2006, 25, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Grzechnik, P.; Kufel, J. Polyadenylation linked to transcription termination directs the processing of snoRNA precursors in yeast. Mol. Cell 2008, 32, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Losh, J.S.; King, A.K.; Bakelar, J.; Taylor, L.; Loomis, J.; Rosenzweig, J.A.; Johnson, S.J.; van Hoof, A. Interaction between the RNA-dependent ATPase and poly(A) polymerase subunits of the TRAMP complex is mediated by short peptides and important for snoRNA processing. Nucleic Acids Res. 2015, 43, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Houseley, J.; Kotovic, K.; El Hage, A.; Tollervey, D. Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J. 2007, 26, 4996–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciais, D.; Bohnsack, M.T.; Tollervey, D. The mRNA encoding the yeast ARE-binding protein Cth2 is generated by a novel 3′ processing pathway. Nucleic Acids Res. 2008, 36, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.M.; Byam, J.; Fang, F.; Butler, J.S. Regulation of Nab2 mRNA 3′-end formation requires the core exosome and the Trf4p component of the TRAMP complex. RNA 2009, 15, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.; Tuck, A.; Staneva, D.; Tollervey, D. Nuclear RNA decay pathways AID rapid remodeling of gene expression in yeast. Mol. Cell 2017, 65, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Patterson, D.N.; Wilson, G.M.; Toth, E.A. Characterization of the essential activities of Saccharomyces cerevisiae Mtr4p, a 3′→5′ helicase partner of the nuclear exosome. J. Biol. Chem. 2008, 283, 4930–4942. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Liu, F.; Guenther, U.P.; Srinivasan, S.; Anderson, J.T.; Jankowsky, E. The RNA helicase Mtr4p modulates polyadenylation in the TRAMP complex. Cell 2011, 145, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.R.; Bonneau, F.; Hentschel, J.; Conti, E. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc. Natl. Acad. Sci. USA 2010, 107, 12139–12144. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Anderson, J.T.; Jankowsky, E. RNA unwinding by the Trf4/Air2/Mtr4 polyadenylation (TRAMP) complex. Proc. Natl. Acad. Sci. USA 2012, 109, 7292–7297. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Weir, J.R.; Hentschel, J.; Reichelt, P.; Bonneau, F.; Conti, E. The molecular architecture of the TRAMP complex reveals the organization and interplay of its two catalytic activities. Mol. Cell 2014, 55, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.M.; Srinivasan, S.; Jankowsky, E.; Comstock, M.J. The RNA helicase Mtr4p is a duplex-sensing translocase. Nat. Chem. Biol. 2017, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, L.; Buratowski, S. Nrd1 interacts with the nuclear exosome for 3′ processing of RNA polymerase II transcripts. Mol. Cell 2006, 21, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Porrua, O.; Hobor, F.; Boulay, J.; Kubicek, K.; D’Aubenton-Carafa, Y.; Gudipati, R.K.; Stefl, R.; Libri, D. In vivo SELEX reveals novel sequence and structural determinants of Nrd1-Nab3-Sen1-dependent transcription termination. EMBO J. 2012, 31, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.J.; Darby, M.M.; Jamonnak, N.; Schaughency, P.; Hao, H.; Wheelan, S.J.; Corden, J.L. Transcriptome-wide binding sites for components of the Saccharomyces cerevisiae non-poly(A) termination pathway: Nrd1, Nab3, and Sen1. PLoS Genet. 2011, 7, e1002329. [Google Scholar] [CrossRef] [PubMed]
- Porrua, O.; Libri, D. A bacterial-like mechanism for transcription termination by the Sen1p helicase in budding yeast. Nat. Struct. Mol. Biol. 2013, 20, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Hazelbaker, D.Z.; Marquardt, S.; Wlotzka, W.; Buratowski, S. Kinetic competition between RNA polymerase II and Sen1-dependent transcription termination. Mol. Cell 2013, 49, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Libri, D.; Porrua, O. Biochemical characterization of the helicase Sen1 provides new insights into the mechanisms of non-coding transcription termination. Nucleic Acids Res. 2017, 45, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Arigo, J.T.; Carroll, K.L.; Ames, J.M.; Corden, J.L. Regulation of yeast NRD1 expression by premature transcription termination. Mol. Cell 2006, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Arigo, J.T.; Eyler, D.E.; Carroll, K.L.; Corden, J.L. Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol. Cell 2006, 23, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.K.; Wilson, S.M.; Steinmetz, E.J.; Patturajan, M.; Brow, D.A.; Swanson, M.S.; Corden, J.L. A yeast heterogeneous nuclear ribonucleoprotein complex associated with RNA polymerase II. Genetics 2000, 154, 557–571. [Google Scholar] [PubMed]
- Mayer, A.; Heidemann, M.; Lidschreiber, M.; Schreieck, A.; Sun, M.; Hintermair, C.; Kremmer, E.; Eick, D.; Cramer, P. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 2012, 336, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.; Schwalb, B.; Kiesel, A.; Baejen, C.; Torkler, P.; Gagneur, J.; Soeding, J.; Cramer, P. Transcriptome surveillance by selective termination of noncoding RNA synthesis. Cell 2013, 155, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, E.J.; Conrad, N.K.; Brow, D.A.; Corden, J.L. RNA-binding protein Nrd1 directs poly(A)-independent 3′-end formation of RNA polymerase II transcripts. Nature 2001, 413, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Grzechnik, P.; Gdula, M.R.; Proudfoot, N.J. Pcf11 orchestrates transcription termination pathways in yeast. Genes Dev. 2015, 29, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, K.; Cerna, H.; Holub, P.; Pasulka, J.; Hrossova, D.; Loehr, F.; Hofr, C.; Vanacova, S.; Stefl, R. Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev. 2012, 26, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, L.; Kim, M.; Mutschler, H.; Buratowski, S.; Meinhart, A. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat. Struct. Mol. Biol. 2008, 15, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Tudek, A.; Porrua, O.; Kabzinski, T.; Lidschreiber, M.; Kubicek, K.; Fortova, A.; Lacroute, F.; Vanacova, S.; Cramer, P.; Stefl, R.; et al. Molecular basis for coordinating transcription termination with noncoding RNA degradation. Mol. Cell 2014, 55, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzawa, H.; Kanda, E.; Ishihama, A. Rpb7 subunit of RNA polymerase II interacts with an RNA-binding protein involved in processing of transcripts. Nucleic Acids Res. 2003, 31, 4696–4701. [Google Scholar] [CrossRef] [PubMed]
- Lemay, J.F.; Marguerat, S.; Larochelle, M.; Liu, X.; van Nues, R.; Hunyadkurti, J.; Hoque, M.; Tian, B.; Granneman, S.; Bahler, J.; et al. The Nrd1-like protein Seb1 coordinates cotranscriptional 3′ end processing and polyadenylation site selection. Genes Dev. 2016, 30, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Marina, D.B.; Shankar, S.; Natarajan, P.; Finn, K.J.; Madhani, H.D. A conserved ncRNA-binding protein recruits silencing factors to heterochromatin through an RNAi-independent mechanism. Genes Dev. 2013, 27, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, S.; Renner, M.; Watts, B.R.; Adams, O.; Huseyin, M.; Baejen, C.; El Omari, K.; Kilchert, C.; Heo, D.H.; Kecman, T.; et al. The conserved protein Seb1 drives transcription termination by binding RNA polymerase II and nascent RNA. Nat. Commun. 2017, 8, 14861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hoque, M.; Larochelle, M.; Lemay, J.F.; Yurko, N.; Manley, J.L.; Bachand, F.; Tian, B. Comparative analysis of alternative polyadenylation in S. cerevisiae and S. pombe. Genome Res. 2017, 27, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, M.; Hunyadkurti, J.; Bachand, F. Polyadenylation site selection: Linking transcription and RNA processing via a conserved carboxy-terminal domain (CTD)-interacting protein. Curr. Genet. 2017, 63, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Patturajan, M.; Wei, X.; Berezney, R.; Corden, J.L. A nuclear matrix protein interacts with the phosphorylated C-terminal domain of RNA polymerase II. Mol. Cell. Biol. 1998, 18, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Loll, B.; Meinhart, A. Snapshots of the RNA processing factor SCAF8 bound to different phosphorylated forms of the carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 2008, 283, 22659–22669. [Google Scholar] [CrossRef] [PubMed]
- Fasken, M.B.; Laribee, R.N.; Corbett, A.H. Nab3 facilitates the function of the TRAMP complex in RNA processing via recruitment of Rrp6 independent of Nrd1. PLoS Genet. 2015, 11, e1005044. [Google Scholar] [CrossRef] [PubMed]
- Thoms, M.; Thomson, E.; Bassler, J.; Gnadig, M.; Griesel, S.; Hurt, E. The exosome is recruited to RNA substrates through specific adaptor proteins. Cell 2015, 162, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Tants, J.N.; Basquin, J.; Thoms, M.; Hurt, E.; Sattler, M.; Conti, E. Structural insights into the interaction of the nuclear exosome helicase Mtr4 with the pre-ribosomal protein Nop53. RNA 2017, 23, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Babour, A.; Shen, Q.; Dos-Santos, J.; Murray, S.; Gay, A.; Challal, D.; Fasken, M.; Palancade, B.; Corbett, A.; Libri, D.; et al. The chromatin remodeler ISW1 is a quality control factor that surveys nuclear mRNP biogenesis. Cell 2016, 167, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Buhler, M.; Haas, W.; Gygi, S.P.; Moazed, D. RNAi-dependent and -independent RNA turnover mechanisms contribute to heterochromatic gene silencing. Cell 2007, 129, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Turcu, F.E.; Zhang, K.; Zofall, M.; Chen, E.; Grewal, S.I. Defects in RNA quality control factors reveal RNAi-independent nucleation of heterochromatin. Nat. Struct. Mol. Biol. 2011, 18, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fischer, T.; Porter, R.L.; Dhakshnamoorthy, J.; Zofall, M.; Zhou, M.; Veenstra, T.; Grewal, S.I. Clr4/Suv39 and RNA quality control factors cooperate to trigger RNAi and suppress antisense RNA. Science 2011, 331, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Stevenson, A.L.; Kearsey, S.E.; Watt, S.; Bahler, J. Global role for polyadenylation-assisted nuclear RNA degradation in posttranscriptional gene silencing. Mol. Cell. Biol. 2008, 28, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Buhler, M.; Spies, N.; Bartel, D.P.; Moazed, D. TRAMP-mediated RNA surveillance prevents spurious entry of RNAs into the Schizosaccharomyces pombe siRNA pathway. Nat. Struct. Mol. Biol. 2008, 15, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, M.; Lemay, J.F.; Bachand, F. The THO complex cooperates with the nuclear RNA surveillance machinery to control small nucleolar RNA expression. Nucleic Acids Res. 2012, 40, 10240–10253. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, P.; Halic, M. Tailing and degradation of Argonaute-bound small RNAs protect the genome from uncontrolled RNAi. Nat. Commun. 2017, 8, 15332. [Google Scholar] [CrossRef] [PubMed]
- Strasser, K.; Masuda, S.; Mason, P.; Pfannstiel, J.; Oppizzi, M.; Rodriguez-Navarro, S.; Rondon, A.G.; Aguilera, A.; Struhl, K.; Reed, R.; et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 2002, 417, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Zenklusen, D.; Vinciguerra, P.; Wyss, J.C.; Stutz, F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol. Cell. Biol. 2002, 22, 8241–8253. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.N.; Chalamcharla, V.R.; Reyes-Turcu, F.; Mehta, S.; Zofall, M.; Balachandran, V.; Dhakshnamoorthy, J.; Taneja, N.; Yamanaka, S.; Zhou, M.; et al. Mtr4-like protein coordinates nuclear RNA processing for heterochromatin assembly and for telomere maintenance. Cell 2013, 155, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Egan, E.D.; Braun, C.R.; Gygi, S.P.; Moazed, D. Post-transcriptional regulation of meiotic genes by a nuclear RNA silencing complex. RNA 2014, 20, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, J.; Schermann, G.; Ohle, C.; Bendrin, K.; Sugioka-Sugiyama, R.; Sugiyama, T.; Fischer, T. The fission yeast MTREC complex targets cuts and unspliced pre-mRNAs to the nuclear exosome. Nat. Commun. 2015, 6, 7050. [Google Scholar] [CrossRef] [PubMed]
- Harigaya, Y.; Tanaka, H.; Yamanaka, S.; Tanaka, K.; Watanabe, Y.; Tsutsumi, C.; Chikashige, Y.; Hiraoka, Y.; Yamashita, A.; Yamamoto, M. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature 2006, 442, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Shichino, Y.; Yamashita, A.; Yamamoto, M. Meiotic long non-coding meiRNA accumulates as a dot at its genetic locus facilitated by Mmi1 and plays as a decoy to lure Mmi1. Open Biol. 2014, 4, 140022. [Google Scholar] [CrossRef] [PubMed]
- Kilchert, C.; Wittmann, S.; Passoni, M.; Shah, S.; Granneman, S.; Vasiljeva, L. Regulation of mRNA levels by decay-promoting introns that recruit the exosome specificity factor Mmi1. Cell Rep. 2015, 13, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Shichino, Y.; Tanaka, H.; Hiriart, E.; Touat-Todeschini, L.; Vavasseur, A.; Ding, D.Q.; Hiraoka, Y.; Verdel, A.; Yamamoto, M. Hexanucleotide motifs mediate recruitment of the RNA elimination machinery to silent meiotic genes. Open Biol. 2012, 2, 120014. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Futcher, B.; Leatherwood, J. The fission yeast RNA binding protein Mmi1 regulates meiotic genes by controlling intron specific splicing and polyadenylation coupled RNA turnover. PLoS ONE 2011, 6, e26804. [Google Scholar] [CrossRef] [PubMed]
- Hiriart, E.; Vavasseur, A.; Touat-Todeschini, L.; Yamashita, A.; Gilquin, B.; Lambert, E.; Perot, J.; Shichino, Y.; Nazaret, N.; Boyault, C.; et al. Mmi1 RNA surveillance machinery directs RNAi complex RITS to specific meiotic genes in fission yeast. EMBO J. 2012, 31, 2296–2308. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Takayama, T.; Iwata, R.; Yamamoto, M. A novel factor Iss10 regulates Mmi1-mediated selective elimination of meiotic transcripts. Nucleic Acids Res. 2013, 41, 9680–9687. [Google Scholar] [CrossRef] [PubMed]
- St-Andre, O.; Lemieux, C.; Perreault, A.; Lackner, D.H.; Bahler, J.; Bachand, F. Negative regulation of meiotic gene expression by the nuclear poly(A)-binding protein in fission yeast. J. Biol. Chem. 2010, 285, 27859–27868. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Wanatabe, N.; Kitahata, E.; Tani, T.; Sugioka-Sugiyama, R. Red5 and three nuclear pore components are essential for efficient suppression of specific mRNAs during vegetative growth of fission yeast. Nucleic Acids Res. 2013, 41, 6674–6686. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.R.; Domanski, M.; Kristiansen, M.S.; Storvall, H.; Ntini, E.; Verheggen, C.; Schein, A.; Bunkenborg, J.; Poser, I.; Hallais, M.; et al. The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nat. Struct. Mol. Biol. 2013, 20, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Christensen, M.S.; Kristiansen, M.S.; Domanski, M.; Falkenby, L.G.; Lykke-Andersen, S.; Andersen, J.S.; Dziembowski, A.; Jensen, T.H. Interaction profiling identifies the human nuclear exosome targeting complex. Mol. Cell 2011, 43, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, C.; Bilen, B.; Zavolan, M.; Keller, W. PAPD5, a noncanonical poly(A) polymerase with an unusual RNA-binding motif. RNA 2011, 17, 1737–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogami, K.; Cho, R.; Hoshino, S. Molecular cloning and characterization of a novel isoform of the non-canonical poly(A) polymerase PAPD7. Biochem. Biophys. Res. Commun. 2013, 432, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Molleston, J.M.; Sabin, L.R.; Moy, R.H.; Menghani, S.V.; Rausch, K.; Gordesky-Gold, B.; Hopkins, K.C.; Zhou, R.; Jensen, T.H.; Wilusz, J.E.; et al. A conserved virus-induced cytoplasmic TRAMP-like complex recruits the exosome to target viral RNA for degradation. Genes Dev. 2016, 30, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Berndt, H.; Harnisch, C.; Rammelt, C.; Stohr, N.; Zirkel, A.; Dohm, J.C.; Himmelbauer, H.; Tavanez, J.P.; Huttelmaier, S.; Wahle, E. Maturation of mammalian H/ACA box snoRNAs: PAPD5-dependent adenylation and PARN-dependent trimming. RNA 2012, 18, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Shcherbik, N.; Wang, M.; Lapik, Y.R.; Srivastava, L.; Pestov, D.G. Polyadenylation and degradation of incomplete RNA polymerase I transcripts in mammalian cells. EMBO Rep. 2010, 11, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Steitz, J.A. Tri-snRNP-associated proteins interact with subunits of the TRAMP and nuclear exosome complexes, linking RNA decay and pre-mRNA splicing. RNA Biol. 2012, 9, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Yamazoe, T.; Hara, Y.; Tani, K.; Tsuji, A.; Tagaya, M. The AAA-ATPase NVL2 is a component of pre-ribosomal particles that interacts with the DExD/H-box RNA helicase DOB1. Biochem. Biophys. Res. Commun. 2006, 346, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Nozaki, A.; Uno, H.; Ishida, Y.; Nagahama, M. Interaction properties of human TRAMP-like proteins and their role in pre-rRNA 5′ETS turnover. FEBS Lett. 2016, 590, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Ishida, Y.; Nagahama, M. AAA-ATPase NVL2 acts on Mtr4-exosome complex to dissociate the nucleolar protein WDR74. Biochem. Biophys. Res. Commun. 2015, 467, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Ishida, Y.I.; Sudo, H.; Nagahama, M. WDR74 participates in an early cleavage of the pre-rRNA processing pathway in cooperation with the nucleolar AAA-ATPase NVL2. Biochem. Biophys. Res. Commun. 2017, 495, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yoshikatsu, Y.; Ishida, Y.; Sudo, H.; Yuasa, K.; Tsuji, A.; Nagahama, M. NVL2, a nucleolar AAA-ATPase, is associated with the nuclear exosome and is involved in pre-rRNA processing. Biochem. Biophys. Res. Commun. 2015, 464, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Macias, S.; Cordiner, R.A.; Gautier, P.; Plass, M.; Caceres, J.F. DGCR8 acts as an adaptor for the exosome complex to degrade double-stranded structured RNAs. Mol. Cell 2015, 60, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Wang, H.F.; Burns, A.M.; Schroeder, M.R.; Gaspari, M.; Baumann, P. Human telomerase RNA processing and quality control. Cell Rep. 2015, 13, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Grenier St-Sauveur, V.; Bergeron, D.; Dupuis-Sandoval, F.; Scott, M.S.; Bachand, F. A polyadenylation-dependent 3′ end maturation pathway is required for the synthesis of the human telomerase RNA. Cell Rep. 2015, 13, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Schmidt, J.C.; Goldfarb, K.C.; Cech, T.R.; Parker, R. Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat. Struct. Mol. Biol. 2016, 23, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Andersen, P.R.; Schein, A.; Dziembowski, A.; Kudla, G.; Jensen, T.H. The human nuclear exosome targeting complex is loaded onto newly synthesized RNA to direct early ribonucleolysis. Cell Rep. 2015, 10, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Hrossova, D.; Sikorsky, T.; Potesil, D.; Bartosovic, M.; Pasulka, J.; Zdrahal, Z.; Stefl, R.; Vanacova, S. RBM7 subunit of the NEXT complex binds U-rich sequences and targets 3′-end extended forms of snRNAs. Nucleic Acids Res. 2015, 43, 4236–4248. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.M.; Conrad, N.K. The human nuclear poly(A)-binding protein promotes RNA hyperadenylation and decay. PLoS Genet. 2013, 9, e1003893. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.M.; Hunter, O.V.; Hunter, A.C.; Conrad, N.K. Canonical poly(A) polymerase activity promotes the decay of a wide variety of mammalian nuclear RNAs. PLoS Genet. 2015, 11, e1005610. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, Y.B.; Kleinman, C.L.; Landry-Voyer, A.M.; Majewski, J.; Bachand, F. Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1. PLoS Genet. 2012, 8, e1003078. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Di Giammartino, D.C.; Taylor, D.; Sarkeshik, A.; Rice, W.J.; Yates, J.R., 3rd; Frank, J.; Manley, J.L. Molecular architecture of the human pre-mRNA 3′ processing complex. Mol. Cell 2009, 33, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, S.; Benbahouche, N.E.H.; Domanski, M.; Robert, M.C.; Meola, N.; Lubas, M.; Bukenborg, J.; Andersen, J.S.; Schulze, W.M.; Verheggen, C.; et al. Mutually exclusive CBC-containing complexes contribute to RNA fate. Cell Rep. 2017, 18, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Iasillo, C.; Schmid, M.; Yahia, Y.; Maqbool, M.A.; Descostes, N.; Karadoulama, E.; Bertrand, E.; Andrau, J.C.; Jensen, T.H. ARS2 is a general suppressor of pervasive transcription. Nucleic Acids Res. 2017, 45, 10229–10241. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Ogami, K.; Chen, Y.; Feng, S.; Moresco, J.J.; Yates, J.R., 3rd; Manley, J.L. NRDE-2, the human homolog of fission yeast Nrl1, prevents DNA damage accumulation in human cells. Manuscript in preparation.
- Morton, D.J.; Kuiper, E.G.; Jones, S.K.; Leung, S.W.; Corbett, A.H.; Fasken, M.B. The RNA exosome and RNA exosome-linked disease. RNA 2017, 24, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Tomecki, R.; Drazkowska, K.; Kucinski, I.; Stodus, K.; Szczesny, R.J.; Gruchota, J.; Owczarek, E.P.; Kalisiak, K.; Dziembowski, A. Multiple myeloma-associated hDIS3 mutations cause perturbations in cellular RNA metabolism and suggest hDIS3 PIN domain as a potential drug target. Nucleic Acids Res. 2014, 42, 1270–1290. [Google Scholar] [CrossRef] [PubMed]
- Blasius, M.; Wagner, S.A.; Choudhary, C.; Bartek, J.; Jackson, S.P. A quantitative 14-3-3 interaction screen connects the nuclear exosome targeting complex to the DNA damage response. Genes Dev. 2014, 28, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, C.; Lubas, M.; Tehrani, M.; Menon, M.B.; Ronkina, N.; Rousseau, S.; Cohen, P.; Kotlyarov, A.; Gaestel, M. p38MAPK/MK2-mediated phosphorylation of RBM7 regulates the human nuclear exosome targeting complex. RNA 2015, 21, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Arai, S.; Song, X.; Reichart, D.; Du, K.; Pascual, G.; Tempst, P.; Rosenfeld, M.G.; Glass, C.K.; Kurokawa, R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 2008, 454, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Lloret-Llinares, M.; Mapendano, C.K.; Martlev, L.H.; Lykke-Andersen, S.; Jensen, T.H. Relationships between PROMPT and gene expression. RNA Biol. 2016, 13, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Cotranscriptional processes and their influence on genome stability. Genes Dev. 2006, 20, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Manley, J.L. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 2005, 122, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Huertas, P.; Aguilera, A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell 2003, 12, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, H.; Aguilera, A. Transcription as a threat to genome integrity. Annu. Rev. Biochem. 2016, 85, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Manley, J.L. R loops and links to human disease. J. Mol. Biol. 2017, 429, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, J.M.; Aguilera, A. R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Proudfoot, N.J. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014, 28, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Sollier, J.; Cimprich, K.A. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015, 25, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.; Jimeno, S.; Marin, M.; Huertas, P.; Garcia-Rubio, M.; Aguilera, A. Interdependence between transcription and mRNP processing and export, and its impact on genetic stability. Mol Cell 2005, 18, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Gavalda, S.; Gallardo, M.; Luna, R.; Aguilera, A. R-loop mediated transcription-associated recombination in trf4Δ mutants reveals new links between RNA surveillance and genome integrity. PLoS ONE 2013, 8, e65541. [Google Scholar] [CrossRef] [PubMed]
- El Hage, A.; French, S.L.; Beyer, A.L.; Tollervey, D. Loss of topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 2010, 24, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Manfrini, N.; Trovesi, C.; Wery, M.; Martina, M.; Cesena, D.; Descrimes, M.; Morillon, A.; d’Adda di Fagagna, F.; Longhese, M.P. RNA-processing proteins regulate Mec1/ATR activation by promoting generation of RPA-coated ssDNA. EMBO Rep. 2015, 16, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wahba, L.; Amon, J.D.; Koshland, D.; Vuica-Ross, M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell 2011, 44, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Skourti-Stathaki, K.; Proudfoot, N.J.; Gromak, N. Human Senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol. Cell 2011, 42, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Feng, S.; Manley, J.L. A SUMO-dependent interaction between Senataxin and the exosome, disrupted in the neurodegenerative disease AOA2, targets the exosome to sites of transcription-induced DNA damage. Genes Dev. 2013, 27, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, B.; Basu, U.; Lim, J. RNA exosome and non-coding RNA-coupled mechanisms in AID-mediated genomic alterations. J. Mol. Biol. 2017, 429, 3230–3241. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Meng, F.L.; Keim, C.; Grinstein, V.; Pefanis, E.; Eccleston, J.; Zhang, T.; Myers, D.; Wasserman, C.R.; Wesemann, D.R.; et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell 2011, 144, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Keim, C.D.; Wang, J.; Kazadi, D.; Oliver, P.M.; Rabadan, R.; Basu, U. E3-ubiquitin ligase Nedd4 determines the fate of AID-associated RNA polymerase II in B cells. Genes Dev. 2013, 27, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, E.; Basu, U. RNA exosome regulates AID DNA mutator activity in the B cell genome. Adv. Immunol. 2015, 127, 257–308. [Google Scholar] [PubMed]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Chao, J.; Rabadan, R.; Economides, A.N.; Basu, U. Noncoding RNA transcription targets AID to divergently transcribed loci in B cells. Nature 2014, 514, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Kazadi, D.; Sun, J.; Federation, A.; Chao, J.; Elliott, O.; Liu, Z.P.; et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 2015, 161, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Giri, P.K.; Kazadi, D.; Laffleur, B.; Zhang, W.; Grinstein, V.; Pefanis, E.; Brown, L.M.; Ladewig, E.; Martin, O.; et al. Nuclear proximity of Mtr4 to RNA exosome restricts DNA mutational asymmetry. Cell 2017, 169, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Wang, Q.; Wu, G.; Tan, M.; Wang, L.; Shi, M.; Chang, X.; Cheng, H. Aly and THO are required for assembly of the human TREX complex and association of TREX components with the spliced mRNA. Nucleic Acids Res. 2013, 41, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Dufu, K.; Lee, C.S.; Hsu, J.L.; Dias, A.; Reed, R. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 2006, 127, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.; Hirose, T.; Kimura, H.; Hagiwara, M. The interaction between cap-binding complex and RNA export factor is required for intronless mRNA export. J. Biol. Chem. 2007, 282, 15645–15651. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Das, R.; Cheng, H.; Hurt, E.; Dorman, N.; Reed, R. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 2005, 19, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Wang, K.; Du, Y.; Gui, B.; Chang, X.; Wang, L.; Fan, J.; Chen, S.; Wu, X.; Li, G.; et al. A sub-element in PRE enhances nuclear export of intronless mRNAs by recruiting the TREX complex via ZC3H18. Nucleic Acids Res. 2014, 42, 7305–7318. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kuai, B.; Wu, G.; Wu, X.; Chi, B.; Wang, L.; Wang, K.; Shi, Z.; Zhang, H.; Chen, S.; et al. Exosome cofactor hMTR4 competes with export adaptor ALYREF to ensure balanced nuclear RNA pools for degradation and export. EMBO J. 2017, 36, 2870–2886. [Google Scholar] [CrossRef] [PubMed]
- Ogami, K.; Manley, J.L. Mtr4/ZFC3H1 protects polysomes through nuclear RNA surveillance. Cell Cycle 2017, 16, 1999–2000. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Cubberley, G.; Bentley, D.L. Cotranscriptional recruitment of the mRNA export factor Yra1 by direct interaction with the 3′ end processing factor Pcf11. Mol. Cell 2009, 33, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ruepp, M.D.; Aringhieri, C.; Vivarelli, S.; Cardinale, S.; Paro, S.; Schumperli, D.; Barabino, S.M. Mammalian pre-mRNA 3′ end processing factor CF Im68 functions in mRNA export. Mol. Biol. Cell. 2009, 20, 5211–5223. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.D.; Saran, S.; Williamson, A.J.; Pierce, A.; Dittrich-Breiholz, O.; Wiehlmann, L.; Koch, A.; Whetton, A.D.; Tamura, T. THOC5 controls 3′end-processing of immediate early genes via interaction with polyadenylation specific factor 100 (CPSF100). Nucleic Acids Res. 2014, 42, 12249–12260. [Google Scholar] [CrossRef] [PubMed]
- Katahira, J.; Okuzaki, D.; Inoue, H.; Yoneda, Y.; Maehara, K.; Ohkawa, Y. Human TREX component THOC5 affects alternative polyadenylation site choice by recruiting mammalian cleavage factor I. Nucleic Acids Res. 2013, 41, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Wu, X.; He, Z.; Wang, L.; Yin, S.; Tian, B.; Li, G.; Cheng, H. ALYREF mainly binds to the 5′ and the 3′ regions of the mRNA in vivo. Nucleic Acids Res. 2017, 45, 9640–9653. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T. Ribosome footprint profiling of translation throughout the genome. Cell 2016, 165, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.L.; Pauli, A.; Rinn, J.L.; Regev, A.; Schier, A.F.; Valen, E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Development 2013, 140, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Y.; Ma, Q.; Gu, F.; Day, D.S.; He, A.; Zhou, B.; Li, J.; Stevens, S.M.; Romo, D.; et al. Interrogating translational efficiency and lineage-specific transcriptomes using ribosome affinity purification. Proc. Natl. Acad. Sci. USA 2013, 110, 15395–15400. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Brar, G.A.; Stern-Ginossar, N.; Harris, M.S.; Talhouarne, G.J.; Jackson, S.E.; Wills, M.R.; Weissman, J.S. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep. 2014, 8, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Van Heesch, S.; van Iterson, M.; Jacobi, J.; Boymans, S.; Essers, P.B.; de Bruijn, E.; Hao, W.; MacInnes, A.W.; Cuppen, E.; Simonis, M. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014, 15, R6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, A.P.; Rodriguez, E.H.; Jovanovic, M.; Stern-Ginossar, N.; Haas, B.J.; Mertins, P.; Raychowdhury, R.; Hacohen, N.; Carr, S.A.; Ingolia, N.T.; et al. A regression-based analysis of ribosome-profiling data reveals a conserved complexity to mammalian translation. Mol. Cell 2015, 60, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Song, R.; Regev, A.; Struhl, K. Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 2015, 4, e08890. [Google Scholar] [CrossRef] [PubMed]
- Calviello, L.; Mukherjee, N.; Wyler, E.; Zauber, H.; Hirsekorn, A.; Selbach, M.; Landthaler, M.; Obermayer, B.; Ohler, U. Detecting actively translated open reading frames in ribosome profiling data. Nat. Methods 2016, 13, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, F.; Gerber, A.; Mauvoisin, D.; Wang, J.; Gatfield, D.; Stubblefield, J.J.; Green, C.B.; Gachon, F.; Schibler, U. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell 2017, 169, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.S.; Chen, Y.; Sen, G.L. Progenitor function in self-renewing human epidermis is maintained by the exosome. Cell Stem Cell 2012, 11, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Skamagki, M.; Zhang, C.; Ross, C.A.; Ananthanarayanan, A.; Liu, Z.; Mu, Q.; Basu, U.; Wang, J.; Zhao, R.; Li, H.; et al. RNA exosome complex-mediated control of redox status in pluripotent stem cells. Stem Cell Rep. 2017, 9, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Taleahmad, S.; Mirzaei, M.; Parker, L.M.; Hassani, S.N.; Mollamohammadi, S.; Sharifi-Zarchi, A.; Haynes, P.A.; Baharvand, H.; Salekdeh, G.H. Proteome analysis of ground state pluripotency. Sci. Rep. 2015, 5, 17985. [Google Scholar] [CrossRef] [PubMed]
- Fazzio, T.G.; Huff, J.T.; Panning, B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell 2008, 134, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Onderak, A.M.; Anderson, J.T. Loss of the RNA helicase SKIV2L2 impairs mitotic progression and replication-dependent histone mRNA turnover in murine cell lines. RNA 2017, 23, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Rialdi, A.; Hultquist, J.; Jimenez-Morales, D.; Peralta, Z.; Campisi, L.; Fenouil, R.; Moshkina, N.; Wang, Z.Z.; Laffleur, B.; Kaake, R.M.; et al. The RNA exosome syncs IAV-RNAPII transcription to promote viral ribogenesis and infectivity. Cell 2017, 169, 679–692. [Google Scholar] [CrossRef] [PubMed]



| Complex | Saccharomyces cerevisiae | Schizosaccharomyces pombe | Homo sapiens |
|---|---|---|---|
| TRAMP | Mtr4 | Mtr4 | Mtr4/SKIV2L2/MTREX |
| Air1, Air2 | Air1 | ZCCHC7 | |
| Trf4, Trf5 | Cid14 | PAPD5, PAPD7 | |
| NNS | Nrd1 | Seb1 | SCAF4, SCAF8 |
| Nab3 | Nab3 | RALY, RALYL, hnRNPC, hnRNPCL1, hnRNPCL2, hnRNPCL3, hnRNPCL4 | |
| Sen1 | Sen1 | SETX | |
| MTREC NURS Mtr4/ZFC3H1 PAXT | Mtr4 | Mtl1 | Mtr4/SKIV2L2/MTREX |
| - | Red1 | ZFC3H1 | |
| - | Iss10 | - | |
| Pho92 | Mmi1 | YTHDF1, YTHDF2, YTHDF3 | |
| Sto1 | Cbc1 | CBP80/NCBP1 | |
| Cbc2 | Cbc2 | CBP20/NCBP2, NCBP2L | |
| - | Ars2/Pir2 | ARS2/SRRT | |
| - | Red5 | ZC3H3 | |
| Sgn1/Rbp1/Rbp29 | Pab2 | PABPN1, PABPN1L | |
| - | Rmn1 | RBM26, RBM27 | |
| Pap1 | Pla1 | PAPOLA, PAPOLB, PAPOLG | |
| Mtl1-Ctr1-Nrl1 | Mtr4 | Mtl1 | Mtr4/SKIV2L2/MTREX |
| - | Ctr1 | CCDC174 | |
| - | Nrl1 | NRDE2 | |
| NEXT | Mtr4 | Mtr4 | Mtr4 |
| - | - | RBM7 | |
| - | - | ZCCHC8 | |
| Other | Utp18 | Utp18 | UTP18 |
| Nop53 | Rrp16 | NOP53 | |
| ISW1 | - | SMARCA5 | |
| Rix7 | Rix7 | NVL/NVL2 | |
| Nsa1 | Wdr74 | WDR74 | |
| - | - | DGCR8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogami, K.; Chen, Y.; Manley, J.L. RNA Surveillance by the Nuclear RNA Exosome: Mechanisms and Significance. Non-Coding RNA 2018, 4, 8. https://doi.org/10.3390/ncrna4010008
Ogami K, Chen Y, Manley JL. RNA Surveillance by the Nuclear RNA Exosome: Mechanisms and Significance. Non-Coding RNA. 2018; 4(1):8. https://doi.org/10.3390/ncrna4010008
Chicago/Turabian StyleOgami, Koichi, Yaqiong Chen, and James L. Manley. 2018. "RNA Surveillance by the Nuclear RNA Exosome: Mechanisms and Significance" Non-Coding RNA 4, no. 1: 8. https://doi.org/10.3390/ncrna4010008
APA StyleOgami, K., Chen, Y., & Manley, J. L. (2018). RNA Surveillance by the Nuclear RNA Exosome: Mechanisms and Significance. Non-Coding RNA, 4(1), 8. https://doi.org/10.3390/ncrna4010008