The Role of Functionalization in the Applications of Carbon Materials: An Overview
Abstract
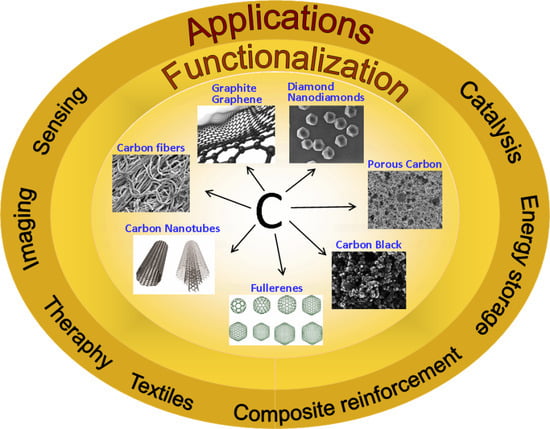
:1. Introduction
2. Functionalization of Carbon Materials and Applications
2.1. Solubilization of Carbon Materials and Bio-Applications
2.1.1. Graphite and Graphene
2.1.2. Carbon Nanotubes
2.1.3. Fullerenes
2.1.4. Diamond
2.2. Electrochemistry, Energy Conversion, Storage and Sensing
2.2.1. Graphite, Graphitic Carbons, and Graphene
2.2.2. Porous Carbon and Carbon Fibers and Felt
2.2.3. Carbon Nanotubes
2.2.4. Fullerenes
2.2.5. D Hybrid Structures
2.3. Composite Materials
2.3.1. Carbon Nanofibers and Carbon Nanotubes
2.3.2. Graphene Oxide
2.3.3. Nanodiamonds
3. Conclusions
Funding
Conflicts of Interest
References
- Prasanth, R.; Ammini, S.K.; Ge, L.; Thakur, M.K.; Thakur, V.K. Carbon Allotropes and Fascinated Nanostructures: The High-Impact Engineering Materials of the Millennium. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016; pp. 3–28. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; da Paz, E.; dos Santos Pinheiro, V. Carbon-Based Materials: Recent Advances, Challenges, and Perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Toktam, N.; Tan, A.; Seifalian, A.M. Different Functionalization Methods of Carbon-Based Nanomaterials. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016; pp. 29–58. [Google Scholar]
- Field, J.E. The Properties of Natural and Synthetic Diamond; Academic: New York, NY, USA, 1992. [Google Scholar]
- Kalish, R. Diamond as a unique high-tech electronic material: Difficulties and prospects. J. Phys. D 2007, 40, 6467–6478. [Google Scholar] [CrossRef]
- Nebel, C.E.; Shin, D.; Rezek, B.; Tokuda, N.; Uetsuka, H.; Watanabe, H. Diamond and biology. J. R. Soc. Interface 2007, 4, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Nebel, C.E.; Kato, H.; Rezek, B.; Shin, D.; Takeuchi, D.; Watanabe, H.; Yamamoto, T. Electrochemical properties of undoped hydrogen terminated CVD diamond. Diam. Relat. Mater. 2006, 15, 264–268. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Burchell, T.D. Graphite: Properties and Characteristics. In Comprehensive Nuclear Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 2, pp. 285–306. [Google Scholar]
- Kelly, B.T. Physics of Graphite; Applied Science: London, UK, 1981. [Google Scholar]
- Elham, A.; Masoumeh, G.; Saeed, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B 2019, 293, 183–209. [Google Scholar]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon nanostructures for nanomedicine: Opportunities and challenges. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qui, L. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Shenderova, O.A.; Zhirnov, V.V.; Brenner, D.V. Carbon Nanostructures. Crit. Rev. Solid State Mater. Sci. 2002, 27, 227–356. [Google Scholar] [CrossRef]
- Chang, Y.R.; Lee, H.Y.; Chen, K.; Chang, C.C.; Tsai, D.S.; Fu, C.C.; Lim, T.S.; Tzeng, Y.K.; Fang, C.Y.; Han, C.C.; et al. Mass production and dynamic imaging of fluorescent nanodiamonds. Nat. NanoTechnol. 2008, 3, 284–288. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond. Nanotechnology 2017, 26, 252001/1–252001/27. [Google Scholar] [CrossRef] [PubMed]
- Rabeau, J.R.; Stacey, A.; Rabeau, A.; Prawer, S.; Jelezko, F.; Mirza, I.; Wrachtrup, J. Single Nitrogen Vacancy Centers in Chemical Vapor Deposited Diamond Nanocrystals. Nano Lett. 2007, 7, 3433–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, O.A.; Douheret, O.; Daenen, M.; Haenen, K.; Osawa, E.; Takahashi, M. Enhanced diamond nucleation on monodispersed nanocrystalline diamond. Chem. Phys. Lett. 2007, 445, 255–258. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Lin, X.; Tang, Y.; Liu, Y.; Wang, T.; Zhu, H. The electronic properties of chiral carbon nanotubes. Comput. Mater. Sci. 2017, 129, 209–294. [Google Scholar] [CrossRef]
- Qureshi, A.; Kang, W.K.; Davidson, J.L.; Gurbuz, Y. Review on carbon-derived, solid-state, micro and nano sensors for electrochemical sensing applications. Diam. Relat. Mater. 2009, 18, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Bethune, D.S.; Klang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vasquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; Carpena-Nuñez, J.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar]
- Camilli, L.; Passacantando, M. Advances on sensors based on carbon nanotubes. Chemosensors 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Loos, M. Carbon Nanotube Reinforced Composites, 1st ed.; Plastic Design Library (PDL) handbook series; William Andrew Applied Science Publishers: Oxford, UK, 2015; ISBN 978-1-4557-3195-4. [Google Scholar]
- Nayak, L.; Rahaman, M.; Giri, R. Surface Modification_Functionalizationof Carbon Materials by DifferentTechniques: An Overview. In Carbon-Containing Polymer Composites; Springer Series on Polymer and Composite Materials; Springer Nature: Singapore, 2019; pp. 65–98. ISBN 978-981-13-2687-5. [Google Scholar]
- Pusch, J.; Wohlmann, B. Carbon Fibers. In Inorganic and Composite Fibers: Production, Properties, and Applications; The Textile Institute Book Series; Woodhead Publishing: Oxford, UK, 2018; pp. 31–51. ISBN 978-0-08-102228-3. [Google Scholar]
- Yao, S.-S.; Jin, F.-L.; Rheeb, K.Y.; Hui, D.; Park, S.-J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Composites B 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Saito, N.; Aoki, K.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; Haniu, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef]
- Du, J.; Zhang, H.; Geng, Y.; Ming, W.; He, W.; Ma, J.; Cao, Y.; Li, X.; Liu, K. A review on machining of carbon fiber reinforced ceramic matrix composites. Ceram. Int. 2019, 45, 18155–18166. [Google Scholar] [CrossRef]
- Galyshev, S.; Gomzin, A.; Musin, F. Aluminum Matrix Composite Reinforced by Carbon Fibers. Mater. Today Proc. 2019, 11, 281–285. [Google Scholar] [CrossRef]
- Hiremath, N.; Mays, J.; Bhata, G. Recent Developments in Carbon Fibers and Carbon Nanotube-Based Fibers: A Review. Polym. Rev. 2017, 57, 339–368. [Google Scholar] [CrossRef]
- Rehman, M.; Park, S.J. Current Progress on the Surface Chemical Modification of Carbonaceous Materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Burg, P.; Cagniant, D. Characterization of carbon surface chemistry. In Chemistry and Physics of Carbon; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008; Volume 30. [Google Scholar]
- Brender, P.; Gadiou, R.; Rietsch, J.C.; Fioux, P.; Dentzer, J.; Ponche, A.; Vix-Guterl, C. Characterization of Carbon Surface Chemistry by Combined Temperature Programmed Desorption with in Situ X-ray Photoelectron Spectrometry and Temperature Programmed Desorption with Mass Spectrometry Analysis. Anal. Chem. 2012, 84, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M. Materials Science and Engineering of Carbon: Characterization, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Naik, R.R.; Dai, L. Carbon Nanomaterials for Biomedical Applications; Springer Series in Biomaterials Science and Engineering; Springer International Publishing: Cham, Switzerland, 2016; Volume 5. [Google Scholar]
- Meenakshi, G. Nonenvironmental industrial applications of activated carbon adsorption. In From Novel Carbon Adsorbents; Elsevier: Oxford, UK, 2012; pp. 605–638. [Google Scholar]
- Wang, M.-J.; Gray, C.A.; Reznek, S.R.; Mahmud, K.; Kutsovsky, Y. Carbon black. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; Volume 2, pp. 426–466. [Google Scholar]
- Kamau, G.N. Surface preparation of glassy carbon electrodes. Anal. Chim. Acta 1988, 207, 1–16. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesin, L.A. Review Structure and properties of glass-like carbon. J. Mater. Sci. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X. Recent advances in porous carbon materials for electrochemical energy storage. Chem.-Asian J. 2018, 13, 1518–1529. [Google Scholar] [CrossRef]
- Li, W.; Fang, R.; Xia, Y.; Zhang, W.; Wang, X.; Xia, X.; Tu, J. Multiscale Porous Carbon Nanomaterials for Applications in Advanced Rechargeable Batteries. Batter. Supercaps 2019, 2, 9–36. [Google Scholar] [CrossRef] [Green Version]
- VanDersarl, J.J.; Mercanzini, A.; Renaud, P. Integration of 2D and 3D Thin Film Glassy Carbon Electrode Arrays for Electrochemical Dopamine Sensing in Flexible Neuroelectronic Implants. Adv. Funct. Mater. 2014, 25, 78–84. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Stable Glassy Carbon Interfaces for Long-Term Neural Stimulation and Low-Noise Recording of Brain Activity. Sci. Rep. 2017, 7, 40332/1–40332/14. [Google Scholar]
- Kurihara, M. Characteristics and applications of carbon black. J. Technol. Assoc. Refract. Jpn. 2011, 31, 152–155. [Google Scholar]
- Kausar, A. Contemporary applications of carbon black-filled polymer composites: An overview of essential aspects. J. Plast. Film. Sheeting 2018, 34, 256–299. [Google Scholar] [CrossRef]
- Radovic, L.R. Chemistry and Physics of Carbon, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; Volume 31. [Google Scholar]
- Zhao, L.; Komatsu, N. Surface Functionalization of Nanodiamond for Biomedical Applications Polyglycerol Grafting and Further Derivatization. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016; pp. 651–664. [Google Scholar]
- Muzammil, I.; Duy, K.D.; Qasim, A.; Muhammad, I.; Harse, S.; Aqrab, U.A. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar]
- Tsubokawa, N. Functionalization of Carbon Material by Surface Grafting of Polymers. Bull. Chem. Soc. Jpn. 2002, 75, 2115–2136. [Google Scholar] [CrossRef]
- Ulman, A. Wetting studies of molecularly engineered surfaces. Thin Solid Film. 1996, 273, 48–53. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Carbon nanomaterial-based biosensors A review of design and applications. IEEE Nanotechnol. Mag. 2009, 18, 1401–1420. [Google Scholar] [CrossRef]
- Nagappa, L.T.; Raz, J. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574/1–1700574/36. [Google Scholar]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Eswaran, S.V. Water soluble nanocarbon materials: A panacea for all? Curr. Sci. 2018, 114, 1846–1850. [Google Scholar] [CrossRef]
- Kozbial, A.; Zhou, F.; Li, Z.; Liu, H.; Li, L. Are Graphitic Surfaces Hydrophobic? Acc. Chem. Res. 2016, 49, 2765–2773. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K. Liquid-Phase Exfoliation of Graphite Towards Solubilized Graphenes. Small 2009, 5, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.F.; Billups, W.E. Synthesis of Soluble Graphite and Graphene. Acc. Chem. Res. 2013, 46, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, M. New Carbons—Control of Structure and Functions, 1st ed.; Elsevier Science: Oxford, UK, 2000; ISBN 978-0-08-043713-2. [Google Scholar]
- Hao, R.; Qian, W.; Zhang, L.; Hou, Y. Aqueous dispersions of TCNQ-anion-stabilized graphene sheets. Chem. Commun. 2008, 48, 6576–6578. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, J.; Jeong, H.; Liu, J.; Lee, K.; Park, J.Y.; Ahn, Y.H.; Lee, S. Reduction of functionalized graphite oxides by trioctylphosphine in non-polar organic solvents. Carbon 2010, 48, 2282–2289. [Google Scholar] [CrossRef]
- Acocella, M.R.; D’Urso, L.; Maggio, M.; Avolio, R.; Errico, M.E.; Guerra, G. Green and Facile Esterification Procedure Leading to Crystalline-Functionalized Graphite Oxide. Langmuir 2017, 33, 6819–6825. [Google Scholar] [CrossRef]
- Ardhaoui, M.; Zheng, M.; Pulpytel, J.; Dowling, D.; Jolivalt, C.; Khonsari, F.A. Plasma functionalized carbon electrode for laccase-catalyzed oxygen reduction by direct electron transfer. Bioelectrochemistry 2013, 91, 52–61. [Google Scholar] [CrossRef]
- Ji, H.B.; He, Z.W.; Song, S.S.; Zhao, Z.D. Scalable preparation of functionalized graphite nanoplatelets via magnetic grinding as lubricity-enhanced additive. J. Cent. South Univ. 2016, 23, 2800–2808. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Santos, C.C.; da Cruz, F.S.; Correa, R.A.M.S.; Verly, R.M.; Da Silva, L.M. Preparation, characterization, and application in biosensors of functionalized platforms with poly (4-aminobenzoic acid). J. Mater. Sci. 2015, 50, 1103–1116. [Google Scholar] [CrossRef]
- Chelaghmia, M.L.; Nacef, M.; Affoune, A.M.; Pontie, M.; Derabla, T. Graphite Electrode and its Application for Highly Sensitive Non-enzymatic Glucose Sensor. Electroanalysis 2018, 30, 1117–1124. [Google Scholar] [CrossRef]
- Kesik, M.; Kanik, F.E.; Hizalan, G.; Kozanoglu, D.; Esenturk, E.N.; Timur, S.; Toppare, L. A functional immobilization matrix based on a conducting polymer and functionalized gold nanoparticles: Synthesis and its application as an amperometric glucose biosensor. Polymer 2013, 54, 4463–4471. [Google Scholar] [CrossRef]
- Park, J.; Grayfer, E.D.; Jung, Y.; Kim, K.; Wang, K.K.; Kim, Y.R.; Yoon, D.; Cheong, H.; Chung, H.E.; Choi, S.J.; et al. Photoluminescent nanographitic/nitrogen-doped graphitic hollow shells as a potential candidate for biological applications. J. Mater. Chem. B 2013, 1, 1229–1234. [Google Scholar] [CrossRef]
- Correa, R.A.M.S.; da Cruz, F.S.; Santos, C.C.; Pimenta, T.C.; Franco, D.L.; Ferreira, L.F. Optimization and application of electrochemical transducer for detection of specific oligonucleotide sequence for Mycobacterium tuberculosis. Biosensors 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azani, M.R.; Hassanpour, A.; Carcelén, V.; Gibaja, C. Highly concentrated and stable few-layers graphene suspensions in pure and volatile organic solvents. Appl. Mater. Today 2016, 2, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Liang, A.; Jiang, X.; Hong, X.; Jiang, Y.; Shao, Z.; Zhu, D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Rao, K.V.; Voggu, R.; George, S.J. Non-covalent functionalization, solubilization of graphene and single-walled carbon nanotubes with aromatic donor and acceptor molecules. Chem. Phys. Lett. 2010, 488, 198–201. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Huang, Y.; Wang, Y.; Ma, Y.; Liu, Z.; Chen, Y. Synthesis and characterization of a graphene–C60 hybrid material. Carbon 2009, 47, 334–337. [Google Scholar] [CrossRef]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y.-P. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 18, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Wu, H.; Cort, J.R.; Buchko, G.W.; Zhang, Y.; Shao, Y.; Aksay, A.; Liu, J.; Lin, Y. Constraint of DNA on Functionalized Graphene Improves its Biostability and Specificity. Small 2010, 6, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Sun, J.; Tian, S.; Yang, S.; Ding, S.; Ding, G.; Xie, X.; Jang, M. Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chem. Mater. 2015, 27, 218–226. [Google Scholar] [CrossRef]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Lei, X.; Song, B.; Fang, H.; Tu, Y.; Tan, Z.-J. Dynamic Cooperation of Hydrogen Binding and π Stacking in ssDNA Adsorption on Graphene Oxide. Chemistry 2017, 23, 13100–13104. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2015, 3, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Kang, H.; Zuo, K.; Wang, Z.; Zhang, L.; Liu, L.; Guo, B. Using a green method to develop graphene oxide/elastomers nanocomposites with combination of high barrier and mechanical performance. Compos. Sci. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-Soluble Poly (N -isopropylacrylamide)–Graphene Sheets Synthesized via Click Chemistry for Drug Delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, J.; Xi, C.; Hu, J. Stimuli-Responsive Polymer Covalent Functionalization of Graphene Oxide by Ce(IV)-Induced Redox Polymerization. J. Phys. Chem. C 2011, 115, 24636–24641. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Dai, J.; Lang, M.; Huang, X. Functionalization of Graphene Oxide Towards Thermo-Sensitive Nanocomposites via Moderate In Situ SET-LRP. J. Polym. Sci. A 2011, 49, 4747–4755. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Karousis, N.; Economopoulos, S.P.; Sarantopoulou, E.; Tagmatarchis, N. Porphyrin counter anion in imidazolium-modified graphene-oxide. Carbon 2010, 48, 854–860. [Google Scholar] [CrossRef]
- Vasilievna, K.O.; Ildusovich, K.B. Solubilization and Dispersion of Carbon Nanotubes, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-62949-0. [Google Scholar]
- Caneba, G.T.; Dutta, C.; Agrawal, V.; Rao, M. Novel Ultrasonic Dispersion of Carbon Nanotubes. J. Miner. Mater. Charact. Eng. 2010, 9, 165–181. [Google Scholar] [CrossRef]
- Vetcher, A.A.; Fan, J.H.; Vetcher, I.A.; Lin, T.; Abramov, S.M.; Draper, R.; Kozlov, M.E.; Baughman, R.H.; Levene, S.D. Electrophoretic fractionation of carbon nanotube dispersion on agarose gels. Int. J. NanoSci. 2007, 6, 1–7. [Google Scholar] [CrossRef]
- Burg, B.R.; Schneider, J.; Muoth, M.; Durrer, L.; Helbling, T.; Schirmer, N.C.; Schwamb, T.; Hierold, C.; Poulikakos, D. Aqueous dispersion and dielectrophoretic assembly of individual surface synthesized single-walled carbon nanotubes. Langmuir 2009, 25, 7778–7782. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lusting, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Zhao, P.; Einarsson, E.; Xiang, R.; Murakami, Y.; Maruyama, S. Controllable expansion of single-walled carbon nanotube dispersions using density gradient ultracentrifugation. J. Phys. Chem. C 2010, 114, 4831–4834. [Google Scholar] [CrossRef]
- Czech, B.; Oleszczuk, P.; Wiącek, A. Advanced oxidation (H₂O₂ and/or UV) of functionalized carbon nanotubes (CNT-OH and CNT-COOH) and its influence on the stabilization of CNTs in water and tannic acid solution. Environ. Pollut. 2015, 200, 161–167. [Google Scholar] [CrossRef]
- Saka, C. Overview on the Surface Functionalization Mechanism and Determination of Surface Functional Groups of Plasma Treated Carbon Nanotubes. Crit. Rev. Anal. Chem. 2018, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ruelle, B.; Bittencourt, C.; Dubois, C. Surface treatment of carbon nanotubes via plasma technology. Chap. 2. In Polymer–Carbon Nanotube Composites Preparation, Properties and Applications; Woodhead Publishing Series in Composites Science and Engineering: Oxford, UK, 2011; pp. 25–54. [Google Scholar]
- Kharissova, O.V.; Kharisov, B.I.; de Casas Ortiz, E.G. Dispersion of carbon nanotubes in water and non-aqueous solvents. RSC Adv. 2013, 3, 24812–24852. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Oxidative acid treatment of carbon nanotubes. Surf. Interface 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Dong, C.; Campbell, A.S.; Eldawud, R.; Perhinschi, G.; Rojanasakul, Y.; Dinu, C.Z. Effects of acid treatment on structure, properties and biocompatibility of carbon nanotubes. Appl. Surf. Sci. 2013, 264, 261–268. [Google Scholar] [CrossRef]
- Cai, J.Y.; Min, J.; McDonnell, J.; Church, J.S.; Easton, C.D.; Humphries, W.; Lucas, S. An improved method for functionalisation of carbon nanotube spun yarns with aryldiazonium compounds. Carbon 2012, 50, 4655–4662. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, Z.; Malhotra, S.V. Functionalization of carbon nanotubes with amines and enzymes. Chem. Phys. Lett. 2005, 402, 96–101. [Google Scholar] [CrossRef]
- Chen, J.; Collier, C.P. Noncovalent Functionalization of Single-Walled Carbon Nanotubes with Water-Soluble Porphyrins. J. Phys. Chem. B 2005, 109, 7605–7609. [Google Scholar] [CrossRef]
- Bayazit, M.K.; Coleman, K.S. Fluorescent Single-Walled Carbon Nanotubes Following the 1,3-Dipolar Cycloaddition of Pyridinium Ylides. J. Am. Chem. Soc. 2009, 131, 10670–10676. [Google Scholar] [CrossRef]
- Fabbro, C.; Chaloin, O.; Moyon, C.M.; Smulski, C.R.; Da Ros, T.; Kostarelos, K.; Prato, M.; Bianco, A. Antibody covalent immobilization on carbon nanotubes and assessment of antigen binding. Small 2011, 7, 2179–2187. [Google Scholar]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R.; Lorents, D.C. Solubility of fullerene C60 in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Chu, K.H.; Son, A.; Heo, J.; Her, N.; Jang, M.; Park, C.M.; Yoon, Y. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: A review. Sep. Purif. Technol. 2015, 156, 861–874. [Google Scholar] [CrossRef]
- Jensen, A.W.; Wilson, S.R.; Schuster, D.I. Biological applications of fullerenes. Bioorg. Med. Chem. 1996, 4, 767–779. [Google Scholar] [CrossRef]
- Da Ros, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 8, 663–669. [Google Scholar] [CrossRef]
- Diederich, F.; Gomez-Lopez, M. Supramolecular fullerene chemistry. Chem. Soc. Rev. 1999, 28, 263–277. [Google Scholar] [CrossRef]
- Diederich, F.; Thilgen, C. Covalent Fullerene Chemistry. Science 1996, 271, 317–323. [Google Scholar] [CrossRef]
- Ikeda, A.; Iizuka, T.; Maekubo, N.; Aono, R.; Kikuchi, J.; Akiyama, M.; Konishi, T.; Ogawa, T.; Ishida-Kitagawa, N.; Tatebe, H.; et al. Cyclodextrin Complexed [60] Fullerene Derivatives with High Levels of Photodynamic Activity by Long Wavelength Excitation. ACS Med. Chem. Lett. 2013, 4, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.Y.; Swirczewski, J.W.; Hsu, C.S.; Chowdhury, S.K.; Cameron, S.; Creegan, K. Multi-hydroxy additions onto C60 fullerene molecules. J. Chem. Soc. Chem. Commun. 1992, 24, 1791–1793. [Google Scholar] [CrossRef]
- Li, J.; Takeuchi, A.; Ozawa, M.; Li, X.; Saigo, K.; Kitazawa, K. C60 fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 1993, 23, 1784–1785. [Google Scholar] [CrossRef]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Gong, Q.J.; Qiao, J.G.; Li, M.D.; Wei, J.J. Spectroscopic study on the photoinduced reaction of fullerene C60 with aliphatic amines and its dynamics—strong short wavelength fluorescence from the adducts. Spectrochim. Acta Part A 2001, 57, 17–25. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H.; Tomita, N.; Sawamura, M.; Jinno, S.; Okayama, H. Functionalized fullerene as an artificial vector for transfection. Angew. Chem. Int. Ed. 2000, 39, 4254–4257. [Google Scholar] [CrossRef]
- Isobe, H.; Tomita, N.; Jinno, S.; Okayama, N.; Nakamura, E. Nonviral gene delivery by tetraamino fullerene. Mol. Pharm. 2006, 3, 124–134. [Google Scholar] [CrossRef]
- Sitharaman, B.; Zakharian, T.Y.; Saraf, A.; Misra, P.; Ashcroft, J.; Pan, S.; Pham, Q.P.; Mikos, A.G.; Wilson, L.J.; Engler, D.A. Water-soluble fullerene (C60) derivatives as nonviral gene-delivery vectors. Mol. Pharm. 2008, 5, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Bingel, C. Cyclopropanierung von fullerenen. Chem. Ber. 1993, 126, 1957–1959. [Google Scholar] [CrossRef]
- Biglova, N.; Mustafin, A.G. Nucleophilic cyclopropanation of [60] fullerene bythe addition–elimination mechanism. RSC Adv. 2019, 9, 22428–22498. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Trochimczyk, P.; Sun, L.; Hou, S.; Li, H. Sugar-Functionalized Fullerenes. Curr. Org. Chem. 2016, 20, 1490–1501. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.; Muñoz, A.; Illescas, B.I.; Rojo, J.; Martín, N. [60] Fullerene as Multivalent Scaffold: Efficient Molecular Recognition of Globular Glycofullerenes by Concanavalin A. Chem. Eur. J. 2011, 17, 766–769. [Google Scholar] [CrossRef]
- Nierengarten, I.; Nierengarten, J.F. Fullerene Sugar Balls: A New Class of Biologically Active FullereneDerivatives. Chem. Asian J. 2014, 9, 1436–1444. [Google Scholar] [CrossRef]
- Luczkowiak, J.; Muñoz, A.; Sánchez-Navarro, M.; Ribeiro-Viana, R.; Ginieis, A.; Illescas, B.M.; Martín, N.; Delgado, R.; Rojo, J. Glycofullerenes Inhibit Viral Infection. Biomacromolecules 2013, 14, 431–437. [Google Scholar] [CrossRef]
- Rísquez-Cuadro, R.; García Fernández, J.; Nierengarten, J.F.; Ortiz Mellet, C. Fullerene-sp2-Iminosugar Balls as Multimodal Ligands for Lectins and Glycosidases: A Mechanistic Hypothesis for the Inhibitory Multivalent Effect. Chem. Eur. J. 2013, 19, 16791–16803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecioni, S.; Oerthel, V.; Iehl, J.; Holler, M.; Goyard, D.; Praly, J.P.; Imbeerty, A.; Nierengarten, J.F.; Vidal, S. Synthesis of Dodecavalent Fullerene-Based Glycoclusters and Evaluation of Their Binding Properties towards a Bacterial Lectin. Chem. Eur. J. 2011, 17, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, K.T.; Perevedentseva, E.; Karmenyan, A.; Lin, M.D.; Cheng, C.L. Nanodiamond for biolabelling and toxicity evaluation in the zebrafish embryo in vivo. J. Biophotonics 2016, 9, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Shergold, H.L.; Hartley, C.J. The surface chemistry of diamond. Int. J. Miner. Process. 1982, 9, 219–233. [Google Scholar] [CrossRef]
- Szunerits, S.; Nebel, C.E.; Hamers, R.J. Surface functionalization and biological applications of CVD diamond. MRS Bull. 2014, 39, 517–524. [Google Scholar] [CrossRef]
- Stavis, C.; Lasseter Clare, T.; Butler, J.E.; Radadia, A.D.; Carr, R.; Zeng, H.; King, W.P.; Carlisle, J.A.; Aksimentiev, A.; Bashir, R.; et al. Surface functionalization of thin-film diamond for highly stable and selective biological interfaces. Proc. Natl. Acad. Sci. USA 2011, 18, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Ho, D. Nanodiamonds Applications in Biology and Nanoscale Medicine; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0531-4. [Google Scholar]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Wang, P.; Su, W.; Ding, X. Control of nanodiamond-doxorubicin drug loading and elution through optimized compositions and release environments. Diam. Relat. Mater. 2018, 88, 43–50. [Google Scholar] [CrossRef]
- Tinwala, H.; Wairkar, S. Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics. Mater. Sci. Eng. C 2019, 97, 913–931. [Google Scholar] [CrossRef]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.-Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-Mediated Delivery of Water-Insoluble Therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef]
- Kong, X.L.; Huang, L.C.L.; Hsu, C.M.; Chen, W.H.; Han, C.C.; Chang, H.C. High-Affinity Capture of Proteins by Diamond Nanoparticles for Mass Spectrometric Analysis. Anal. Chem. 2005, 77, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.L.; Chang, H.C. Adsorption and Immobilization of Cytochrome c on Nanodiamonds. Langmuir 2004, 20, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.B.; Chang, H.C.; Wu, V.W.K. Adsorption and hydrolytic activity of lysozyme on diamond nanocrystallites. Diam. Relat. Mater. 2007, 16, 872–876. [Google Scholar] [CrossRef]
- Yang, W.S.; Auciello, O.; Butler, J.E.; Cai, W.; Carlisle, J.A.; Gerbi, J.; Gruen, D.M.; Knickerbocker, T.; Lasseter, T.L.; Russell, J.N.; et al. DNA-modified nanocrystalline diamond thin-films as stable, biologically active substrates. Nat. Mater. 2002, 1, 253–257. [Google Scholar] [CrossRef]
- Krueger, A.; Liang, Y.; Jarre, G.; Stegk, J. Surface functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Ando, T.; Yamamoto, K.; Matsuzawa, M.; Takamatsu, Y.; Kawasaki, S.; Okino, F.; Touhara, H.; Kamo, M.; Sato, Y. Direct interaction of elemental fluorine with diamond surfaces. Diam. Relat. Mater. 1996, 5, 1021–1025. [Google Scholar] [CrossRef]
- Ando, T.; Yamamoto, K.; Suehara, S.; Kamo, M.; Sato, Y.; Shimosaki, S.; Gamo, M.N. Interaction of Chlorine with Hydrogenated Diamond Surface. J. Chin. Chem. Soc. 1995, 42, 285–292. [Google Scholar] [CrossRef]
- Zhou, J.; Laube, C.; Knolle, W.; Naumov, S.; Prager, A.; Kopinke, F.D.; Abel, B. Efficient chlorine atom functionalization at nanodiamond surfaces by electron beam irradiation. Diam. Relat. Mater. 2018, 82, 150–159. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Z.; Margrave, J.L.; Khabashesku, V.N. Functionalization of Nanoscale Diamond Powder: Fluoro-, Alkyl-, Amino-, and Amino Acid-Nanodiamond Derivatives. Chem. Mater. 2004, 16, 3924–3930. [Google Scholar] [CrossRef]
- Barras, A.; Lyskawa, J.; Szunerits, S.; Woisel, P.; Boukherroub, R. Direct Functionalization of Nanodiamond Particles Using Dopamine Derivatives. Langmuir 2011, 27, 12451–12457. [Google Scholar] [CrossRef]
- Hajiali, F.; Shojaei, A. Silane functionalization of nanodiamond for polymer nanocomposites-effect of degree of silanization. Colloids Surf. A 2016, 506, 254–263. [Google Scholar] [CrossRef]
- Ma, W.; Yu, X.; Qu, X.; Zhang, Q. Functionalization of agglomerating nanodiamonds with biodegradable poly(ε-caprolactone) through surface-initiated polymerization. Diam. Relat. Mater. 2016, 62, 14–21. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Recent advances in carbon dots. C-J. Carbon Res. 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Hui, Y.Y.; Cheng, C.-L.; Chang, H.-C. Nanodiamonds for optical bioimaging. J. Phys. D 2010, 43, 374021/1–374021/11. [Google Scholar] [CrossRef]
- Reineck, P.; Lau, D.W.M.; Wilson, E.R.; Fox, K.; Filed, M.R.; Deeleepojananan, C.; Mochaklin, V.N.; Gibson, B.C. Effect of Surface Chemistry on the Fluorescence of Detonation Nanodiamonds. ACS Nano 2017, 11, 10924–10934. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Stenzel, M.H.; Xiao, P. Surface engineering and applications of nanodiamonds in cancer treatment and imaging. Int. Mater. Rev. 2019, 1–37, Published online. [Google Scholar] [CrossRef]
- Huang, H.; Pierstorff, E.; Osawa, E.; Dean, H. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Yan, J.; Guo, Y.; Altawashi, A.; Moosa, B.; Lecommandoux, S.; Khashab, N.M. Experimental and theoretical evaluation of nanodiamonds as pH triggered drug carriers. New J. Chem. 2012, 36, 1479–1484. [Google Scholar] [CrossRef]
- Toh, T.B.; Lee, D.K.; Hou, W.; Adullah, L.N.; Nguyen, J.; Ho, D.; Chow, E.K.H. Nanodiamond–mitoxantrone complexes enhance drug retention in chemoresistant breast cancer cells. Mol. Pharm. 2014, 11, 2683–2691. [Google Scholar] [CrossRef] [Green Version]
- Pandolfo, A.G.; Hollenkamp, A.F. Review Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Babu, S.N.; Gopiraman, M.; Karvembu, R.; Kim, I.S. Carbon Material Supported Nanostructures in Catalysis. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2016; pp. 147–172. [Google Scholar]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.A.; Kneten, K.; McCreery, R.L. Electron Transfer Kinetics of Aquated Fe +3/+2, Eu +3/+2, and V +3/+2 at Carbon Electrodes. Inner Sphere Catalysis by Surface Oxides. J. ElectroChem. Soc. 1993, 140, 2593–2599. [Google Scholar] [CrossRef]
- Chen, P.; Fryling, M.; McCreery, R.L. Electron Transfer Kinetics at Modified Carbon Electrode Surfaces: The Role of Specific Surface Sites. Anal. Chem. 1995, 67, 3115–3122. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.J.; Lee, J.W.; Park, S.J. Functionalized Carbon Materials for Electronic Devices: A Review. Micromachines 2019, 10, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Banks, C.E. Defining the origins of electron transfer at screen-printed graphene-like and graphite electrodes: MoO2 nanowire fabrication on edge plane sites reveals electrochemical insights. Nanoscale 2016, 8, 15241–15251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005, 7, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.D.; Rees, N.V.; Klymenko, O.V.; Coles, B.A.; Compton, R.G. Marcus Theory of Outer-Sphere Heterogeneous Electron Transfer Reactions: Dependence of the Standard Electrochemical Rate Constant on the Hydrodynamic Radius from High Precision Measurements of the Oxidation of Anthracene and Its Derivatives in Nonaqueous Solvents Using the High-Speed Channel Electrode. J. Am. Chem. Soc. 2004, 126, 6185–6192. [Google Scholar]
- Chang, H.; Bard, A.J. Observation and characterization by scanning tunneling microscopy of structures generated by cleaving highly oriented pyrolytic graphite. Langmuir 1991, 7, 1143–1153. [Google Scholar] [CrossRef]
- Peng, W.; Han, G.; Huang, Y.; Cao, Y.; Son, S. Insight the effect of crystallinity of natural graphite on the electrochemical performance of reduced graphene oxide. Res. Phys. 2018, 11, 131–137. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, H.; Kong, X.; Wang, J. Reversible redox reaction on the oxygen-containing functional groups of an electrochemically modified graphite electrode for the pseudo-capacitance. J. Mater. Chem. 2011, 21, 18753–18760. [Google Scholar]
- Chiang, H.L.; Huang, C.P.; Chiang, P.C. The surface characteristics of activated carbon as affected by ozone and alkaline treatment. Chemosphere 2002, 47, 257–265. [Google Scholar] [CrossRef]
- Evans, J.F.; Kuwana, T. Radiofrequency oxygen plasma treatment of pyrolytic graphite electrode surfaces. Anal. Chem. 1977, 49, 1632–1635. [Google Scholar] [CrossRef]
- Khataee, A.; Sajjadi, S.; Pouran, S.R.; Hasanzadeh, A. Efficient electrochemical generation of hydrogen peroxide by means of plasma-treated graphite electrode and activation in electro-Fenton. J. Ind. Eng. Chem. 2017, 56, 312–320. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, D.; Jiang, L.; Dongand, H.; Hu, W. Tuning electrical properties of graphite oxide by plasma. Philos. Trans. R. Soc. A 2013, 371, 20120308/1–20120308/8. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Huang, J.L.; Chi, C.H. Conjugated polymer-functionalized graphite oxide sheets thin films for enhanced photovoltaic properties of polymer solar cells. J. Polym. Sci. Part B 2013, 51, 137–148. [Google Scholar] [CrossRef]
- Sathyamoorthi, S.; Suryanarayanan, V.; Velayutham, D. Electrochemical exfoliation and in situ carboxylic functionalization of graphite in non-fluoro ionic liquid for supercapacitor application. J. Solid State Electrochem. 2014, 18, 2789–2796. [Google Scholar] [CrossRef]
- Jeong, K.H.; Jeong, S.M. Enhanced capacitance of unexfoliated graphite oxide by coupled electro-deoxidation/functionalization in an alkali solution. Electrochim. Acta 2013, 108, 801–807. [Google Scholar] [CrossRef]
- Luo, J.; Jang, H.D.; Huang, J. Effect of sheet morphology on the scalability of graphene-based ultracapacitors. ACS Nano 2013, 7, 1464–1471. [Google Scholar] [CrossRef]
- Slate, A.J.; Brownson, D.A.C.; Abo Dena, A.S.; Smith, G.C.; Whitehead, K.A.; Banks, C.E. Exploring the electrochemical performance of graphite and graphene paste electrodes composed of varying lateral flake sizes. Phys. Chem. Chem. Phys. 2018, 20, 20010–20022. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, A.; Pumera, M. Precise Tuning of Surface Composition and Electron-Transfer Properties of Graphene Oxide Films through Electroreduction. Chemistry 2013, 19, 4748–4753. [Google Scholar] [CrossRef]
- Sinitskii, A.; Dimiev, A.; Corley, D.A.; Fursina, A.A.; Kosynkin, D.V. Kinetics of Diazonium Functionalization of Chemically Converted Graphene Nanoribbons. ACS Nano 2010, 4, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ryu, S.; Chen, Z.; Steigerwald, M.L.; Nuckolls, C.; Brus, L.E. Photochemical Reactivity of Graphene. J. Am. Chem. Soc. 2009, 131, 7099–17101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and Scalable One-Step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef] [Green Version]
- Neustroev, E.P. Plasma Treatment of Graphene Oxide chap. 2. In Raphene Oxide—Applications and Opportunities; IntechOpen: London, UK, 2018. [Google Scholar]
- Imamura, G.; Saiki, K. Synthesis of Nitrogen-Doped Graphene on Pt (111) by Chemical Vapor Deposition. J. Phys. Chem. C 2011, 115, 10000–10005. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Santiago, F.F.; Bisquert, J. A perspective on the production of dye-sensitized solar modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- Song, L.; Luo, Q.; Zhao, F.; Li, Y.; Lin, H.; Qu, L.; Zhang, Z. Dually functional, N-doped porous graphene foams as counter electrodes for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 21820–21826. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef]
- Cui, T.; Lv, R.; Huang, Z.H.; Chen, S.; Zhang, Z.; Gan, X.; Jia, Y.; Li, X.; Wang, K.; Wu, D. Enhanced efficiency of graphene/silicon heterojunction solar cells by molecular doping. J. Mater. Chem. A 2013, 1, 5736–5740. [Google Scholar] [CrossRef]
- Hai, X.; Mao, Q.X.; Wang, W.J.; Wang, X.F.; Chen, X.W.; Wang, J.H. An acid free microwave approach to prepare highly luminescent boron-doped graphene quantum dots for cell imaging. J. Mater. Chem. B 2015, 3, 9109–9114. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Dhar, S.; Majumder, T.; Chakraborty, P.; Mondal, S.P. DMSO modified PEDOT: PSS polymer/ZnO nanorods Schottky junction ultraviolet photodetector: Photoresponse, external quantum efficiency, detectivity, and responsivity augmentation using N doped graphene quantum dots. Org. Electron. 2018, 53, 101–110. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.W. Highly-efficient organic light-emitting devices based on poly (N, N’-bis-4-butylphenyl-N, N’-bisphenyl) benzidine: Octadecylamine graphene quantum dots. Org. Electron. 2018, 57, 305–310. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Ali, M.; Zhou, F.; Zhang, X.; McFarlane, D.R. Sulfated carbon quantum dots as efficient visible-light switchable acid catalysts for room temperature ring-opening reactions. Angew. Chem. Int. Ed. 2015, 54, 8420–8424. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, 1902136/1–1902136/19. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, B.C.; Tan, W.; Ye, B.C. A versatile graphene based fluorescence “on/off” switch for multiplex detection ofvarious targets. Biosens. Bioelectron. 2011, 26, 3260–3265. [Google Scholar] [CrossRef]
- Lim, S.K.; Chen, P.; Lee, F.L.; Moochhala, S.; Liedberg, B. Peptide-assembled graphene oxide as a fluorescent turn-on sensor for lipopolysaccharide (endotoxin) detection. Anal. Chem. 2015, 87, 9408–9412. [Google Scholar] [CrossRef]
- Garion, C. Mechanical Properties for Reliability Analysis of Structures in Glassy Carbon. World J. Mech. 2014, 4, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.J.F. Fullerene-related structure of commercial glassy carbons. Philos. Mag. 2004, 84, 3159–3167. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Zhang, J.; Kenny-Benson, C.; Park, C.; Wang, Y.; Shen, G. Nanoarchitectured materials composed of fullerene-like spheroids and disordered graphene layers with tunable mechanical properties. Nat. Commun. 2015, 6, 6212/1–6212/10. [Google Scholar]
- Jouikov, V.; Simonet, J. Electrochemical conversion of glassy carbon into a poly-nucleophilic reactive material. Applications for carbon chemical functionalization. A mini-review. ElectroChem. Commun. 2014, 45, 32–36. [Google Scholar] [CrossRef]
- Evans, J.F.; Kuwana, T. Introduction of functional groups onto carbon electrodes via treatment with radio-frequency plasmas. Anal. Chem. 1979, 51, 358–365. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Dogan-Topal, B.; Bozal-Palabıyık, B.; Uslu, B.; Ozkan, S.A. Multi-walled carbon nanotube modified glassy carbon electrode as a voltammetric nanosensor for the sensitive determination of anti-viral drug valganciclovir in pharmaceuticals. Sens. Actuators B 2013, 177, 841–847. [Google Scholar] [CrossRef]
- De Clements, R.; Swain, G.M.; Dallas, T.; Holtz, M.W.; Herick, R.D.; Stickney, J.L. Electrochemical and Surface Structural Characterization of Hydrogen Plasma Treated Glassy Carbon Electrodes. Langmuir 1996, 12, 6578–6586. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-like models for microporous carbon. J. Mater. Sci. 2013, 48, 565–577. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Yan, Y.; Guo, T.; Pang, H.; Xue, H. Porous high specific surface area-activated carbon with co-doping N, S and P for high-performance supercapacitors. RSC Adv. 2017, 7, 43780–43788. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Zhao, G. Preparation and Characterization of High Surface Area Activated Carbon Fibers from Lignin. Polymers 2016, 8, 369. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ahn, H.J.; Cho, D.; Young, J.I.; Kim, Y.J.; Oh, H.J. Effect of surface modification of carbon felts on capacitive deionization for desalination. Carbon Lett. 2015, 16, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Lee, S.W.; Kim, J.G.; Choi, J.W.; Kim, J.H.; Park, M.S. A new strategy for integrating abundant oxygen functional groups into carbon felt electrode for vanadium redox flow batteries. Sci. Rep. 2014, 4, 6906/1–6906/6. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.J.; Kim, J.H.; Park, M.S. The effects of modification on carbon felt electrodes for use in vanadium redox flow batteries. Mater. Chem. Phys. 2011, 131, 547–553. [Google Scholar] [CrossRef]
- Frysz, C.A.; Chung, D.D.L. Improving the electrochemical behavior of carbon black and carbon filaments by oxidation. Carbon 1997, 35, 1111–1127. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Modification of graphite electrode materials for vanadium redox flow battery application—Part I. Thermal treatment. Electrochim. Acta 1192, 35, 1253–1260. [Google Scholar]
- Flox, C.; Rubio-Garcia, J.; Skoumal, M.; Andreu, T.; Morante, J.R. Thermo-chemical treatments based on NH3/O2 for improved graphite-based fiber electrodes in vanadium redox flow batteries. Carbon 2013, 60, 280–288. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Ferro-García, M.A.; Joly, J.P.; Bautista-Toledo, I.; Carrasco-Marín, F.; Rivera-Utrilla, J. Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Quian, H.; Diao, H.; Shirshova, N.; Greenhalgh, E.S.; Steinke, J.G.H.; Shaffer, M.S.P.; Bismarck, A. Activation of structural carbon fibres for potential applications in multifunctional structural supercapacitors. J. Colloid Interface Sci. 2013, 395, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application.—Part II. Acid treatments. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Wu, T.; Huang, K.; Liu, S.; Zhuang, S.; Fang, D.; Li, S.; Dan, L.; Su, A. Hydrothermal ammoniated treatment of PAN-graphite felt for vanadium redox flow battery. J. Solid State Electron. 2012, 16, 579–585. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, X.; Engelhard, M.; Wang, C.; Dai, S.; Liu, J.; Yang, Z.; Lin, Y. Nitrogen-doped mesoporous carbon for energy storage in vanadium redox flow batteries. J. Pow Sources 2010, 195, 4375–4379. [Google Scholar] [CrossRef]
- Kinoshita, K.; Chu, X. Electrochemical Supercapacitors—Scientific Fundamentals and Technological Applications; Kluwer: New York, NY, USA, 1999. [Google Scholar]
- Delnick, F.M.; Tomkiewicz, M. Proceedings of the Symposium on Electrochemical Capacitors; Electrochemical Society Publisher: Pennington, NJ, USA, 1995; Volume 95-25. [Google Scholar]
- Inagaki, M.; Radovic, L.R. Nanocarbons. Carbon 2002, 40, 2279–2282. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F. Active Carbon—Chapter 2; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond and Fullerenes; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Lee, H.; Jung, Y.; Kim, S. Effect of plasma treatments to graphite nanofibers supports on electrochemical behaviors of metal catalyst electrodes. J. NanoSci. Nanotechnol. 2012, 12, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, W.; Chen, M.; Liu, C. Enhancement of the electrochemical properties of commercial coconut shell-based activated carbon by H2O dielectric barrier discharge plasma. R. Soc. Open Sci. 2019, 6, 180872/1–180872/11. [Google Scholar]
- Hsieh, C.T.; Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 2002, 40, 667–674. [Google Scholar] [CrossRef]
- Lin, T.; Chen, I.W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhang, M.; Liu, T.; Li, T.; Guo, D.; Liu, X.X. Cobalt-Containing Nanoporous Nitrogen-Doped Carbon Nanocuboids from Zeolite Imidazole Frameworks for Supercapacitors. Nanomaterials 2019, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Biniak, S.; Swiatkowski, A.; Pakula, M.; Radovic, L.R. Chemistry and Physics of Carbon; Marcel Dekker: New York, NY, USA, 2001; Volume 27. [Google Scholar]
- Hess, W.M.; Herd, C.R.; Donet, J.B.; Bansal, R.C.; Wang, M.J. Carbon Black, 2nd ed.; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Radeke, K.H.; Backhaus, K.O.; Swaitkowski, A. Microporosity of a Graphitized Rayon Fabric Oxidized in Air. Carbon 1991, 29, 122–123. [Google Scholar] [CrossRef]
- Abouelamaiem, D.I.; Mostazo-López, M.J.; He, G.; Patel, D.; Neville, T.P.; Parkin, I.P.; Lozano-Castelló, D. New insights into the electrochemical behaviour of porous carbon electrodes for supercapacitors. J. Energy Storage 2018, 19, 337–347. [Google Scholar] [CrossRef]
- Deinhammer, R.S.; Ho, M.; Anderegg, J.W.; Porter, M.D. Electrochemical oxidation of amine-containing compounds: A route to the surface modification of glassy carbon electrodes. Langmuir 1994, 10, 1306–1313. [Google Scholar] [CrossRef]
- Buttry, D.A.; Peng, J.C.; Donnet, J.B.; Rebouillat, S. Immobilization of amines at carbon fiber surfaces. Carbon 1999, 37, 1929–1940. [Google Scholar] [CrossRef]
- Ogata, A.F.; Song, S.W.; Cho, S.H.; Koo, W.T.; Jang, J.S.; Jeong, Y.J.; Kim, M.H.; Cheong, J.Y. An Impedance-Transduced Chemiresistor with a porous carbon channel for rapid, nonenzymatic, glucose sensing. Anal. Chem. 2018, 90, 9338–9346. [Google Scholar] [CrossRef]
- Huang, T.; Warsinke, A.; Koroljova-Skorobogatko, O.V.; Makower, A.; Kuwana, T.; Scheller, F.W. A Bienzyme Carbon Paste Electrode for the Sensitive Detection of NADPH and the Measurement of Glucose-6-phosphate Dehydrogenase. Electroanalysis 1999, 11, 295–300. [Google Scholar] [CrossRef]
- Musameh, M.; Wang, J.; Merkoci, A.; Lin, Y. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. ElectroChem. Commun. 2002, 4, 743–746. [Google Scholar] [CrossRef]
- Yang, H.H.; McCreery, L.M. Elucidation of the Mechanism of Dioxygen Reduction on Metal-Free Carbon Electrodes. J. ElectroChem. Soc. 2000, 147, 3420–3428. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Bajpai, V.; Ji, T.; Dai, L. Chemistry of carbon nanotubes. Aust. J. Chem. 2003, 56, 635–651. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltaniana, M. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Rana, A.; Baig, N.; Saleh, T.A. Electrochemically pretreated carbon electrodes and their electroanalytical applications—A review. J. Electroanal. Chem. 2019, 833, 313–332. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly activated screen-printed carbon electrodes by electrochemical treatment with hydrogen peroxide. ElectroChem. Commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- Santiago-Rodrıguez, L.; Sanchez-Pomales, G.; Cabrera, C.R. DNA-functionalized carbon nanotubes: Synthesis, self-assembly, and applications. Isr. J. Chem. 2010, 50, 277–290. [Google Scholar] [CrossRef]
- Tasis, N.; Tagmatarchis, N.; Georgakilas, V.; Prato, M. Soluble carbon nanotubes. Chemistry 2003, 9, 4000–4008. [Google Scholar] [CrossRef]
- Lu, F.; Gu, L.; Meziani, M.J.; Wang, X.; Luo, P.G.; Veca, L.M.; Cao, L.; Sun, Y.P. Advances in Bioapplications of Carbon Nanotubes. Adv. Mater. 2009, 21, 139–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgakilas, V.; Gournis, D.; Tzitzios, V.; Pasquato, L.; Guldie, D.M.; Prato, M. Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater. Chem. 2007, 17, 2679–2694. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon Nanotubes in Biology and Medicine: In vitro and in vivo Detection, Imaging and Drug Delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z. An Overview of Carbon Nanotubes and Graphene for Biosensing Applications. Nano-Micro Lett. 2017, 9, 25/1–25/24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Star, A.; Joshi, V.; Han, T.R.; Altoe, M.V.P.; Gruner, G.; Stoddart, J.F. Electronic Detection of the Enzymatic Degradation of Starch. Org. Lett. 2004, 6, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. BioChem. 2004, 332, 75–83. [Google Scholar] [CrossRef]
- Wang, S.G.; Zhang, Q.; Wang, R.; Yoon, S.F.; Ahn, J.; Yang, D.J.; Tian, J.Z.; Li, J.Q.; Zhou, Q. Multi-walled carbon nanotubes for the immobilization of enzyme in glucose biosensors. ElectroChem. Commun. 2003, 5, 800–803. [Google Scholar] [CrossRef]
- Cai, H.; Cao, X.; Jiang, Y.; He, P.; Fang, Y. Carbon nanotube enhanced electrochemical DNA biosensor for DNA hybridization detection. Anal. Bioanal. Chem. 2003, 375, 287–293. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, M.; Ren, R.; Zhang, S. Carbon nanotube-enhanced DNA biosensor for DNA hybridization detection using manganese(II)-Schiff base complex as hybridization indicator. J. Inorg. BioChem. 2009, 103, 43–49. [Google Scholar] [CrossRef]
- Weber, J.E.; Pillai, S.; Ram, M.K.; Kumar, A.; Singh, S.R. Electrochemical impedance-based DNA sensor using a modified single walled carbon nanotube electrode. Mater. Sci. Eng. C 2011, 31, 821–825. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X. Carbon nanomaterials field-effect-transistor based biosensors. NPG Asia Mater. 2012, 4, e23/1–e23/10. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.T.; Tseng, Y.C.; Ormategui, N.; Loinaz, I.; Eritja, R.; Bokor, J. Label-free DNA biosensors based on functionalized carbon nanotube field effect transistors. Nano Lett. 2009, 9, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Sorgenfrei, S.; Chiu, C.; Gonzales, R.L.; Yu, Y.J.; Kim, P.; Nukolls, C.; Shepard, K.L. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat. Nanotechnol. 2011, 6, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Huang, L.; Zhang, H.; Sun, Z.; Zhang, Z.; Zhang, G.J. Fabrication of ultrasensitive field-effect transistor DNA biosensors by a directional transfer technique based on CVD-grown graphene. ACS Appl. Mater. Interface 2015, 7, 16953–16959. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Wang, S.; Huang, L.; Ning, Y.; Zhang, Z.; Zhang, G.J. Ultrasensitive label-free detection of PNA–DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 2014, 8, 2632–2638. [Google Scholar] [CrossRef]
- Yin, Z.; He, Q.; Huang, X.; Zhang, J.; Wu, S.; Chen, P.; Zhang, Q.; Yan, Q.; Zhang, H. Real-time DNA detection using Pt nanoparticle-decorated reduced graphene oxide field-effect transistors. Nanoscale 2012, 4, 293–297. [Google Scholar] [CrossRef]
- Ramnani, P.; Gao, Y.; Ozsoz, M.; Mulchandani, A. Electronic detection of MicroRNA at attomolar level with high specificity. Anal. Chem. 2013, 85, 8061–8064. [Google Scholar] [CrossRef]
- Park, M.; Cella, L.N.; Chen, W.; Myung, N.V.; Mulchandani, A. Carbon nanotubes based chemiresistive immunosensor for small molecules: Detection of nitroaromatic explosives. Biosens. Bioelectron. 2010, 26, 1297–1301. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Saucedo, M.N.; Ramnani, P.; Mulchandani, A. Label-free electrical immunosensor for highly sensitive and specific detection of microcystin-LR in water samples. Environ. Sci. Technol. 2015, 49, 9256–9263. [Google Scholar] [CrossRef]
- Peña-Bahamond, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. NanobioTechnol. 2018, 16, 75/1–75/17. [Google Scholar]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Jeng, E.S.; Yeung, T.K.; Martinez, B.M.; Moll, A.E.; Gastala, J.B.; Strano, M.S. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science 2006, 311, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Daranciang, D.; Dai, H. Selective probing and imaging of cells with single walled carbon nanotubes as near-infrared fluorescent molecules. Nano Lett. 2008, 8, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Erten-Ela, S.; Cogal, S.; Cogal, G.C.; Oksuz, A.U. Highly conductive polymer materials based multi-walled carbon nanotubes as counter electrodes for dye-sensitized solar cells. J. Fuller. Nanotub. Carb Nanostruct 2016, 24, 380–384. [Google Scholar] [CrossRef]
- Yu, F.; Shi, Y.; Yao, W.; Han, S.; Ma, J. A new breakthrough for graphene/carbon nanotubes as counter electrodes of dye-sensitized solar cells with up to a 10.69% power conversion efficiency. J. Power Sources 2019, 412, 366–373. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube ink for writing on cellulose paper. Mater. Res. Bull. 2014, 50, 249–253. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Matochova, D.; Medved, M.; Bakandritsos, A.; Stekly, T.; Zbiril, R.; Otyepka, M. 2D Chemistry: Chemical Control of Graphene Derivatization. J. Phys. Chem. Lett. 2018, 9, 3580–3585. [Google Scholar] [CrossRef]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, J.; Lebedeva, M.A.; Porfyrakis, K.; Stimming, U.; Chamberlain, T.W. All-Fullerene-Based Cells for Nonaqueous Redox Flow Batteries. J. Am. Chem. Soc. 2018, 140, 401–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imahori, H.; Sakata, Y. Fullerenes as Novel Acceptors in Photosynthetic Electron Transfer. Eur. J. Org. Chem. 1999, 1999, 2445–2457. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jin, X.; Hwang, S.J. Fullerene as an efficient hybridization matrix for exploring high performance layered-double-hydroxide-based electrodes. J. Mater. Chem. A 2019, 7, 10971–10979. [Google Scholar] [CrossRef]
- Kamat, P. Carbon Nanomaterials: Building Blocks in Energy Conversion Devices. ElectroChem. Soc. Interface 2006, 15, 45–47. [Google Scholar]
- Coro, J.; Suarez, M.; Silva, L.S.R.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Fullerene applications in fuel cells: A review. Int. J. Hydrog. Energy 2016, 41, 17944–17959. [Google Scholar] [CrossRef]
- Calamba, K.; Ringor, C.; Pascua, C.; Miyazawua, K. Pleated surface morphology of C60 fullerene nanowhiskers incorporated by polyaniline in N-methyl-2-pyrrolidone. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 709–714. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Chen, X.; Yang, S. Single-crystalline C60 crossing microplates: Preparation, characterization, and applications as catalyst supports for methanol oxidation. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 424–430. [Google Scholar] [CrossRef]
- Anafcheh, M.; Ghafouri, R.; Hadipour, N.L. A computational proof toward correlation between the theoretical chemical concept of electrophilicity index for the acceptors of C60 and C70 fullerene derivatives with the open-circuit voltage of polymer-fullerene solar cells. Solar Energy Mater. Solar Cells 2012, 105, 125–131. [Google Scholar] [CrossRef]
- Collavini, S.; Delgado, J.L. Fullerenes: The Stars of Photovoltaics. Sustain. Energy Fuels 2018, 2, 2480–2493. [Google Scholar] [CrossRef]
- Choi, J.H.; Son, K.; Kim, T.; Kim, K.; Ohkuboa, K.; Fukuzumi, S. Thienyl-substituted methanofullerene derivatives for organic photovoltaic cells. J. Mater. Chem. 2010, 20, 475–482. [Google Scholar] [CrossRef]
- Chang, C.L.; Liang, C.W.; Syu, J.J.; Wang, L.; Leung, M.K. Triphenylamine-substituted methanofullerene derivatives for enhanced open-circuit voltages and efficiencies in polymer solar cells. Solar Energy Mater. Solar Cells 2011, 95, 2371–2379. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.B.; Dai, L. Soluble P3HT-Grafted Graphene for Efficient Bilayer−Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, R.; de la Cruz, P.; Langa, F. Basic principles of the chemical reactivity of fullerenes. In In Fullerenes: Principles and Applications; The Royal Society of Chemistry: London, UK, 2012; pp. 66–124. [Google Scholar]
- Shiraishi, H.; Itoh, T.; Hayashi, H.; Takagi, K.; Sakane, M.; Mori, T.; Wang, J. Electrochemical detection of E. coli 16s rDNA sequence using air plasma activated fullerene impregnated screen printed electrodes. Bioelectrochemistry 2007, 70, 481–487. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochem. Biosens Based on C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Jang, Z.; Jia, Z.; Huang, S.; Yang, X.; Li, Y.; Gan, L.; Zhang, S.; Zhu, D. Amination of [60] fullerene by ammonia and by primary and secondary aliphatic amines--preparation of amino [60] fullerene peroxides. Chemistry 2007, 13, 1129–11241. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, Z.; Lei, X.; Tian, W.; Bi, Y.; Jia, Y.; Han, N.; Gao, T.; Zhang, Q. NiCoFe-Layered Double Hydroxides/N-Doped Graphene Oxide Array Colloid Composite as an Efficient Bifunctional Catalyst for Oxygen Electrocatalytic Reactions. Adv. Mater. 2017, 8, 1701905/1–1701905/7. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Zhuo, Y.; Ma, N.; Chai, Q.; Zhao, M.; Yuan, R. Amplified electrochemiluminescent aptasensor using mimicking bi enzyme nanocomplexes as signal enhancement. Anal. Chim. Acta 2014, 809, 47–53. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, R.; Chai, Y.; Niu, H.; Cao, Y.; Liu, H. Bi enzyme synergetic catalysis to in situ generate coreactant of peroxydisulfate solution for ultrasensitive electrochemiluminescence immunoassay. Biosens. Bioelectron. 2012, 37, 6–10. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Deng, J.; Jiang, L.; Huang, H.; Zengh, J. An electrochemical immunosensor for sensitive detection of Escherichia coli o157:H7 using C60 based biocompatible platform and enzyme functionalized pt nanochains tracing tag. Biosens. Bioelectron. 2013, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.; Shih, S. Preparation and application of immobilized c60 glucose oxidase enzyme in fullerene C60 coated piezoelectric quartz crystal glucose sensor. Sens. Actuators B 2001, 81, 1–8. [Google Scholar] [CrossRef]
- Cui, C.; Qian, W.; Yu, Y.; Kong, C.; Yu, B.; Xiang, L.; Wei, F. Highly electroconductive mesoporous graphene nanofibers and their capacitance performance at 4 V. J. Am. Chem. Soc. 2014, 136, 2256–2259. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Dubal, D.P.; Nagar, B.; Ranc, V.; Tomanec, O.; Petr, M.; Datta, K.K.R.; Zboril, R.; Gómez-Romero, P.; Fisher, R.A. Ultrathin hierarchical porous carbon nanosheets for high-performance supercapacitors and redox electrolyte energy storage. Adv. Mater. 2018, 30, 1705789/1–1705789/9. [Google Scholar] [CrossRef]
- Park, H.; Ambae, R.B.; Noh, S.H.; Eom, W.; Koh, K.H.; Ambade, S.B.; Lee, W.J.; Kim, S.H. Porous Graphene-Carbon Nanotube Scaffolds for Fiber Supercapacitors. ACS Appl. Mater. Interface 2019, 11, 9011–9022. [Google Scholar] [CrossRef]
- Penza, M.; Martin, P.J.; Yeow, J.T.W. Carbon Nanotube Gas Sensors. In Gas Sensing Fundamentals; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Springer: Berlin/Heidelberg, Germany, 2014; Volume 15. [Google Scholar]
- Hoa, N.D.; Van Quy, N.; Cho, Y. Porous single-wall carbon nanotube films formed by in situ arc-discharge deposition for gas sensors application. Sens. Actuators B 2009, 135, 656–663. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Chapline, M.G.; Dai, H. Functionalized carbon nanotubes for molecular hydrogen sensors. Adv. Mater. 2001, 13, 1384–1386. [Google Scholar] [CrossRef]
- Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. Chemiresistive Graphene Sensors for Ammonia Detection. ACS Appl. Mater. Interface 2018, 10, 16169–16176. [Google Scholar] [CrossRef]
- Gutes, A.; Hsia, B.; Sussman, A.; Mickelson, W.; Zettl, A.; Carraro, C.; Maboudian, R. Graphene decoration with metal nanoparticles: Towards easy integration for sensing applications. Nanoscale 2012, 4, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Hirsh, T.; Mirsky, V.M.; Wolfbeis, S. Hydrogen sensor based on a graphene—palladium nanocomposite. Electrochim. Acta 2011, 56, 3707–3712. [Google Scholar] [CrossRef]
- Choi, K.Y.; Park, J.S.; Park, K.B.; Kim, H.J.; Park, H.D.; Kim, S.D. Low power micro gas sensors using mixed SnO2 nanoparticles and MWCNTs to detect NO2, NH3, and xylene gases for ubiquitous sensor network applications. Sens. Actuators B 2010, 150, 65–72. [Google Scholar] [CrossRef]
- Kerdcharoen, T.; Wongchoosuk, C. Carbon nanotube and metal oxide hybrid materials for gas sensing. In Semiconductor Gas Sensors; Woodhead Publishing Limited: Oxford, UK, 2013; pp. 386–407. ISBN 978-0-85709-236-6. [Google Scholar]
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2011, 2479–2482. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Cnossen, A.; Coleman, A.C.; Rudolf, P.; van Wees, B.J.; Browne, W.R.; Feringa, B.L. One-pot functionalization of graphene with porphyrin through cycloaddition reaction. Chem. Eur. J. 2011, 17, 8957–8964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, J.; Meng, L.; Huang, Y.; Liu, L. A highly efficient chemical sensor material for ethanol: Al2O3/graphene nanocomposites fabricated from graphene oxide. Chem. Commun. 2011, 47, 6350–6352. [Google Scholar] [CrossRef]
- Barkade, S.S.; Gajare, G.R.; Mishra, S.; Naik, J.B.; Gogate, P.R.; Pinjari, D.V.; Sonawane, S.H. Recent Trends in Carbon Nanotubes/Graphene Functionalization for Gas/Vapor Sensing: A Review. In Chemical Functionalization of Carbon Nanomaterials, Chemistry and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 869–898. [Google Scholar]
- Hasanzadeh, A.; Khataee, A.; Zarei, M.; Zhang, Y. Two-electron oxygen reduction on fullerene C60-carbon nanotubes covalent hybrid as a metal-free electrocatalyst. Sci. Rep. 2019, 9, 13780/1–13780/12. [Google Scholar] [CrossRef] [Green Version]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Khoshroo, A. Label-free electrochemical immunosensor for detection of tumor necrosis factor α based on fullerene-functionalized carbon nanotubes/ionic liquid. J. Electroanal. Chem. 2015, 757, 58–64. [Google Scholar] [CrossRef]
- Qu, Y.; Piao, G.; Zhao, J.; Jiao, K. Reduced working electrode based on fullerene C60 nanotubes@DNA: Characterization and application. Mater. Sci. Eng. B 2010, 175, 159–163. [Google Scholar]
- Ma, J.; Guo, Q.; Gao, H.L.; Qin, X. Synthesis of C60/Graphene Composite as Electrode in Supercapacitors. Fuller Nanotub. Carbon Nanostruct. 2015, 23, 477–482. [Google Scholar] [CrossRef]
- Wan, Y.J.; Tang, L.C.; Yan, D.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites, 1st ed.; Materials Engineering; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-8247-0983-9. [Google Scholar]
- Xie, X.L.; Mai, Y.W.; Zhou, X.P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Chand, S. Review Carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Alam, P.; Mamalis, D.; Robert, C.; Floreani, C.; Brádaigh, C.M.O. The fatigue of carbon fibre reinforced plastics—A review. Composites B 2019, 166, 555–579. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Bijwe, J. Surface Treatment of Carbon Fibers—A Review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Li, J. The effect of surface modification with nitric acid on the mechanical and tribological properties of carbon. Surf. Interface Anal. 2009, 41, 759–763. [Google Scholar] [CrossRef]
- Zhang, X.R.; Pei, X.Q.; Wang, Q.H. The effect of fiber oxidation on the friction and wear behaviors of short-cut CFs/polyimide composites. Express Polym. Lett. 2007, 1, 318–325. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Silva, L.L.G.; Santos, A.L.; Nascente, P.A.P.; Kostov, K.G. Atmospheric Plasma Treatment of Carbon Fibers for Enhancement of Their Adhesion Properties. IEEE Trans. Plasma Sci. 2013, 41, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Zaldivar, R.J.; Nokes, J.P.; Kim, H.I. The effect of surface treatment on graphite nanoplatelets used in fiber reinforced composites. Appl. Polym. 2014, 131, 39994/1–39994/10. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon Nanotubes and Related Structures: New Materials for the Twenty-first Century; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Wu, S.; Peng, S.; Wang, C.H. Multifunctional Polymer Nanocomposites Reinforced by Aligned Carbon Nanomaterials. Polymers 2018, 10, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, D.; Dickey, E.C.; Andrews, R. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef] [Green Version]
- Biercuk, M.J.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.K.; Johnson, A.T. Carbon nanotube composites for thermal management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Lopez-Barroso, J.; Martinez-Hernandez, A.L.; Rivera-Armenta, J.L.; Velasco-Santos, C. Multidimensional Nanocomposites of Epoxy Reinforced with 1D and 2D Carbon Nanostructures for Improve Fracture Resistance. Polymers 2018, 18, 281. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, T.; Kosaka, A.; Yamamoto, Y.; Aimiya, T.; Notazawa, S.; Takigawa, T.; Inabe, T. Dramatic Effect of Dispersed Carbon Nanotubes on the Mechanical and Electroconductive Properties of Polymers Derived from Ionic Liquids. Small 2006, 2, 554–560. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Khanam, P.N.; Ponnamma, D.; AL-Madeed, M.A. Electrical Properties of Graphene Polymer Nanocomposites. In Graphene-Based Polymer Nanocomposites in Electronics; Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-13874-9. [Google Scholar]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielwaski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, J.; Liu, J.; Yang, X.; Zhao, H. Exfoliated Graphite Oxide Decorated by PDMAEMA Chains and Polymer Particles. Langmuir 2009, 25, 11808–11814. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tahara, K.; Sugie, Y. Structure and Thermal Properties of Poly (ethylene oxide)-intercalated Graphite Oxide. Carbon 1997, 35, 113–120. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Gomez, M.A.; Martmez, G. Polymeric Modification of Graphene through Esterification of Graphite Oxide and Poly(vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommet, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, L.; Li, L.; Zhang, Y.; Chen, F.; Zhong, M. Preparation of polystyrene/graphene oxide composites and their supercritical carbon dioxide foaming. J. Polym. Res. 2013, 173/1–173/9. [Google Scholar] [CrossRef]
- Pham, V.H.; Dang, T.T.; Hur, S.H.; Kim, E.J.; Chung, J.S. Highly Conductive Poly (methyl methacrylate) (PMMA)-Reduced Graphene Oxide Composite Prepared by Self-Assembly of PMMA Latex and Graphene Oxide through Electrostatic Interaction. ACS Appl. Mater. Interface 2012, 4, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, X.; He, X.; Yan, Y. Preparation and Characterization of Graphene Oxide-Modified Sapium sebiferum Oil-Based Polyurethane Composites with Improved Thermal and Mechanical Properties. Polymers 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavgani, J.N.; Adelnia, H.; Zaarei, D.; Gudarzi, M.M. Lightweight flexible polyurethane/reduced ultralarge graphene oxide composite foams for electromagnetic interference shielding. RSC Adv. 2016, 6, 27517–27527. [Google Scholar] [CrossRef]
- Li, Y.; Umer, R.; Samad, Y.A.; Zheng, L.; Liao, K. The effect of the ultrasonication pre-treatment of graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon 2013, 55, 321–327. [Google Scholar] [CrossRef]
- Soheilmoghaddam, M.; Adelnia, H.; Bidsorkhi, H.C.; Sharifzadeh, G.; Wahit, M.U.; Akos, N.I. Development of Ethylene-Vinyl Acetate Composites Reinforced with Graphene Platelets. Macromol. Mater. Eng. 2017, 302, 1600260/1–1600260/9. [Google Scholar] [CrossRef]
- Cheng, X.; Kumar, V.; Yokozeki, T.; Goto, T.; Takahashi, T.; Koyanagi, J.; Wu, L.; Wang, R. Highly conductive graphene oxide/polyaniline hybrid polymernanocomposites with simultaneously improved mechanical properties. Composites A 2016, 82, 100–107. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and application of sunset yellow imprinted ionic liquid polymer − ionic liquid functionalized graphene composite film coated glassy carbon electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Electrochemical determination of methyl parathion using a molecularly imprinted polymer–ionic liquid–graphene composite film coated electrode. Sens. Actuators B 2013, 176, 818–824. [Google Scholar] [CrossRef]
- Horn, M.; Gupta, B.; MacLeod, J.; Liu, J.; Motta, N. Graphene-based supercapacitor electrodes: Addressing challenges in mechanisms and materials. Curr. Opin. Green Sustain. Chem. 2019, 17, 42–48. [Google Scholar] [CrossRef]
- Gupta, A.; Akhtar, A.J.; Saha, S.K. In-situ growth of P3HT/graphene composites for supercapacitor application. Mater. Chem. Phys. 2013, 140, 616–621. [Google Scholar] [CrossRef]
- Luo, R.P.; Lyu, W.Q.; Wen, K.C.; He, W.D. Overview of Graphene as Anode in Lithium-Ion Batteries. J. Electron. Sci. Technol. 2018, 16, 57–68. [Google Scholar]
- Cheng, Q.; Okamoto, Y.; Tamura, N.; Tsuji, M.; Maruyama, S.; Matsuo, Y. Graphene-Like-Graphite as Fast-Chargeable and High-Capacity Anode Materials for Lithium Ion Batteries. Sci. Rep. 2017, 7, 14782/1–14782/14. [Google Scholar] [CrossRef] [Green Version]
- Adil, S.F.; Khan, M.; Kalpana, D. Graphene-based nanomaterials for solar cells. In Multifunctional Photocatalytic Materials for Energy; Woodhead Publishing in Materials: Oxford, UK, 2018; pp. 127–152. [Google Scholar]
- Hsieh, Y.P.; Hong, B.J.; Ting, C.C.; Hofmann, M. Ultrathin graphene-based solar cells. RSC Adv. 2015, 5, 99627–99631. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-Based Sensors for Human Health Monitoring. Front. Chem. 2019, 11, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Mitra, A.; Mukhopadhyaya, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Shareena, T.P.D.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghigha, A.; Alizadeha, V.; Wonga, H.Y.; Islamb, S.; Aminc, N.; Zaman, M. Recent advances in utilization of graphene forfiltration and desalinationof water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered graphene oxide membranes for water treatment: A review. Carbon 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Boretti, A.; Al-Zubaidy, S.; Vaclavikova, M.; Al-Abri, M.; Castelletto, S.; Mikhalovsky, S. Outlook for graphene-based desalination membranes. NPJ Clean Water 2018, 1, 5/1–5/11. [Google Scholar] [CrossRef]
- Khalilnezhad, S.; Sajjadi, S.A.; Zebarjad, S.M. Effect of nanodiamond surface functionalization using oleylamine on the scratch behavior of polyacrylic/nanodiamond nanocomposite. Diam. Relat. Mater. 2014, 45, 7–11. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Oliva González, C.M.; Kharisov, B.I. Solubilization and Dispersion of Carbon Allotropes in Water and Non-aqueous Solvents. Ind. Eng. Chem. Res. 2018, 57, 12624–12645. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Karami, P.; Khasraghi, S.S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites—A comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef]
- Astuti, Y.; Saputra, F.D.; Wuning, S.; Bhaduri, G.; Arnelli. Enrichment of nanodiamond surfaces with carboxyl groups for doxorubicin loading and release. IOP Conf. Ser. Mater. Sci. Eng. 2017, 172, 012066/1–012066/8. [Google Scholar] [CrossRef]
- Jee, A.Y.; Lee, M. Surface functionalization and physicochemical characterization of diamond nanoparticles. Curr. Appl. Phys. 2009, 9, e144–e147. [Google Scholar] [CrossRef]
- Raymakers, J.; Haenen, K.; Maes, W. Diamond surface functionalization: From gemstone to photoelectrochemical applications. J. Mater. Chem. C 2019, 7, 10134–10165. [Google Scholar] [CrossRef]
- Karami, P.; Shojaei, A. Morphological and mechanical properties of polyamide 6/nanodiamond composites prepared by melt mixing: Effect of surface functionality of nanodiamond. Poly. Int. 2017, 66, 557–565. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. A Structure and chemistry of polymer/nanodiamond composites. In Hybrid Polymer Composite Materials, Structure and Chemistry; Woodhead Publishing: Oxford, UK, 2017. [Google Scholar]
- Avazkonandeh-Gharavol, M.H.; Sajjadi, S.A.; Zebarjad, S.M.; Mohammadtaheri, M.; Abbasi, M.; Alimardani, M.; Mossaddegh, K. Effect of heat treatment of nanodiamonds on the scratch behavior of polyacrylic/nanodiamond nanocomposite clear coats. Prog. Org. Coat. 2013, 76, 1258–1264. [Google Scholar] [CrossRef]
- Haleem, Y.A.; Song, P.; Liu, D.; Wang, C.; Gan, W.; Saleem, M.F.; Song, L. The Effect of High Concentration and Small Size of Nanodiamonds on the Strength of Interface and Fracture Properties in Epoxy Nanocomposite. Materials 2016, 9, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haleem, Y.A.; Liu, D.; Chen, W.; Wang, C.; Hong, C.; He, Z.; Liu, J.; Song, P.; Yu, S.; Song, L. Surface functionalization and structure characterizations of nanodiamond and its epoxy based nanocomposites. Compos. Part. B 2015, 78, 480–487. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, K.; Kim, C.K.; Kang, E. Reinforcement of nylon 6, 6/nylon 6, 6 grafted nanodiamond composites by in situ reactive extrusion. Nat. Sci. Rep. 2016, 6, 37010/1–37010/10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Paterson, A.; Palmese, G.; Gogotsi, Y. Covalent incorporation of aminated nanodiamond into an epoxy polymer network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M. The effect of amino functionalized and polyethylene glycol grafted nanodiamond on anti-biofouling properties of cellulose acetate membrane in membrane bioreactor systems. Sep. Purif. Technol. 2017, 177, 350–362. [Google Scholar] [CrossRef]
- Loktev, V.F.; Makal’skii, V.I.; Stoyanova, I.V.; Kalinkin, A.V.; Likholobov, V.A.; Mit’kin, V.N. Surface modification of ultradispersed diamonds. Carbon 1991, 29, 817–819. [Google Scholar] [CrossRef]
- Tasaki, T.; Guo, Y.; Machida, H.; Akasaka, S.; Fujimori, A. Nano-dispersion of fluorinated phosphonate-modified nanodiamond in crystalline fluoropolymer matrix to achieve a transparent polymer/nanofiller hybrid. Polym. Compos. 2019, 40(S1), E842–E855. [Google Scholar] [CrossRef]
- Li, C.C.; Huang, C.L. Preparation of clear colloidal solutions of detonation nanodiamond in organic solvents. Colloids Surf. A 2010, 353, 52–56. [Google Scholar] [CrossRef]
- Aris, A.; Shojaei, A.; Bagheri, R. Cure kinetics of nanodiamond-filled epoxy resin: Influence of nanodiamond surface functionality. Ind. Eng. Chem. Res. 2015, 54, 8954–8962. [Google Scholar] [CrossRef]

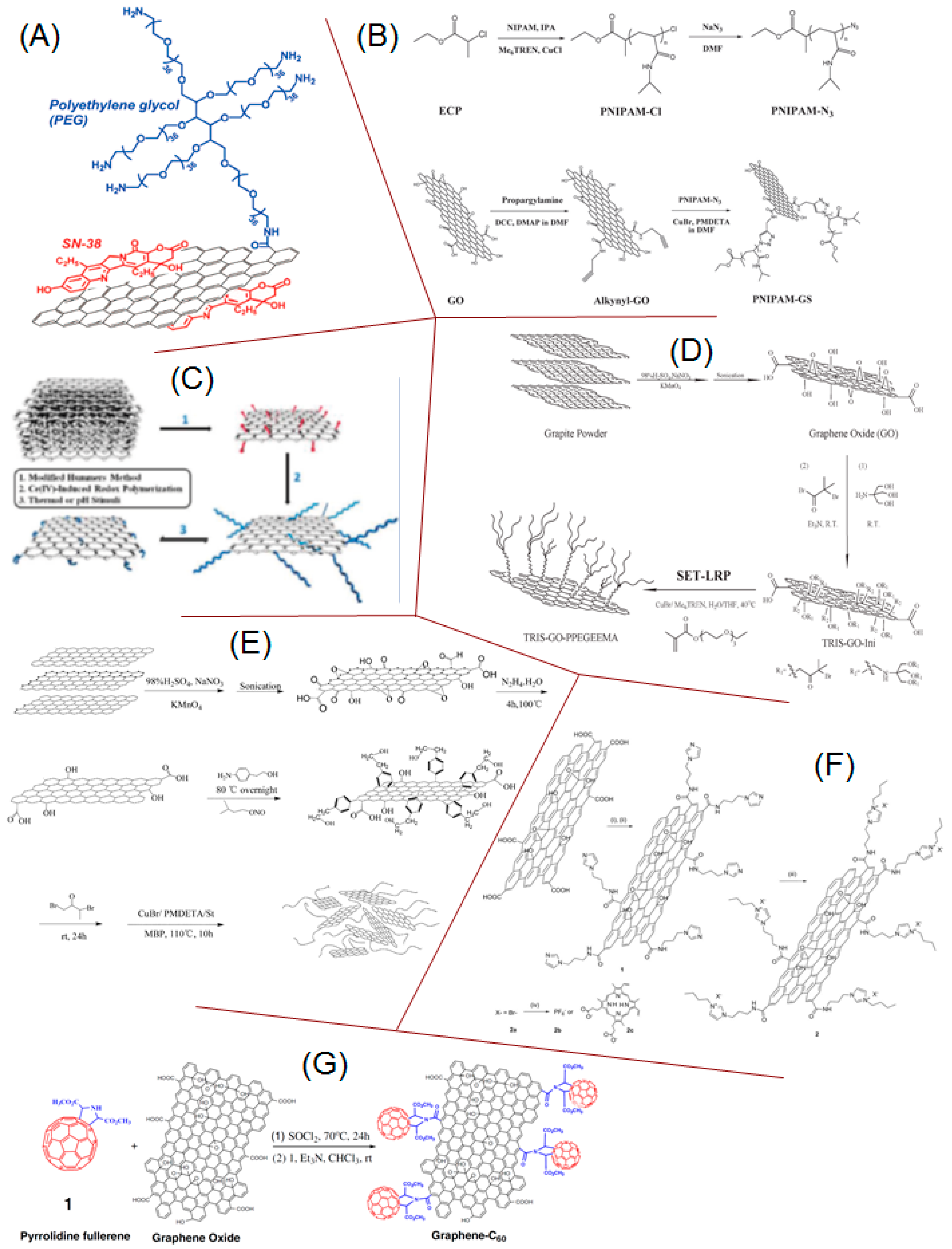










© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C 2019, 5, 84. https://doi.org/10.3390/c5040084
Speranza G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C. 2019; 5(4):84. https://doi.org/10.3390/c5040084
Chicago/Turabian StyleSperanza, Giorgio. 2019. "The Role of Functionalization in the Applications of Carbon Materials: An Overview" C 5, no. 4: 84. https://doi.org/10.3390/c5040084
APA StyleSperanza, G. (2019). The Role of Functionalization in the Applications of Carbon Materials: An Overview. C, 5(4), 84. https://doi.org/10.3390/c5040084