Experimental Investigation of Use of Monoethanolamine with Iron Oxide Nanoparticles in a 10 kg per Day Pilot CO2 Capture Plant: Implications for Commercialization
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanoparticles
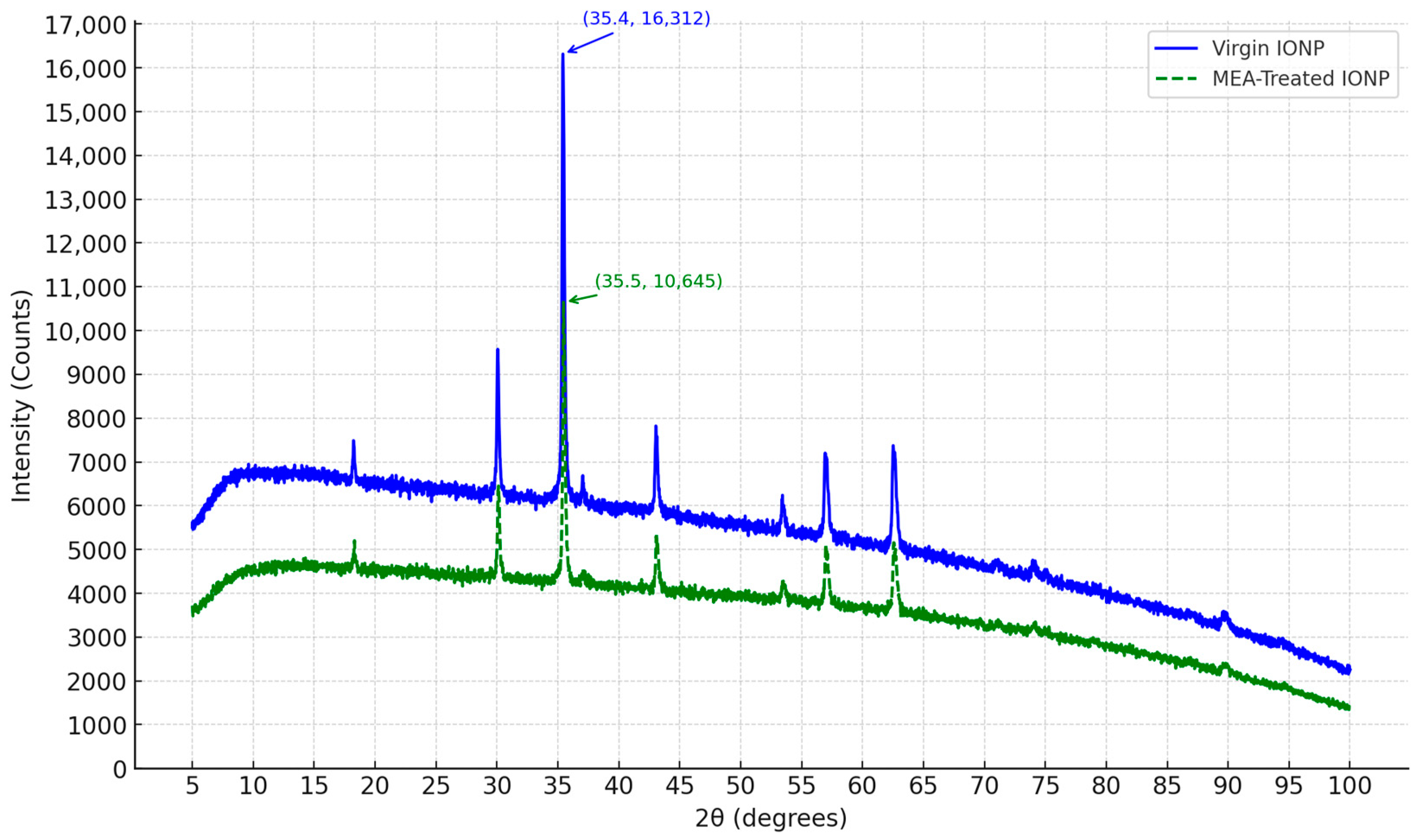
2.2. XRD Analysis
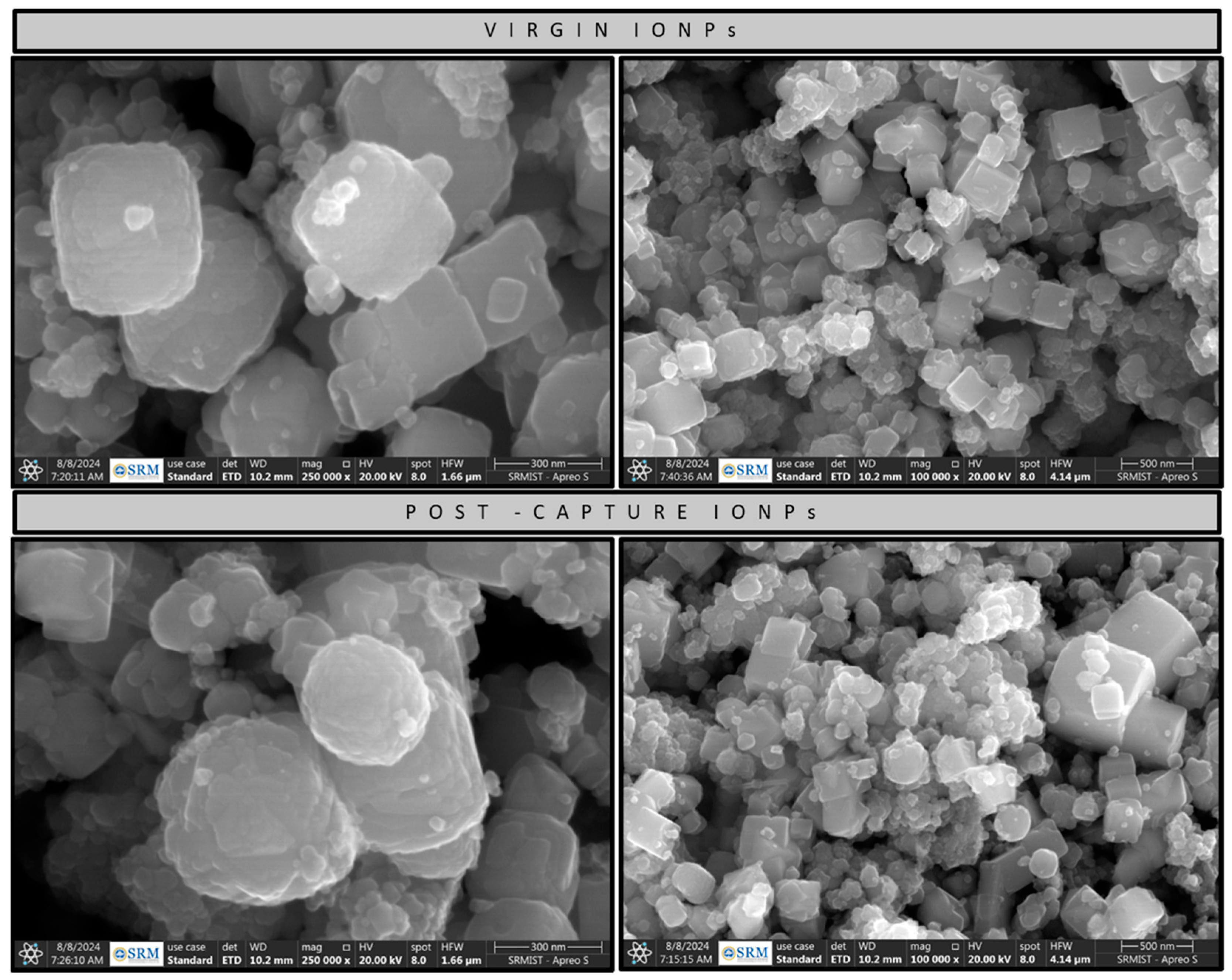
2.3. HR-SEM Analysis
3. Experimental Setup
3.1. Materials and Reagents
3.2. CO2 Absorption and Desorption–Pilot CO2 Capture Plant
3.3. Nanoparticle Integration
3.4. CO2 Loading Measurement in Solvent–UOP Method 829-82
4. Results and Discussion
4.1. Characterization of IONPs
4.1.1. XRD Characterization
4.1.2. Virgin IONP Characterization
4.1.3. Post-CO2 Capture Process IONP Characterization
4.1.4. Impacts of Crystallite Size of IONPs on CO2 Capture Process Using MEA as Solvent
4.1.5. HR-SEM Characterization
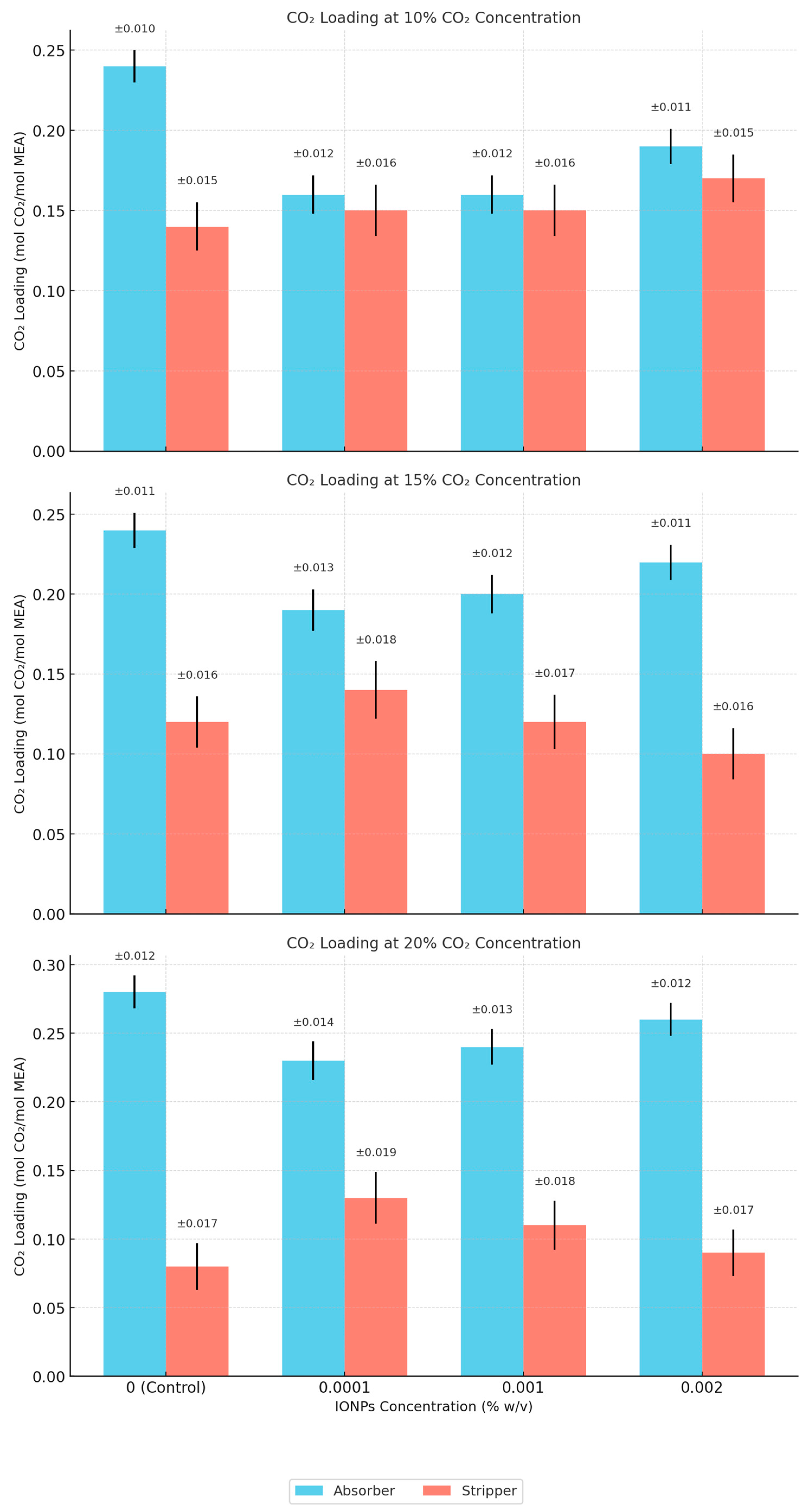
4.2. CO2 Capture and Separation
4.3. Commercial Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEA | Monoethanolamine |
| CO2 | Carbon dioxide |
| IONPs | Iron oxide nanoparticles |
| PLC | Programmable Logic Controller |
| XRD | X-Ray Diffraction |
| HR-SEM | High-Resolution Transmission Electron Microscopy |
| CCS | Carbon capture and storage |
| PCC | Post-combustion capture |
| IPCC | Intergovernmental Panel on Climate Change |
| INDC | Intended Nationally Determined Contributions |
| TRL | Technology Readiness Level |
| OPEX | Operating Expenses |
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Government of India. Submission on the Road to COP21 Paris: Intended Nationally Determined Contributions (INDCs); Ministry of Environment, Forest and Climate Change, Government of India: New Delhi, India, 2015. [Google Scholar]
- IEA. Coal 2021, IEA, Paris. 2021. Available online: https://www.iea.org/reports/coal-2021 (accessed on 10 April 2025).
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, P.V. The absorption of carbon dioxide into aqueous solutions of alkali. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Iliuta, M.C.; Larachi, F.; Păvăloiu, R.D. Carbon dioxide capture by reactive absorption with chemical solvents: A review. Ind. Eng. Chem. Res. 2007, 46, 6413–6437. [Google Scholar]
- Rubin, E.S.; Chen, C. Technological learning in CCS: Lessons from historical development trajectories. Int. J. Greenh. Gas Control 2019, 88, 246–261. [Google Scholar]
- Kim, S.; Shi, H.; Lee, J.Y. CO2 absorption mechanism in amine solvents and enhancement of CO2 capture capability in blended amine solvent. Int. J. Greenh. Gas Control 2016, 45, 181–188. [Google Scholar] [CrossRef]
- Frimpong, R.A.; Johnson, D.; Richburg, L.; Hogston, B.; Remias, J.E.; Neathery, J.K.; Liu, K. Comparison of solvent performance for CO2 capture from coal-derived flue gas: A pilot scale study. Chem. Eng. Res. Des. 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Omoregbe, O.; Mustapha, A.N.; Steinberger-Wilckens, R.; El-Kharouf, A.; Onyeaka, H. Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018. Energy Rep. 2020, 6, 1200–1212. [Google Scholar] [CrossRef]
- Blomen, E.; Hendriks, C.; Neele, F. Capture technologies: Improvements and promising developments. Energy Procedia 2009, 1, 1505–1512. [Google Scholar] [CrossRef]
- Ashkanani, H.E.; Wang, R.; Shi, W.; Siefert, N.S.; Thompson, R.L.; Smith, K.; Steckel, J.A.; Gamwo, I.K.; Hopkinson, D.; Resnik, K.; et al. Levelized cost of CO2 captured using fve physical solvents in pre-combustion applications. Int. J. Greenh. Gas Control 2020, 101, 103135. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Chakma, A. CO2 capture processes: Opportunities for improved energy efficiencies. Energy Convers. Manag. 1997, 38, S51–S56. [Google Scholar] [CrossRef]
- Selvi, P.P.; Baskar, R. CO2 mitigation studies in packed absorption column using iron oxide nano fluid: Scientific paper. Chem. Ind. Chem. Eng. Q. 2023, 29, 161–167. [Google Scholar] [CrossRef]
- Zarei, F.; Keshavarz, P. High performance CO2 Absorption/Desorption using Amine-Functionalized magnetic nanoparticles. Sep. Purif. Technol. 2023, 323, 124438. [Google Scholar] [CrossRef]
- ProMarket Reports. (Year). Iron(III) oxide nanoparticles/nanopowder (Report No. 68934). ProMarket Reports. Available online: https://www.promarketreports.com/reports/ironiii-oxide-nanoparticles-nanopowder-68934 (accessed on 10 April 2025).
- Ito, A.; Yoshioka, K.; Masumoto, S.; Sato, K.; Hatae, Y.; Nakai, T.; Yamazaki, T.; Takahashi, M.; Tanoue, S.; Horie, M. Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells. Sci. Rep. 2020, 10, 13605. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shao, B.; Zhu, J.; Pan, F.; Hu, J.; Wang, M.; Liu, H. In situ electromagnetic induction heating for CO2 temperature swing adsorption on magnetic Fe3O4/N-doped porous carbon. Energy Fuels 2020, 34, 14439–14446. [Google Scholar] [CrossRef]
- Shakir, S.W.; Ahmed, S.M.R.; Wiheeb, A.D. Improvement of CO2 absorption/desorption rate using new nano-fluid. Int. J. Heat Technol. 2021, 39, 851–857. [Google Scholar] [CrossRef]
- Hafizi, A.; Rajabzadeh, M.; Khalifeh, R. Enhanced CO2 absorption and desorption efficiency using DETA functionalized nanomagnetite/water nano-fluid. J. Environ. Chem. Eng. 2020, 8, 103845. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Zavabeti, A.; Christofferson, A.J.; Meftahi, N.; Russo, S.P.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Engineered assembly of water-dispersible nanocatalysts enables low-cost and green CO2 capture. Nat. Commun. 2022, 13, 1249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarei, F.; Keshavarz, P. Intensification of CO2 absorption and desorption by metal/non-metal oxide nanoparticles in bubble columns. Environ. Sci. Pollut. Res. 2022, 30, 19278–19291. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Alobaid, F.; Almoslh, A.; Epple, B. Application of Nanofluids in CO2 Absorption: A Review. Appl. Sci. 2022, 12, 3200. [Google Scholar] [CrossRef]
- Komati, S.; Suresh, A.K. CO2 absorption into amine solutions: A novel strategy for intensification based on the addition of ferrofluids. J. Chem. Technol. Biotechnol. 2008, 83, 1094–1100. [Google Scholar] [CrossRef]
- Wesolowski, D.J.; Palmer, D.A.; Machesky, M.L. Effect of Amines on the Surface Charge Properties of Iron Oxides. J. Solut. Chem. 2009, 38, 925–945. [Google Scholar] [CrossRef]
- Zarei, F.; Jahromi, F.B.; Elhambakhsh, A.; Keshavarz, P. Enhanced CO2 absorption and reduced regeneration energy consumption using modified magnetic NPs. Energy 2023, 278, 127776. [Google Scholar] [CrossRef]
- UOP Method 829-82; Titrimetric Determination of CO2 in Ethanolamines. ASTM International Publications: West Conshohocken, PA, USA, 1982.
- Arnold, J.L.; Pearce, R.L. Analysis of Glycol and Amine Solutions and the Interpretation Thereof; Gas conditioning Inst.: Liberal, Kansas, 1960. [Google Scholar]
- Rozaiddin, S.A.M.; Lau, K.K. A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles. Sustainability 2022, 14, 4750. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical analysis of CO2 capture pathways and technologies. J. Environ. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- Bellal, A.; Hou, X.; Nasah, J.; Van der Watt, J.; Laudal, D.; Hoffman, J. Performance and economic viability assessment of a novel CO2 adsorbent for manufacturing and integration with coal power plants. Chem. Eng. J. 2024, 498, 155604. [Google Scholar] [CrossRef]
- Plaza, J.M.; Van Wagener, D.; Rochelle, G.T. Modeling CO2 capture with aqueous monoethanolamine. Energy Procedia 2009, 1, 1171–1178. [Google Scholar] [CrossRef]
- Sanpasertparnich, T.; Idem, R.; Bolea, I.; DeMontigny, D.; Tontiwachwuthikul, P. Integration of post-combustion capture and storage into a pulverized coal-fired power plant. Int. J. Greenh. Gas Control 2010, 4, 499–510. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]




| Element | Net Counts | Weight % |
|---|---|---|
| Virgin IONPs | ||
| O | 8544 | 33.34 |
| Fe | 13,827 | 66.66 |
| Total | 100 | |
| Post Capture IONPs | ||
| O | 10,051 | 33.22 |
| Na | 176 | 1.03 |
| S | 508 | 0.7 |
| Fe | 16,244 | 65.04 |
| Total | 100 | |
| Parameter (1) | Variation Range |
|---|---|
| Solvent Concentration | 20% and 30% wt/wt |
| IONP addition | 0.0001% w/v, 0.001% w/v, and 0.002% w/v |
| CO2 Concentration | 10%, 15%, 20% vol/vol |
| 30% MEA | 20% MEA | 30% MEA | ||||||
|---|---|---|---|---|---|---|---|---|
| Without IONPs | 0.0001% w/v | 0.001% w/v | 0.002% w/v | 0.0001% w/v | 0.001% w/v | 0.002% w/v | ||
| 10% CO2 | CO2 Loading Absorber [1] | 0.24 | 0.16 | 0.16 | 0.19 | 0.25 | 0.25 | 0.27 |
| CO2 Loading Stripper [1] | 0.14 | 0.15 | 0.15 | 0.17 | 0.15 | 0.13 | 0.09 | |
| Stripper Temperature (°C) [2] | 106 | 109 | 108 | 107 | 106 | 105 | 100 | |
| CO2 Separation Efficiency (%) | 42% | 6% | 6% | 11% | 40% | 48% | 67% | |
| 15% CO2 | CO2 Loading Absorber [1] | 0.24 | 0.19 | 0.2 | 0.22 | 0.25 | 0.26 | 0.29 |
| CO2 Loading Stripper [1] | 0.12 | 0.14 | 0.12 | 0.1 | 0.12 | 0.1 | 0.06 | |
| Stripper Temperature (°C) [2] | 104 | 108 | 107 | 105 | 105 | 103 | 98 | |
| CO2 Separation Efficiency (%) | 50% | 26% | 40% | 55% | 52% | 62% | 79% | |
| 20% CO2 | CO2 Loading Absorber [1] | 0.28 | 0.23 | 0.24 | 0.26 | 0.3 | 0.31 | 0.34 |
| CO2 Loading Stripper [1] | 0.08 | 0.13 | 0.11 | 0.09 | 0.09 | 0.08 | 0.06 | |
| Stripper Temperature (°C) [2] | 105 | 108 | 106 | 103 | 104 | 102 | 98 | |
| CO2 Separation Efficiency (%) | 71% | 43% | 54% | 65% | 70% | 74% | 82% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, S.; Soupramaniane, G.; Saravanane, R. Experimental Investigation of Use of Monoethanolamine with Iron Oxide Nanoparticles in a 10 kg per Day Pilot CO2 Capture Plant: Implications for Commercialization. C 2025, 11, 29. https://doi.org/10.3390/c11020029
Prabhu S, Soupramaniane G, Saravanane R. Experimental Investigation of Use of Monoethanolamine with Iron Oxide Nanoparticles in a 10 kg per Day Pilot CO2 Capture Plant: Implications for Commercialization. C. 2025; 11(2):29. https://doi.org/10.3390/c11020029
Chicago/Turabian StylePrabhu, Sriniwasa, Govindaradjane Soupramaniane, and Raman Saravanane. 2025. "Experimental Investigation of Use of Monoethanolamine with Iron Oxide Nanoparticles in a 10 kg per Day Pilot CO2 Capture Plant: Implications for Commercialization" C 11, no. 2: 29. https://doi.org/10.3390/c11020029
APA StylePrabhu, S., Soupramaniane, G., & Saravanane, R. (2025). Experimental Investigation of Use of Monoethanolamine with Iron Oxide Nanoparticles in a 10 kg per Day Pilot CO2 Capture Plant: Implications for Commercialization. C, 11(2), 29. https://doi.org/10.3390/c11020029







