Emission Ellipsometry Study in Polymeric Interfaces Based on Poly(3-Hexylthiophene), [6,6]-Phenyl-C61-Butyric Acid Methyl Ester, and Reduced Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
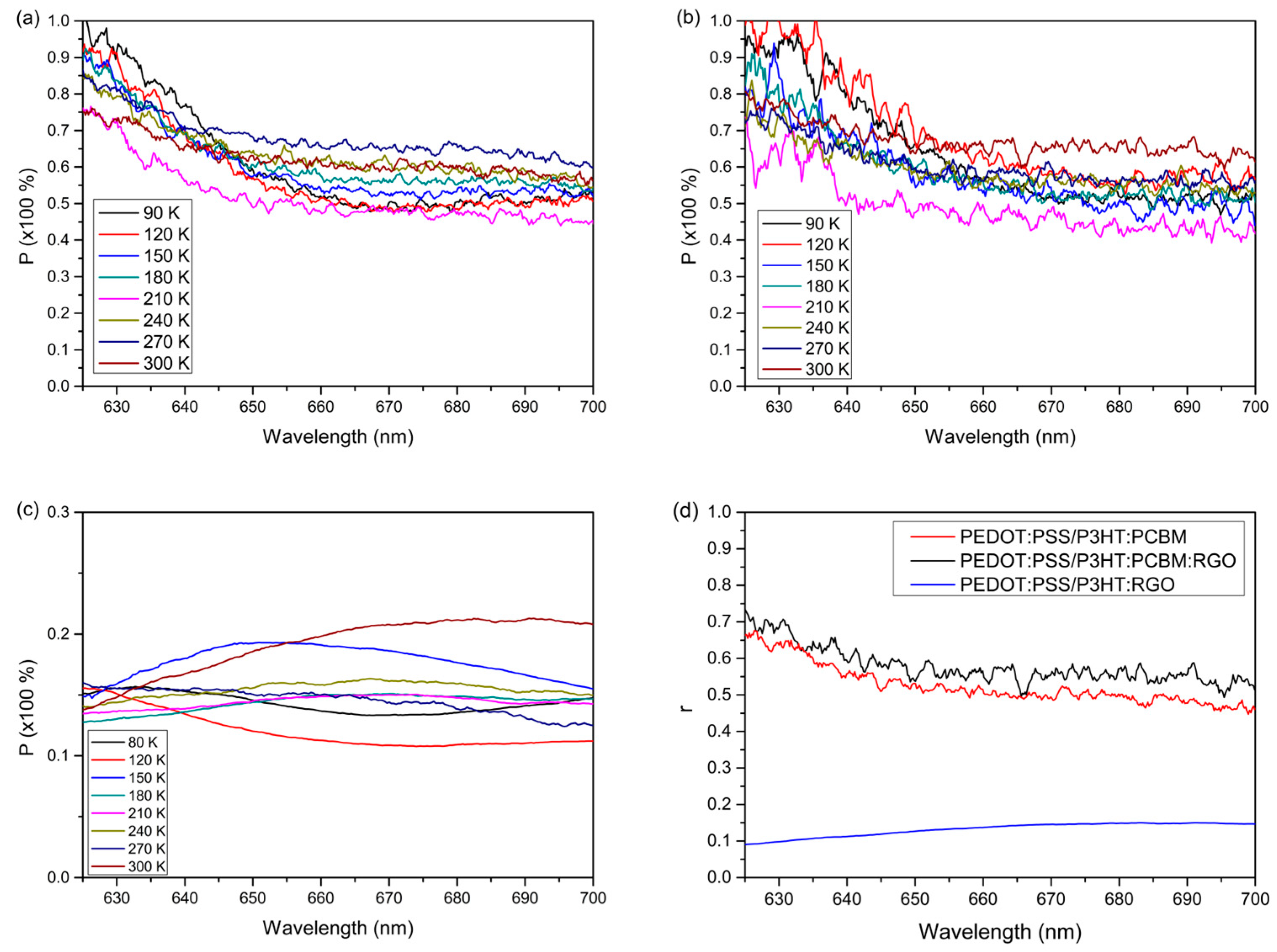
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bégué, D.; Guille, E.; Metz, S.; Arnaud, M.A.; Silva, H.S.; Seck, M.; Fayon, P.; Dagron-Lartigau, C.; Iratcabal, P.; Hiorns, R.C. Graphene-Based Acceptor Molecules for Organic Photovoltaic Cells: A Predictive Study Identifying High Modularity and Morphological Stability. RSC Adv. 2016, 6, 13653–13656. [Google Scholar] [CrossRef]
- Tang, H.; Hessel, C.M.; Wang, J.; Yang, N.; Yu, R.; Zhao, H.; Wang, D. Two-Dimensional Carbon Leading to New Photoconversion Processes. Chem. Soc. Rev. 2014, 43, 4281–4299. [Google Scholar] [CrossRef] [PubMed]
- Oklobia, O.; Shafai, T.S. A Study of Donor/Acceptor Interfaces in a Blend of P3HT/PCBM Solar Cell: Effects of Annealing and PCBM Loading on Optical and Electrical Properties. Solid. State Electron. 2013, 87, 64–68. [Google Scholar] [CrossRef]
- Kalonga, G.; Chinyama, K.; Munyati, O.; Maaza, M. Characterization and Optimization of P3HT and PCBM Blends for Photo-Absorption. J. Chem. Eng. Mater. Sci. 2013, 4, 93–102. [Google Scholar] [CrossRef]
- Shrotriya, V.; Ouyang, J.; Tseng, R.J.; Li, G.; Yang, Y. Absorption Spectra Modification in Poly(3-Hexylthiophene):Methanofullerene Blend Thin Films. Chem. Phys. Lett. 2005, 411, 138–143. [Google Scholar] [CrossRef]
- Khan, M.T.; Bhargav, R.; Kaur, A.; Dhawan, S.K.; Chand, S. Effect of Cadmium Sulphide Quantum Dot Processing and Post Thermal Annealing on P3HT/PCBM Photovoltaic Device. Thin Solid Films 2010, 519, 1007–1011. [Google Scholar] [CrossRef]
- Benatto, L.; de Jesus Bassi, M.; de Menezes, L.C.W.; Roman, L.S.; Koehler, M. Kinetic Model for Photoluminescence Quenching by Selective Excitation of D/A Blends: Implications for Charge Separation in Fullerene and Non-Fullerene Organic Solar Cells. J. Mater. Chem. C 2020, 8, 8755–8769. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Xu, Y.; Xian, K.; Gao, B.; Qin, J.; Zhang, J.; Wei, Z.; et al. Achieving Over 15% Efficiency in Organic Photovoltaic Cells via Copolymer Design. Adv. Mater. 2019, 31, e1808356. [Google Scholar] [CrossRef]
- Salleo, A. Charge Transport in Polymeric Transistors. Mater. Today 2007, 10, 38–45. [Google Scholar] [CrossRef]
- McCullough, R.D. The Chemistry of Conducting Polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Delgado, J.L.; Filippone, S.; Giacalone, F.; Herranz, M.Á.; Illescas, B.; Pérez, E.M.; Martín, N. Buckyballs. In Polyarenes II; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–64. [Google Scholar]
- Brunetti, F.G. Fullerene and Its Derivatives for Organic Solar Cells. In Progress in High-Efficient Solution Process Organic Photovoltaic Devices: Fundamentals, Materials, Devices and Fabrication; Springer: Berlin/Heidelberg, Germany, 2015; pp. 221–247. [Google Scholar]
- Li, C.-Z.; Yip, H.-L.; Jen, A.K.-Y. Functional Fullerenes for Organic Photovoltaics. J. Mater. Chem. 2012, 22, 4161. [Google Scholar] [CrossRef]
- Narra, S.; Tsai, S.; Awasthi, K.; Rana, S.; Diau, E.W.; Ohta, N. Photoluminescence of P3HT:PCBM Bulk Heterojunction Thin Films and Effect of External Electric Field. J. Chin. Chem. Soc. 2022, 69, 140–151. [Google Scholar] [CrossRef]
- Huang, P.; Huang, P.; Horng, Y.; Yang, C. Photoluminescence Study on Charge Transfer Behavior of Poly(3-hexylthiophene) and PCBM Blends. J. Chin. Chem. Soc. 2013, 60, 467–472. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Xiao, L.; Qiao, Q.; Dhakal, R.; Zhang, Z.; Gong, Q.; Galipeau, D.; Yan, X. Femtosecond Time-Resolved Fluorescence Study of P3HT/PCBM Blend Films. J. Phys. Chem. C 2010, 114, 14590–14600. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, J.; He, Q.; Cao, X.; Tan, C.; Chen, H.; Yan, Q.; Zhang, H. Graphene-Based Materials for Solar Cell Applications. Adv. Energy Mater. 2014, 4, 1300574. [Google Scholar] [CrossRef]
- Singh, E.; Nalwa, H.S. Stability of Graphene-Based Heterojunction Solar Cells. RSC Adv. 2015, 5, 73575–73600. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. 2009, 114, 832–842. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Zeng, G.; Wu, Y.; Liu, Y.; Jiang, Q.; Gu, S. Three dimensional graphene based materials: Synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Colloid Interface Sci. 2015, 221, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. Dimension-tailored functional graphene structures for energy conversion and storage. Nanoscale 2013, 5, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of Reduced Graphene Oxide: Effects of Reduction Variables on Electrical Conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar] [CrossRef]
- Mekki, A.; Dere, A.; Mensah-Darkwa, K.; Al-Ghamdi, A.; Gupta, R.K.; Harrabi, K.; Farooq, W.A.; El-Tantawy, F.; Yakuphanoglu, F. Graphene Controlled Organic Photodetectors. Synth. Met. 2016, 217, 43–56. [Google Scholar] [CrossRef]
- Öztürk, T. Effect of Various PCBM Doping on the Interfacial Layer of Al/PCBM:ZnO/p-Si Photodiodes. J. Mater. Sci. Mater. Electron. 2021, 32, 10180–10193. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Huang, Y.; Ma, Y.; Yin, S.; Zhang, X.; Sun, W.; Chen, Y. Organic Photovoltaic Devices Based on a Novel Acceptor Material: Graphene. Adv. Mater. 2008, 20, 3924–3930. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer Photovoltaic Cells Based on Solution-Processable Graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Z.; Li, L.; Agbolaghi, S.; Mousavi, S. Improved Stability in P3HT:PCBM Photovoltaics by Incorporation of Well-Designed Polythiophene/Graphene Compositions. Polym. Int. 2020, 69, 833–846. [Google Scholar] [CrossRef]
- Schura, A.B.; Sá, S.; Silva, R.; de Santana, H.; Marletta, A.; Therézio, E. Energy Transfer Processes in Electrochemical P3HT Thin Films. J. Mater. Sci. Mater. Electron. 2019, 30, 4289–4295. [Google Scholar] [CrossRef]
- Therézio, E.M.; Franchello, F.; Dias, I.F.L.; Laureto, E.; Foschini, M.; Bottecchia, O.L.; de Santana, H.; Duarte, J.L.; Marletta, A. Emission Ellipsometry as a Tool for Optimizing the Electrosynthesis of Conjugated Polymers Thin Films. Thin Solid Films 2013, 527, 255–260. [Google Scholar] [CrossRef]
- Basilio, F.C.; Campana, P.T.; Therézio, E.M.; Neto, N.M.B.; Serein-Spirau, F.; Silva, R.A.; Oliveira, O.N., Jr.; Marletta, A. Ellipsometric Raman Spectroscopy. J. Phys. Chem. C 2016, 120, 25101–25109. [Google Scholar] [CrossRef]
- Marchezi, P.E.; Sonai, G.G.; Hirata, M.K.; Schiavon, M.A.; Nogueira, A.F. Understanding the Role of Reduced Graphene Oxide in the Electrolyte of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 23368–23376. [Google Scholar] [CrossRef]
- Gwydion. Available online: http://gwyddion.net (accessed on 10 August 2024).
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness Parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Abdullahi, S.; Güner, S.; Koseoglu, Y.; Musa, I.; Adamu, B.; Abdulhamid, M. Sımple Method For The Determınatıon of Band Gap of a Nanopowdered Sample Usıng Kubelka Munk Theory. J. Niger. Assoc. Math. Phys. 2016, 35, 241–246. [Google Scholar]
- Stöckert, D.; Kessel, R.; Schultze, J.W. Absorption, Photocurrent and Photoelectron Spectra of Heterocyclic Polymers. Synth. Met. 1991, 41, 1295–1300. [Google Scholar] [CrossRef]
- Ohmori, Y.; Uchida, M.; Muro, K.M.K.; Yoshino, K.Y.K. Visible-Light Electroluminescent Diodes Utilizing Poly(3-Alkylthiophene). Jpn. J. Appl. Phys. 1991, 30, L1938. [Google Scholar] [CrossRef]
- Marrocchi, A.; Lanari, D.; Facchetti, A.; Vaccaro, L. Poly(3-Hexylthiophene): Synthetic Methodologies and Properties in Bulk Heterojunction Solar Cells. Energy Environ. Sci. 2012, 5, 8457–8474. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kobayashi, T.; Nagase, T.; Naito, H. Photoinduced Absorption in P3HT/PCBM Bulk Heterostructures. Mater. Sci. Forum 2010, 658, 503–506. [Google Scholar] [CrossRef]
- Müllerová, J.; Kaiser, M.; Nádaždy, V.; Šiffalovič, P.; Majková, E. Optical Absorption Study of P3HT:PCBM Blend Photo-Oxidation for Bulk Heterojunction Solar Cells. Sol. Energy 2016, 134, 294–301. [Google Scholar] [CrossRef]
- Panzer, F.; Sommer, M.; Bässler, H.; Thelakkat, M.; Köhler, A. Spectroscopic Signature of Two Distinct H-Aggregate Species in Poly(3-Hexylthiophene). Macromolecules 2015, 48, 1543–1553. [Google Scholar] [CrossRef]
- Palacios, R.E.; Barbara, P.F. Single Molecule Spectroscopy of Poly 3-Octyl-Thiophene (P3OT). J. Fluoresc. 2007, 17, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Mahakul, P.C.; Mahanandia, P. RGO Induced Structural and Microstructural Properties of P3HT in the Performance and Stability of Polymer Solar Cells. Mater. Res. Express 2019, 6, 125338. [Google Scholar] [CrossRef]
- Bakour, A.; Baitoul, M.; Bajjou, O.; Massuyeau, F.; Faulques, E. Improving Optical Properties of in Situ Reduced Graphene Oxide/Poly(3-Hexylthiophene) Composites. Mater. Res. Express 2017, 4, 25031. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Spyropoulos, G.D.; Stratakis, E.; Kymakis, E. Solution-Processable Graphene Linked to 3,5-Dinitrobenzoyl as an Electron Acceptor in Organic Bulk Heterojunction Photovoltaic Devices. Carbon 2012, 50, 5554–5561. [Google Scholar] [CrossRef]
- Ehrenreich, P.; Birkhold, S.T.; Zimmermann, E.; Hu, H.; Kim, K.-D.; Weickert, J.; Pfadler, T.; Schmidt-Mende, L. H-Aggregate Analysis of P3HT Thin Films-Capability and Limitation of Photoluminescence and UV/Vis Spectroscopy. Sci. Rep. 2016, 6, 32434. [Google Scholar] [CrossRef]
- Chang, S.H.; Chiang, C.-H.; Cheng, H.-M.; Tai, C.-Y.; Wu, C.-G. Broadband Charge Transfer Dynamics in P3HT:PCBM Blended Film. Opt. Lett. 2013, 38, 5342. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Nonaka, K. Light Depolarization in Off-Specular Reflection on Submicro Rough Metal Surfaces with Imperfectly Random Roughness. Rev. Sci. Instrum. 2015, 86, 023107. [Google Scholar] [CrossRef]
- Janssen, G.; Aguirre, A.; Goovaerts, E.; Vanlaeke, P.; Poortmans, J.; Manca, J. Optimization of Morphology of P3HT/PCBM Films for Organic Solar Cells: Effects of Thermal Treatments and Spin Coating Solvents. Eur. Phys. J. Appl. Phys. 2007, 37, 287–290. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Liu, F.Q.; Wang, L.J.; Yin, W.; Wang, Z.G. Blue-Shift Photoluminescence from Porous InAlAs. Semicond. Sci. Technol. 2010, 25, 115006. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakajima, S.; Park, D.H.; Sugimoto, R. Thermochromism, Photochromism and Anomalous Temperature Dependence of Luminescence in Poly(3-Alkylthiophene) Film. Jpn. J. Appl. Phys. 1988, 27, L716. [Google Scholar] [CrossRef]
- Yoshino, K.; Manda, Y.; Sawada, K.; Onoda, M.; Sugimoto, R. Anomalous Dependences of Luminescence of Poly(3-Alkylthiophene) on Temperature and Alkyl Chain Length. Solid. State Commun. 1989, 69, 143–146. [Google Scholar] [CrossRef]
- Kanemoto, K.; Yasui, M.; Higuchi, T.; Kosumi, D.; Akai, I.; Karasawa, T.; Hashimoto, H. Spectroscopic Investigation of Excitons, Photocarriers, and Bias-Induced Carriers in Regioregular Poly(3-Alkylthiophene). Phys. Rev. B 2011, 83, 205203. [Google Scholar] [CrossRef]
- Therézio, E.M.; Rodrigues, P.C.; Tozoni, J.R.; Marletta, A.; Akcelrud, L. Energy-Transfer Processes in Donor–Acceptor Poly(Fluorenevinylene-Alt-4,7-Dithienyl-2,1,3-Benzothiadiazole). J. Phys. Chem. C 2013, 117, 13173–13180. [Google Scholar] [CrossRef]
- Therézio, E.M.; Piovesan, E.; Anni, M.; Silva, R.A.; Oliveira, O.N.; Marletta, A. Substrate/Semiconductor Interface Effects on the Emission Efficiency of Luminescent Polymers. J. Appl. Phys. 2011, 110, 044504. [Google Scholar] [CrossRef]
- Clark, J.; Silva, C.; Friend, R.H.; Spano, F.C. Role of Intermolecular Coupling in the Photophysics of Disordered Organic Semiconductors: Aggregate Emission in Regioregular Polythiophene. Phys. Rev. Lett. 2007, 98, 206406. [Google Scholar] [CrossRef]
- Cimrová, V.; Remmers, M.; Neher, D.; Wegner, G. Polarized Light Emission from LEDs Prepared by the Langmuir-Blodgett Technique. Adv. Mater. 1996, 8, 146–149. [Google Scholar] [CrossRef]
- Ishihara, H.; Kimura, I.; Saito, K.; Ono, H. Infrared Studies on Segmented Polyurethane-Urea Elastomers. J. Macromol. Sci. Part B 1974, 10, 591–618. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, Best Seller in Polymer Photovoltaic Research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef]
- Kobayashi, T.; Terada, Y.; Nagase, T.; Naito, H. Determination of Carrier Lifetime in Bulk-Heterojunction Solar Cells by Continuous-Wave Photoinduced Absorption Spectroscopy. Appl. Phys. Express 2011, 4, 126602. [Google Scholar] [CrossRef]

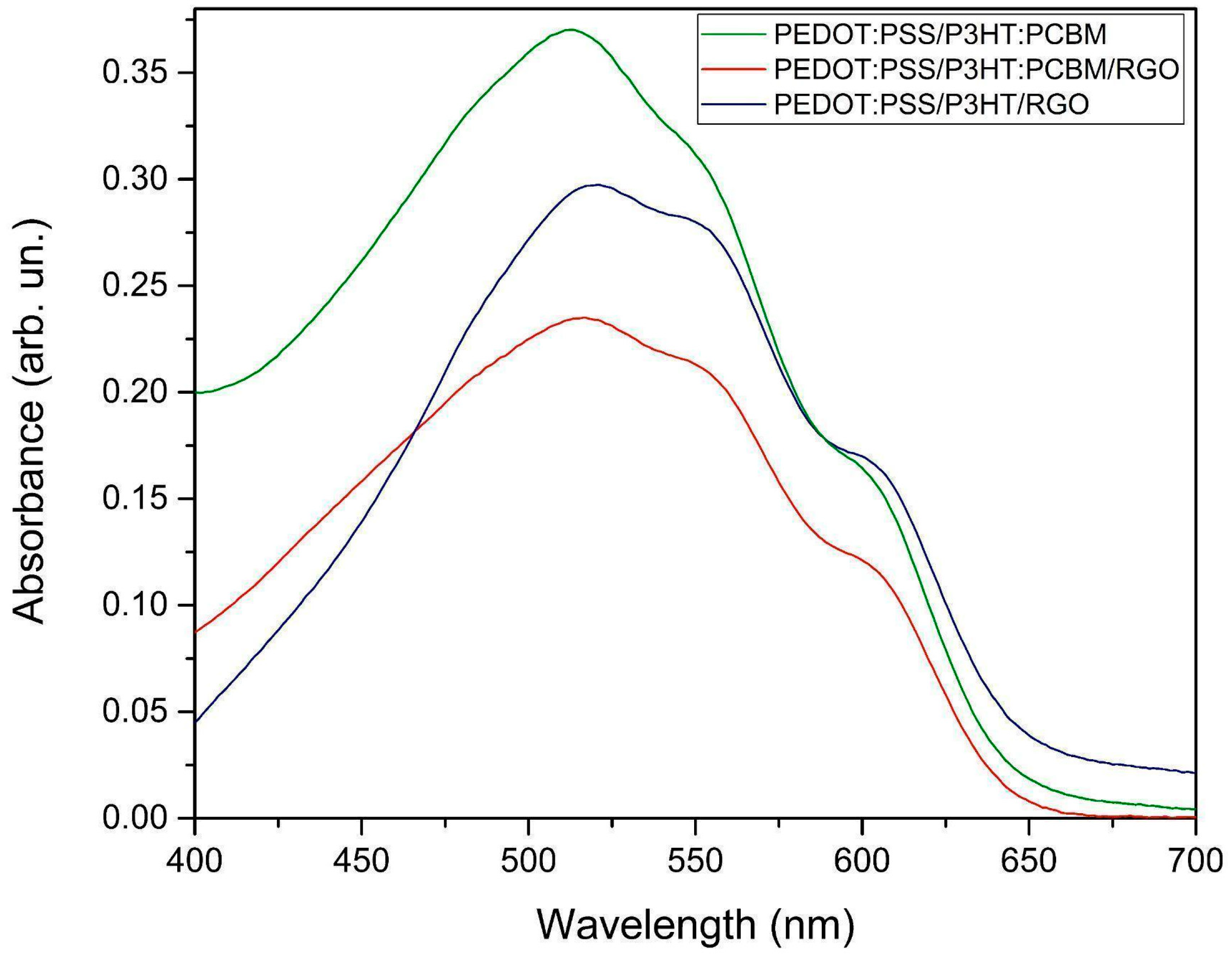

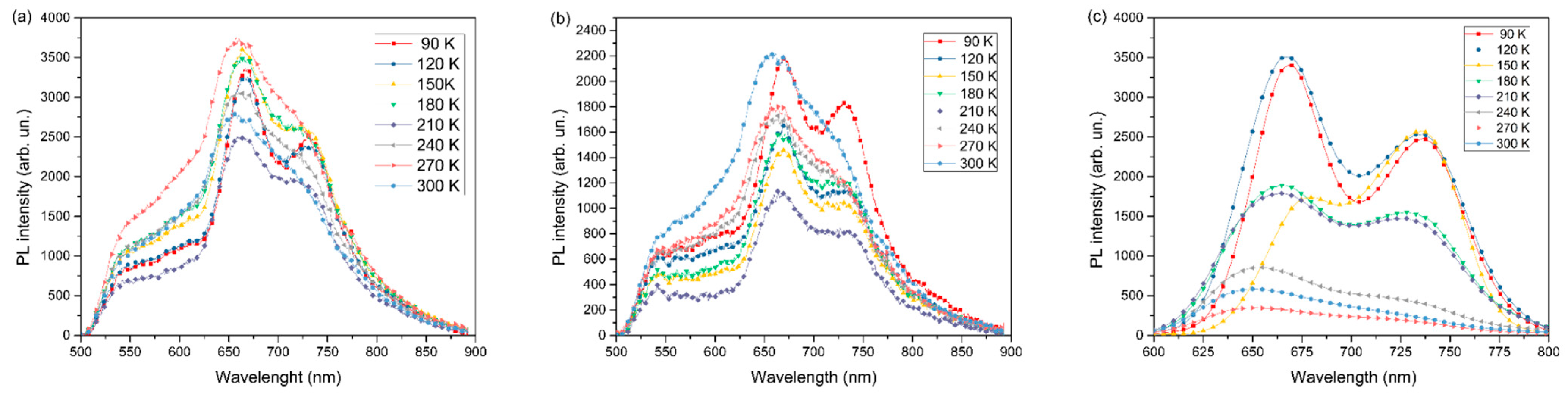
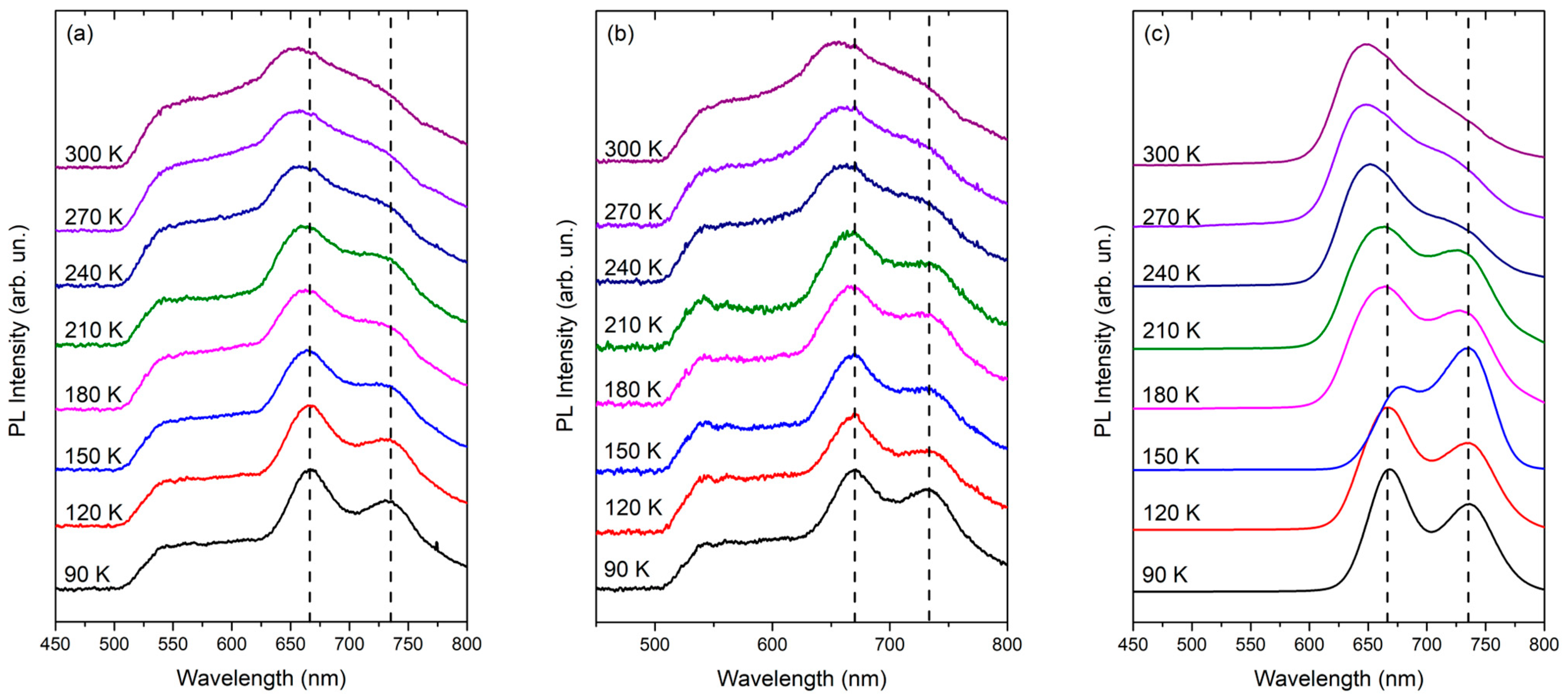
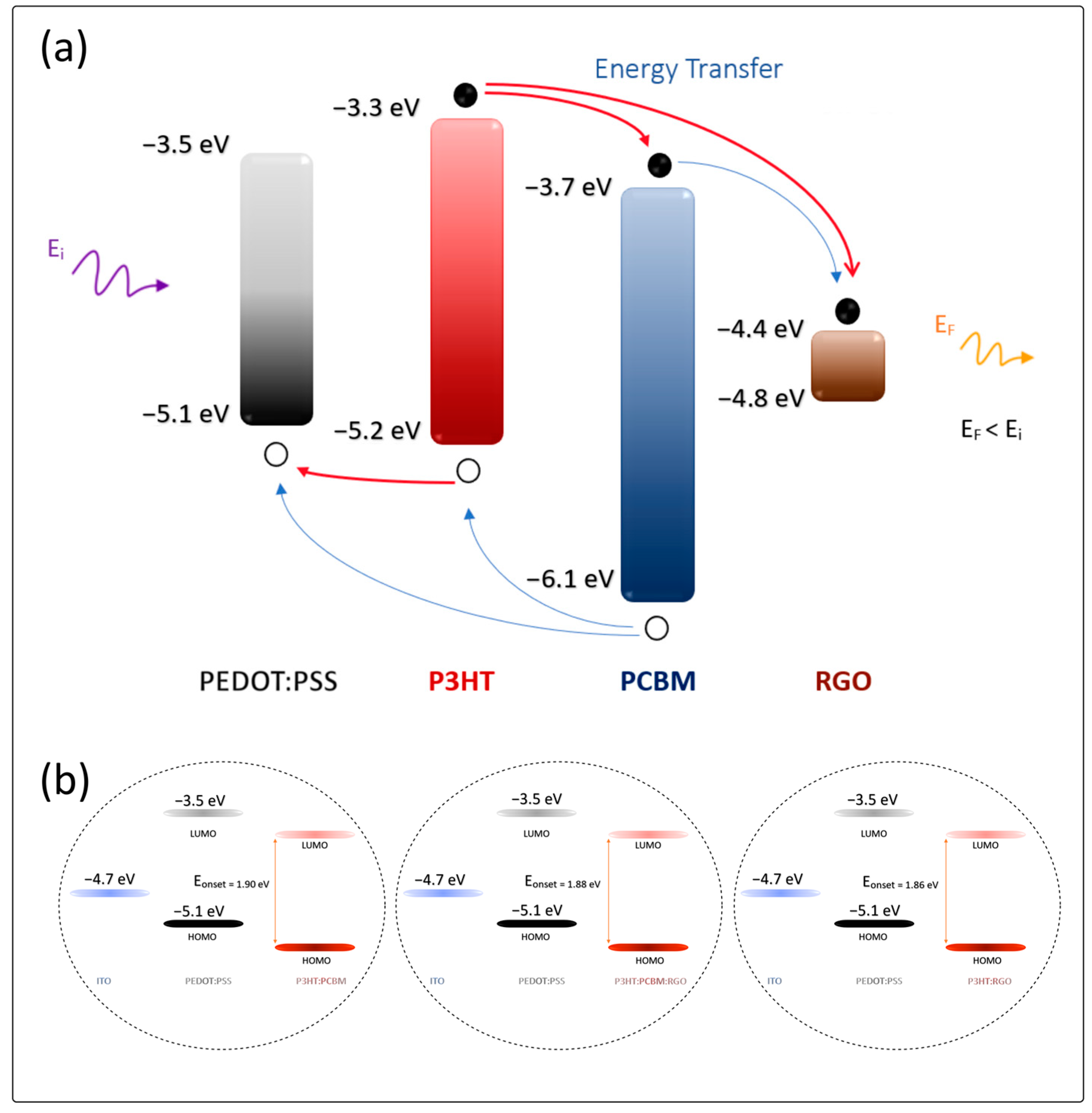

| Sample | Wavelength λc (nm) | Eonset (eV) |
|---|---|---|
| PEDOT:PSS/P3HT:PCBM | 651.00 ± 0.06 | 1.90 ± 0.09 |
| PEDOT:PSS/P3HT:PCBM:RGO | 660.00 ± 0.06 | 1.88 ± 0.09 |
| PEDOT:PSS/P3HT:RGO | 665.00 ± 0.06 | 1.86 ± 0.07 |
| Sample | Mean Roughness | Skewness | Kurtosis |
|---|---|---|---|
| PEDOT:PSS/P3HT:PCBM | 0.70 ± 0.01 | 5.32 ± 0.06 | 110 ± 1 |
| PEDOT:PSS/P3HT:PCBM:RGO | 1.26 ± 0.02 | −0.19 ± 0.01 | 4.22 ± 0.05 |
| PEDOT:PSS/P3HT:RGO | 4.66 ± 0.06 | −0.16 ± 0.01 | 3.40 ± 0.04 |
| S ≃ I1/I0 | |||
|---|---|---|---|
| Temperature (K) | PEDOT:PSS/ P3HT:PCBM | PEDOT:PSS/ P3HT:PCBM:RGO | PEDOT:PSS/ P3HT:RGO |
| 90 ± 1 | 0.74 ± 0.01 | 0.84 ± 0.02 | 0.73 ± 0.01 |
| 120 ± 1 | 0.86 ± 0.02 | 0.85 ± 0.02 | 0.87 ± 0.02 |
| 150 ± 1 | 0.91 ± 0.02 | 0.91 ± 0.02 | 1.12 ± 0.02 |
| 180 ± 1 | 0.93 ± 0.02 | 0.95 ± 0.02 | 0.96 ± 0.02 |
| 210 ± 1 | 0.95 ± 0.02 | 0.95 ± 0.02 | 0.96 ± 0.02 |
| 240 ± 1 | 0.95 ± 0.02 | 0.96 ± 0.02 | 0.92 ± 0.02 |
| 270 ± 1 | 0.96 ± 0.02 | 0.96 ± 0.02 | 0.94 ± 0.02 |
| 300 ± 1 | 0.95 ± 0.02 | 0.96 ± 0.02 | 0.93 ± 0.01 |
| Δν = ν0 − ν1 (eV) | |||
|---|---|---|---|
| Temperature (K) | PEDOT:PSS/ P3HT:PCBM | PEDOT:PSS/ P3HT:PCBM:RGO | PEDOT:PSS/ P3HT:RGO |
| 90 ± 1 | 0.166 ± 0.003 | 0.165 ± 0.003 | 0.175 ± 0.004 |
| 120 ± 1 | 0.172 ± 0.003 | 0.171 ± 0.003 | 0.175 ± 0.004 |
| 150 ± 1 | 0.182 ± 0.004 | 0.174 ± 0.003 | 0.148 ± 0.003 |
| 180 ± 1 | 0.189 ± 0.004 | 0.183 ± 0.004 | 0.181 ± 0.004 |
| 210 ± 1 | 0.186 ± 0.004 | 0.189 ± 0.004 | 0.185 ± 0.004 |
| 240 ± 1 | 0.197 ± 0.004 | 0.166 ± 0.003 | 0.199 ± 0.004 |
| 270 ± 1 | 0.202 ± 0.004 | 0.188 ± 0.004 | 0.205 ± 0.004 |
| 300 ± 1 | 0.206 ± 0.004 | 0.209 ± 0.004 | 0.211 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolbow, A.C.H.; Rambo, E.C.; Santos, M.R.N.d.; Marchezi, P.E.; Nogueira, A.F.; Marletta, A.; Ramos, R.J.; Therézio, E.M. Emission Ellipsometry Study in Polymeric Interfaces Based on Poly(3-Hexylthiophene), [6,6]-Phenyl-C61-Butyric Acid Methyl Ester, and Reduced Graphene Oxide. C 2024, 10, 83. https://doi.org/10.3390/c10030083
Kolbow ACH, Rambo EC, Santos MRNd, Marchezi PE, Nogueira AF, Marletta A, Ramos RJ, Therézio EM. Emission Ellipsometry Study in Polymeric Interfaces Based on Poly(3-Hexylthiophene), [6,6]-Phenyl-C61-Butyric Acid Methyl Ester, and Reduced Graphene Oxide. C. 2024; 10(3):83. https://doi.org/10.3390/c10030083
Chicago/Turabian StyleKolbow, Ana Clarissa Henrique, Everton Crestani Rambo, Maria Ruth Neponucena dos Santos, Paulo Ernesto Marchezi, Ana Flávia Nogueira, Alexandre Marletta, Romildo Jerônimo Ramos, and Eralci Moreira Therézio. 2024. "Emission Ellipsometry Study in Polymeric Interfaces Based on Poly(3-Hexylthiophene), [6,6]-Phenyl-C61-Butyric Acid Methyl Ester, and Reduced Graphene Oxide" C 10, no. 3: 83. https://doi.org/10.3390/c10030083
APA StyleKolbow, A. C. H., Rambo, E. C., Santos, M. R. N. d., Marchezi, P. E., Nogueira, A. F., Marletta, A., Ramos, R. J., & Therézio, E. M. (2024). Emission Ellipsometry Study in Polymeric Interfaces Based on Poly(3-Hexylthiophene), [6,6]-Phenyl-C61-Butyric Acid Methyl Ester, and Reduced Graphene Oxide. C, 10(3), 83. https://doi.org/10.3390/c10030083









