Investigation into the Role of Long-Non-Coding RNA MIAT in Leukemia
Abstract
:1. Introduction
2. Results
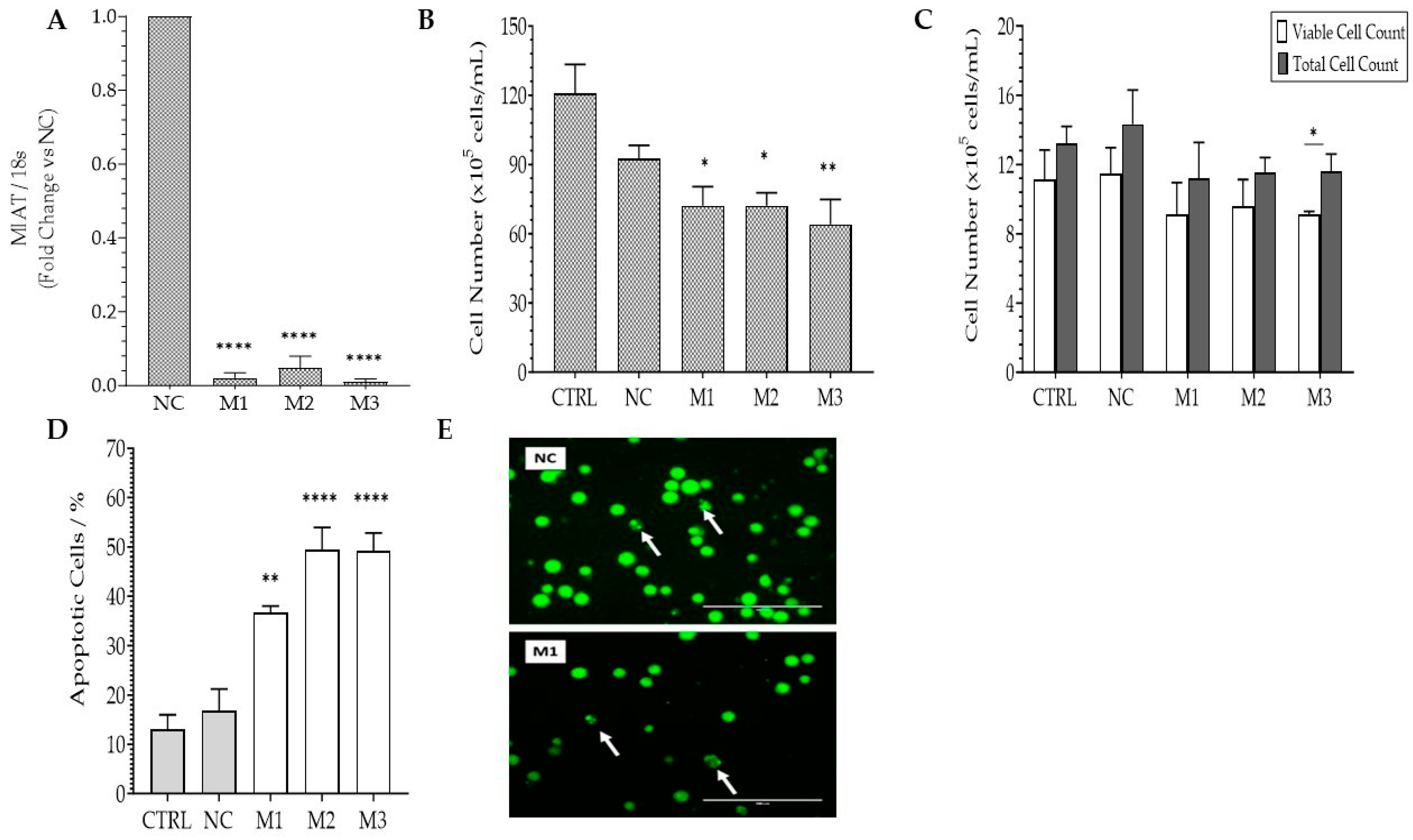
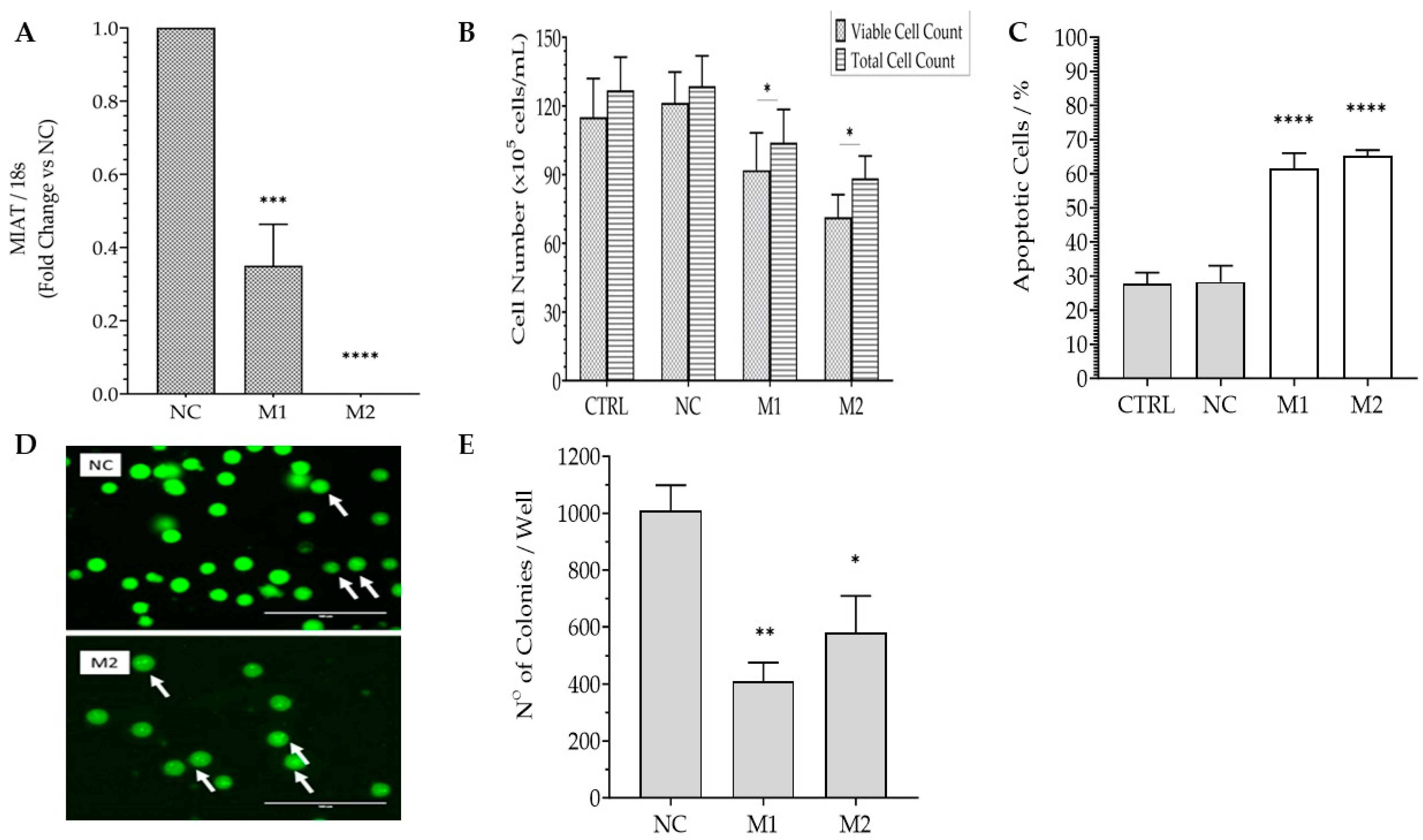
2.1. Effects of MIAT Knockdown on the Survival of Leukemic Cells
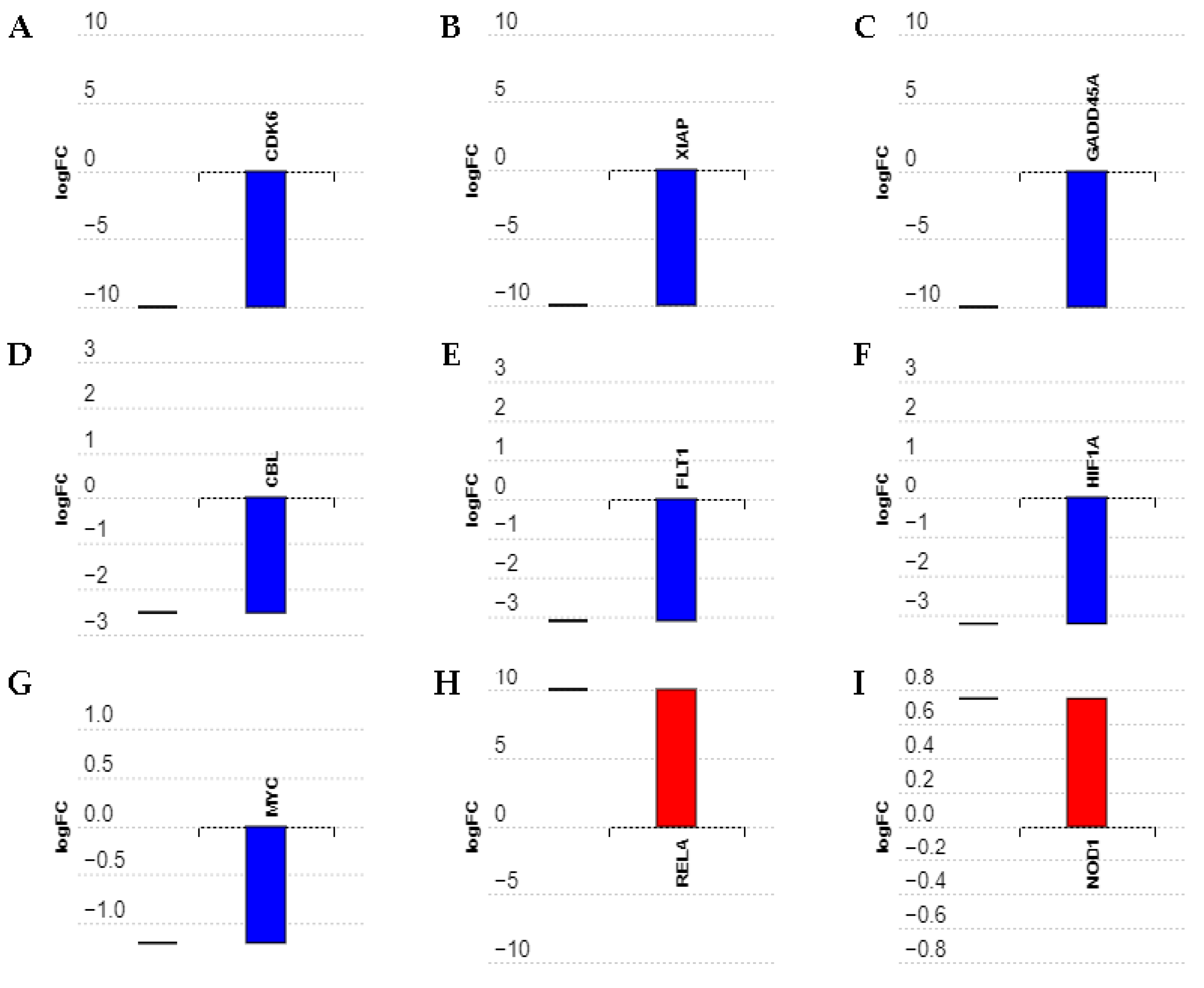
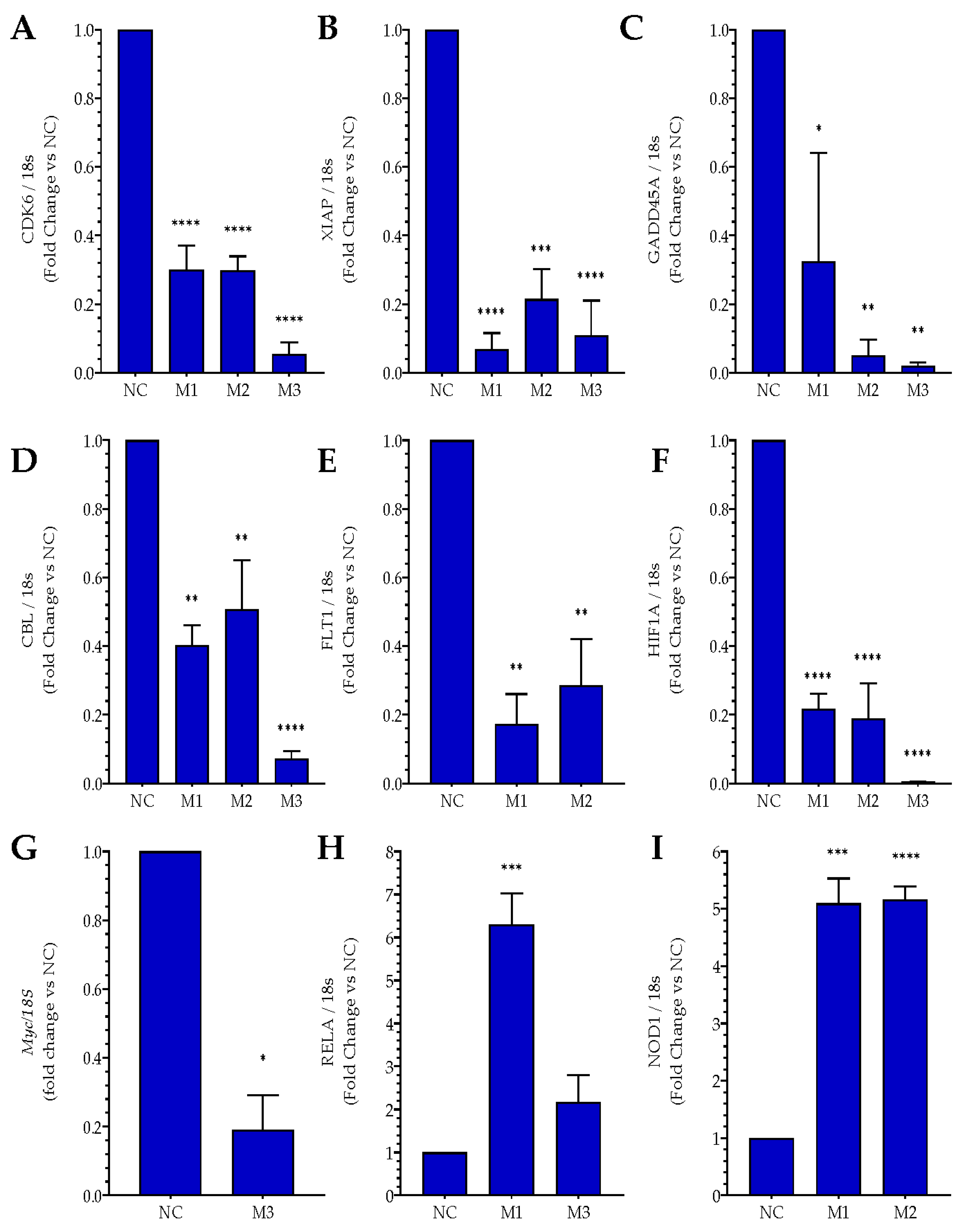
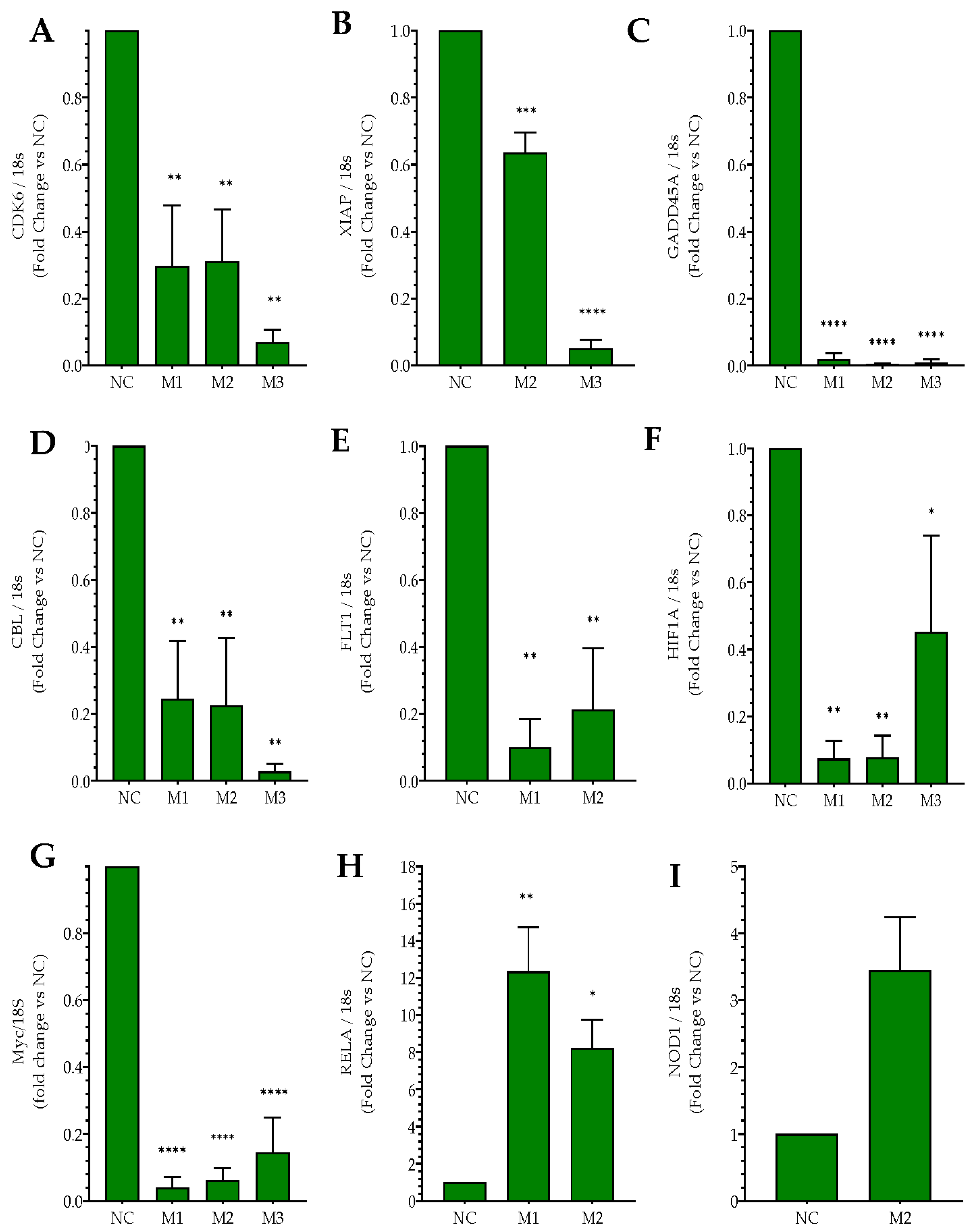
2.2. Effects of MIAT Knockdown on the Dysregulation of Oncogenes and Tumor Suppressor Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Interference Using siRNAs
4.3. RNA Extraction
4.4. Reverse Transcription and Real-Time Polymerase Chain Reaction (RT-qPCR)
4.5. Cell Survival and Apoptosis Determination
4.6. Cell Cycle Analysis
4.7. Long-Term Survival Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salzberg, S. Open questions: How many genes do we have? BMC Biol. 2018, 16, 94. [Google Scholar]
- The Human Genome Project—Genome.gov. Available online: https://www.genome.gov/human-genome-project (accessed on 11 June 2023).
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16, 0027. [Google Scholar]
- Su, Y.; Wu, H.; Pavlosky, A.; Zou, L.L.; Deng, X.; Zhang, Z.X.; Jevnikar, A.M. Regulatory non-coding RNA: New instruments in the orchestration of cell death. Cell Death Dis. 2016, 7, e2333. [Google Scholar]
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long non-coding RNA in the pathogenesis of cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef] [Green Version]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.; et al. The GENCODE v7 catalogue of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar]
- Slack, F.J.; Chinnaiyan, A.M. The role of non-coding RNAS in oncology. Cell 2019, 179, 1033–1055. [Google Scholar]
- Hulshoff, M.S.; del Monte-Nieto, G.; Kovacic, J.; Krenning, G. Non-coding RNA in endothelial-to-mesenchymal transition. Cardiovasc. Res. 2019, 115, 1716–1731. [Google Scholar]
- Gudenas, B.L.; Wang, L. Prediction of LncRNA Subcellular Localization with Deep Learning from Sequence Features. Sci. Rep. 2018, 8, 16385. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; He, Q.; Li, M.; Chen, Y.; Liu, Y.; Wang, J. LncRNA MIAT: Myocardial infarction associated and more. Gene 2016, 578, 158–161. [Google Scholar]
- Boon, R.A.; Ja, N.; Holdt, L.; Dimmeler, S. Long Noncoding RNAs from Clinical Genetics to Therapeutic Targets. J. Am. Coll. Cardiol. 2016, 67, 1214–1226. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Azimi, T.; Taheri, M. Myocardial Infarction Associated Transcript (MIAT): Review of its impact in the tumorigenesis. Biomed. Pharmacother. 2020, 133, 111040. [Google Scholar]
- Da, C.; Gong, C.; Nan, W.; Zhou, K.; Wu, Z.; Zhang, H. The role of long non-coding RNA MIAT in cancers. Biomed. Pharmacother. 2020, 129, 110359. [Google Scholar] [CrossRef]
- Bountali, A.; Tonge, D.; Mourtada-Maarabouni, M. RNA sequencing reveals a key role for the long non-coding RNA MIAT in regulating neuroblastoma and glioblastoma cell fate. Int. J. Biol. Macromol. 2019, 130, 878–891. [Google Scholar] [CrossRef]
- GeneCard Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=MIAT#summaries (accessed on 11 June 2023).
- Hao, J.; Jin, X.; Chai, W.; Yin, Z.; Li, Y.; Dong, F.; Wang, W. Long non-coding RNA MIAT competitively binds miR-150-5p to regulate ZEB1 expression in osteosarcoma. Oncol. Lett. 2019, 17, 1229–1236. [Google Scholar]
- Jin, S.; Yang, X.; Li, J.; Yang, W.; Ma, H.; Zhang, Z. p53-targeted lincRNA-p21 acts as a tumor suppressor by inhibiting JAK2/STAT3 signaling pathways in head and neck squamous cell carcinoma. Mol. Cancer 2019, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Sattari, A.; Siddiqui, H.; Moshiri, F.; Ngankeu, A.; Nakamura, T.; Kipps, T.J.; Croce, C.M. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget 2016, 7, 54174–54182. [Google Scholar] [CrossRef] [Green Version]
- Fabris, L.; Juracek, J.; Calin, G. Non-Coding RNAs as Cancer Hallmarks in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2020, 21, 6720. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Song, L.; Pan, H.; Jiang, J.; Sun, L. Long noncoding RNA MIAT promotes the progression of acute myeloid leukemia by negatively regulating mir-495. Leuk. Res. 2019, 87, 106265. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wu, P.; Chen, Y.; Jia, Y. Aberrant lncrna expression in leukemia. J. Cancer 2020, 11, 4284–4296. [Google Scholar] [CrossRef]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef] [Green Version]
- Fassl, A.; Geng, Y.; Sicinski, P. Cdk4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, abc1495. [Google Scholar]
- Nebenfuehr, S.; Kollmann, K.; Sexl, V. The role CDK6 in cancer. Int. J. Cancer 2020, 147, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Ishio, T.; Kumar, S.; Shimono, J.; Daenthanasanmak, A.; Dubois, S.; Lin, Y.; Bryant, B.; Petrus, M.N.; Bachy, E.; Huang, D.W.; et al. Genome-wide CRISPR screen identifies CDK6 as a therapeutic target in adult T-cell leukemia/lymphoma. Blood 2022, 139, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Schmoellerl, J.; Barbosa IA, M.; Eder, T.; Brandstoetter, T.; Schmidt, L.; Maurer, B.; Troester, S.; Pham, H.T.T.; Sagarajit, M.; Ebner, J.; et al. CDK6 is an essential direct target of NUP98 fusion proteins in acute myeloid leukemia. Blood 2020, 136, 387–400. [Google Scholar] [CrossRef]
- Tu, H.; Costa, M. Xiap’s profile in human cancer. Biomolecules 2020, 10, 1493. [Google Scholar]
- Abbas, R.; Larisch, S. Targeting xiap for promoting cancer cell death—The story of arts and SMAC. Cells 2020, 9, 663. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Long, F.; Yan, F.; Wang, N.; Wang, Y. The expression and clinical significance of GADD45A in breast cancer patients. PeerJ 2018, 6, e5344. [Google Scholar] [CrossRef] [Green Version]
- Pietrasik, S.; Zajac, G.; Morawiec, J.; Soszynski, M.; Fila, M.; Blasiak, J. Interplay between BRCA1 and GADD45A and its potential for nucleotide excision repair in breast cancer pathogenesis. Int. J. Mol. Sci. 2020, 21, 870. [Google Scholar] [CrossRef] [Green Version]
- Liebermann, D.; Tront, J.S.; Sha, X.; Mukherjee, K.; Mohamed-Hadley, A.; Hoffman, B. Gadd45 stress sensors in malignancy and Leukemia. Crit. Rev. Oncog. 2011, 16, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sun, Q.; Sun, D.; Willette-Brown, J.; Anderson, M.J.; Gu, Q.; Lewandoski, M.; Hu, Y.; Zhu, F.; Wei, F.; et al. C-CBL is required for inhibition of angiogenesis through modulating JAK2/STAT3 activity in ROP development. Biomed. Pharmacother. 2020, 132, 110856. [Google Scholar] [CrossRef]
- Lyle, C.L.; Belghasem, M.; Chitalia, V.C. C-CBL: An important regulator and a target in angiogenesis and tumorigenesis. Cells 2019, 8, 498. [Google Scholar] [PubMed] [Green Version]
- Lutz-Nicoladoni, C.; Wolf, D.; Sopper, S. Modulation of immune cell functions by the E3 ligase cbl-b. Front. Oncol. 2015, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.J.; Kole, H.K.; Brown, K.; Naramura, M.; Fukuhara, S.; Hu, R.J.; Jang, I.K.; Gutkind, J.S.; Shevach, E.; Gu, H. CBL-B regulates the CD28 dependence of T-cell activation. Nature 2000, 403, 216–220. [Google Scholar] [CrossRef]
- Augustin, R.C.; Bao, R.; Luke, J.J. Targeting CBL-B in cancer immunotherapy. J. ImmunoTherapy Cancer 2023, 11, 006007. [Google Scholar] [CrossRef]
- Lacal, P.M.; Graziani, G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol. Res. 2018, 136, 97–107. [Google Scholar]
- Mohammad Rezaei, F.; Hashemzadeh, S.; Ravanbakhsh Gavgani, R.; Hosseinpour Feizi, M.; Pouladi, N.; Samadi Kafil, H.; Rostamizadeh, L.; Kholghi Oskooei, V.; Taheri, M.; Sakhinia, E. Dysregulated KDR and FLT1 Gene Expression in Colorectal Cancer Patients. Rep. Biochem. Mol. Biol. 2019, 8, 244–252. [Google Scholar] [PubMed]
- Casalou, C.; Fragoso, R.; Nunes, J. VEGF/PLGF induces leukemia cell migration via P38/ERK1/2 kinase pathway, resulting in Rho GTPases activation and caveolae formation. Leukemia 2007, 21, 1590–1594. [Google Scholar]
- Fragoso, R.; Pereira, T.; Wu, Y.; Zhu, Z.; Cabeçadas, J.; Dias, S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood 2005, 107, 1608–1616. [Google Scholar]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar]
- Geismann, C.; Hauser, C.; Grohmann, F.; Schneeweis, C.; Bölter, N.; Gundlach, J.P.; Schneider, G.; Röcken, C.; Meinhardt, C.; Schäfer, H.; et al. NF-κB/RELA controlled A20 limits trail-induced apoptosis in pancreatic cancer. Cell Death Dis. 2023, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, P.; Li, B.; Miao, D.; Deng, Q. RELA promotes proliferation but inhibits osteogenic and chondrogenic differentiation of mesenchymal stem cells. FEBS Lett. 2020, 594, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricca, A.; Biroccio, A.; Trisciuoglio, D.; Cippitelli, M.; Zupi, G. relA over-expression reduces tumorigenicity and activates apoptosis in human cancer cells. Br. J. Cancer 2001, 85, 1914–1921. [Google Scholar]
- Zhang, W.; Wang, Y. Activation of RIPK2-mediated NOD1 signaling promotes proliferation and invasion of ovarian cancer cells via NF-κB pathway. Histochem. Cell Biol. 2021, 157, 173–182. [Google Scholar] [CrossRef]
- Mey, L.; Jung, M.; Roos, F.; Blaheta, R.; Hegele, A.; Kinscherf, R.; Urbschat, A. NOD1 and nod2 of the innate immune system is differently expressed in human clear cell renal cell carcinoma, corresponding healthy renal tissue, its vasculature and primary isolated renal tubular epithelial cells. J. Cancer Res. Clin. Oncol. 2019, 145, 1405–1416. [Google Scholar] [CrossRef]
- Wang, D. NOD1 and NOD2 are potential therapeutic targets for cancer immunotherapy. Comput. Intell. Neurosci. 2022, 2022, 2271788. [Google Scholar]
- Zhu, Y.; Liu, Z.; Wan, Y.; Zou, L.; Liu, L.; Ding, S.; Lu, C.; Qiu, F. PARP14 promotes the growth and glycolysis of acute myeloid leukemia cells by regulating hif-1α expression. Clin. Immunol. 2022, 242, 109094. [Google Scholar]
- Griggio, V.; Vitale, C.; Todaro, M.; Riganti, C.; Kopecka, J.; Salvetti, C.; Bomben, R.; Bo, M.D.; Magliulo, D.; Rossi, D.; et al. HIF-1α is over-expressed in leukemic cells from tp53-disrupted patients and is a promising therapeutic target in chronic lymphocytic leukemia. Haematologica 2019, 105, 1042–1054. [Google Scholar]
- Frolova, O.; Samudio, I.; Benito, J.M.; Jacamo, R.; Kornblau, S.M.; Markovic, A.; Schober, W.; Lu, H.; Qiu, Y.H.; Buglio, D.; et al. Regulation of hif-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol. Ther. 2012, 13, 858–870. [Google Scholar]
- Kontos, C.K.; Papageorgiou, S.G.; Diamantopoulos, M.A.; Scorilas, A.; Bazani, E.; Vasilatou, D.; Gkontopoulos, K.; Glezou, E.; Stavroulaki, G.; Dimitriadis, G.; et al. MRNA overexpression of the hypoxia inducible factor 1 alpha subunit gene (HIF1A): An independent predictor of poor overall survival in chronic lymphocytic leukemia. Leuk. Res. 2017, 53, 65–73. [Google Scholar]
- Zhou, H.; Jiang, Y.; Huang, Y.; Zhong, M.; Qin, D.; Xie, C.; Pan, G.; Tan, J.; Deng, M.; Zhao, H.; et al. Therapeutic inhibition of PPARA-HIF1A-PGK1 signaling targets leukemia stem and progenitor cells in acute myeloid leukemia. Cancer Lett. 2023, 554, 215997. [Google Scholar] [CrossRef]
- Fatma, H.; Maurya, S.K.; Siddique, H.R. Epigenetic modifications of c-myc: Role in cancer cell reprogramming, progression and chemoresistance. Semin. Cancer Biol. 2022, 83, 166–176. [Google Scholar]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Goswami, S.; Mani, R.; Nunes, J.; Chiang, C.-L.; Zapolnik, K.; Hu, E.; Frissora, F.; Mo, X.; Walker, L.A.; Yan, P.; et al. PP2A is a therapeutically targetable driver of cell fate decisions via a c-myc/p21 axis in human and murine acute myeloid leukemia. Blood 2022, 139, 1340–1358. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, Y.; Fang, F.; Ling, J.; Chu, X.; Zhang, Z.; Tao, Y.; Li, X.; Tian, Y.; et al. BRD4 PROTAC degrader MZ1 exerts anticancer effects in acute myeloid leukemia by targeting C-Myc and ANP32B genes. Cancer Biol. Ther. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.X.; Qian, D.Z.; Dai, M.S. The Molecular Crosstalk Between MYC and HIF in Cancer. Front. Cell Dev. Biol. 2020, 8, 590576. [Google Scholar] [CrossRef]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quarantino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. LncRNA MIAT promotes proliferation and invasion of HCC cells via sponging mir-214. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G559–G565. [Google Scholar]
- Xiang, Y.; Huang, Y.; Sun, H.; Pan, Y.; Wu, M.; Zhang, J. (2019) Deregulation of mir-520d-3p promotes hepatocellular carcinoma development via lncRNA MIAT regulation and EPHA2 signaling activation. Biomed. Pharmacother. 2018, 109, 1630–1639. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Williams, G.T. Protein phosphatase 4 regulates apoptosis, proliferation and mutation rate of human cells. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 1490–1502. [Google Scholar] [CrossRef] [Green Version]
- Kavousi, N.; Tonge, D.P.; Mourtada-Maarabouni, M. New insights into the functional role of protein phosphatase 4 regulatory subunit PP4R3A/SMEK1 in the regulation of Leukemic Cell Fate. Int. J. Biol. Macromol. 2023, 233, 123467. [Google Scholar] [CrossRef]
- Almnaseer, Z.A.; Mourtada-Maarabouni, M. Long noncoding RNA MIAT regulates apoptosis and the apoptotic response to chemotherapeutic agents in breast cancer cell lines. Biosci. Rep. 2018, 38, BSR20180704. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostini, A.; Mourtada-Maarabouni, M. Investigation into the Role of Long-Non-Coding RNA MIAT in Leukemia. Non-Coding RNA 2023, 9, 47. https://doi.org/10.3390/ncrna9040047
Ostini A, Mourtada-Maarabouni M. Investigation into the Role of Long-Non-Coding RNA MIAT in Leukemia. Non-Coding RNA. 2023; 9(4):47. https://doi.org/10.3390/ncrna9040047
Chicago/Turabian StyleOstini, Alessia, and Mirna Mourtada-Maarabouni. 2023. "Investigation into the Role of Long-Non-Coding RNA MIAT in Leukemia" Non-Coding RNA 9, no. 4: 47. https://doi.org/10.3390/ncrna9040047
APA StyleOstini, A., & Mourtada-Maarabouni, M. (2023). Investigation into the Role of Long-Non-Coding RNA MIAT in Leukemia. Non-Coding RNA, 9(4), 47. https://doi.org/10.3390/ncrna9040047




