Impacts of Mn Content and Mass Loading on the Performance of Flexible Asymmetric Solid-State Supercapacitors Using Mixed-Phase MnO2/N-Containing Graphene Composites as Cathode Materials
Abstract
:1. Introduction
2. Experimental
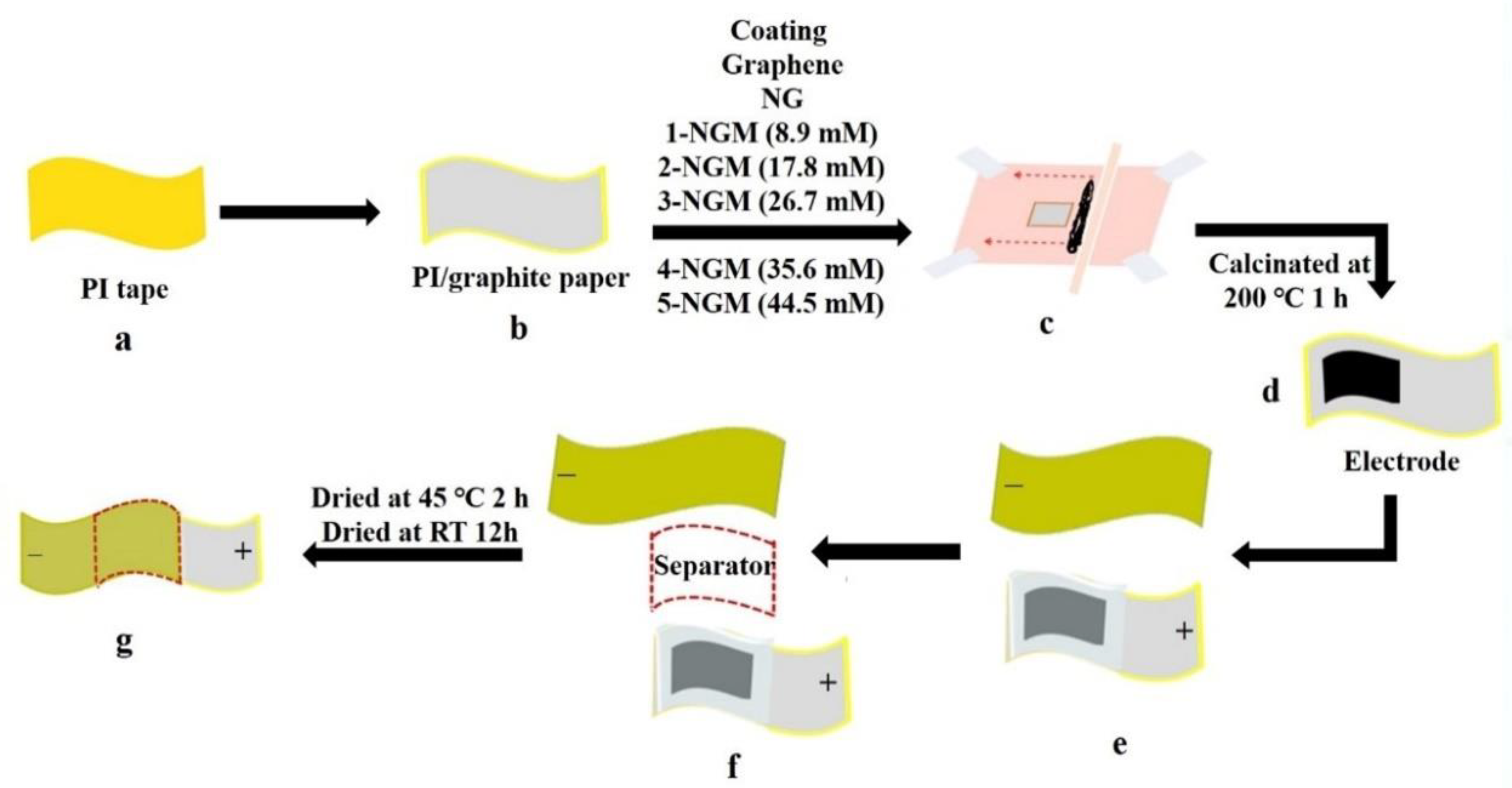
2.1. Preparation of G
2.2. Preparation of N-Doped G (NG) Composites
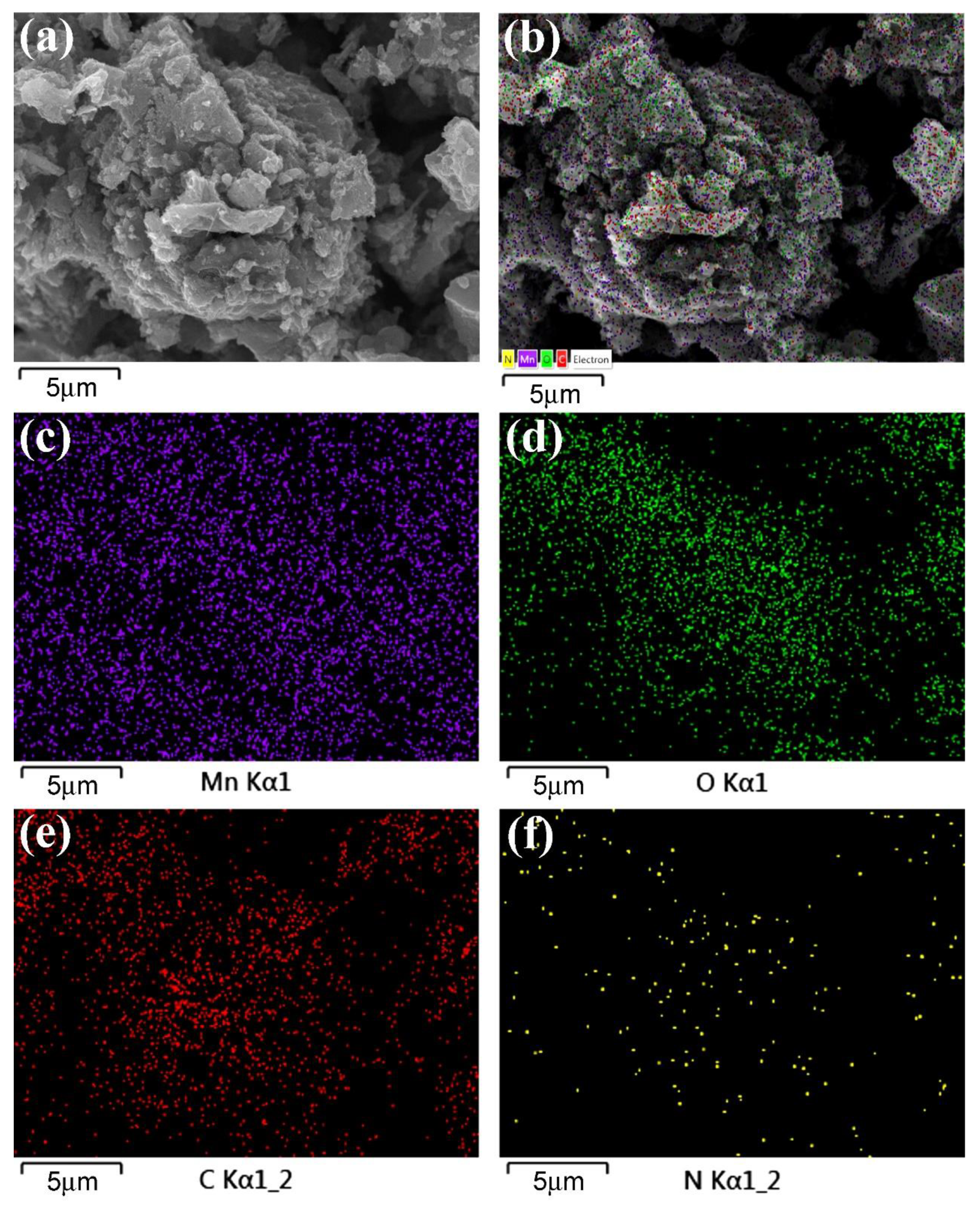
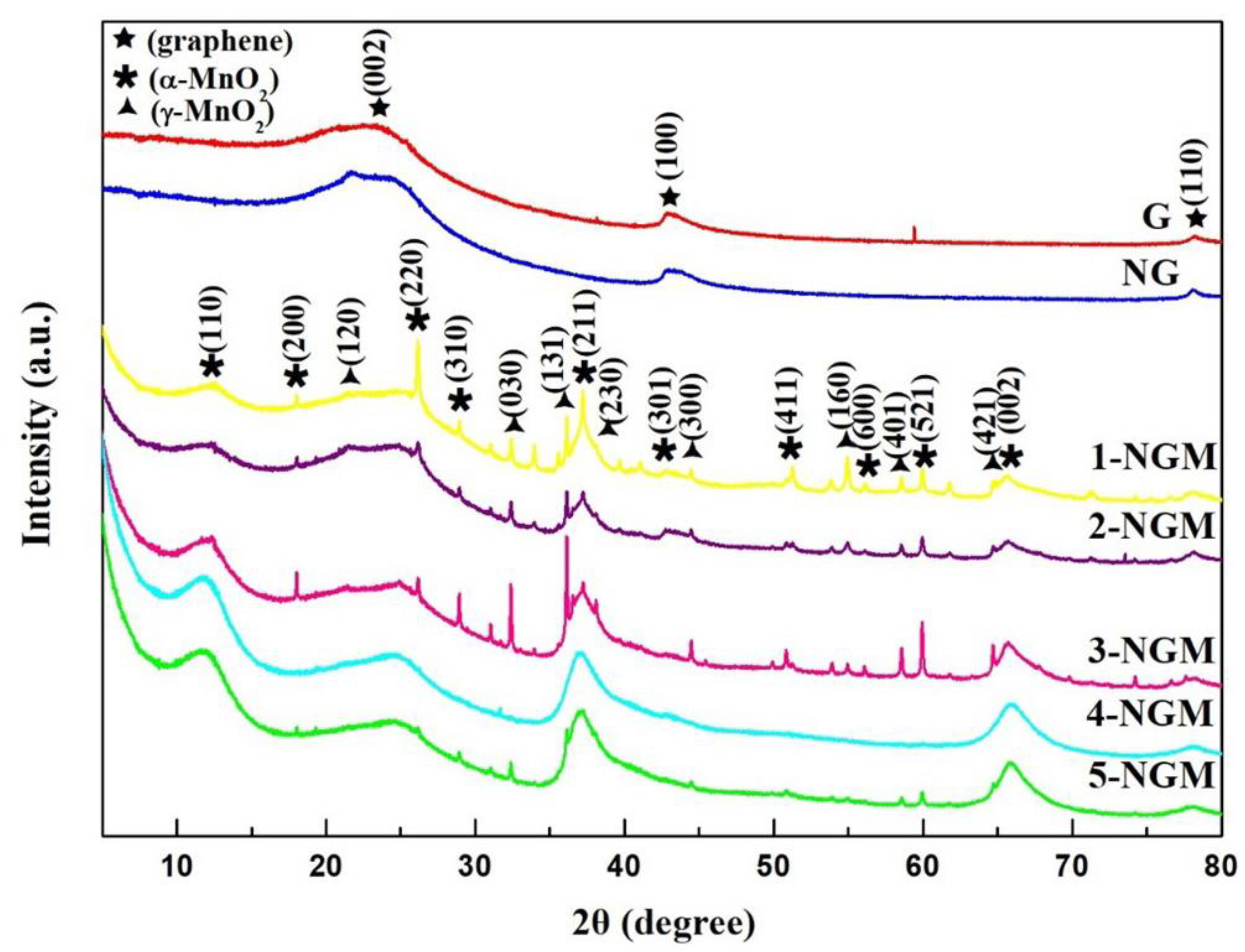
2.3. Preparation of NG/MnO2 (NGM) Composites
2.4. Fabrication of Electrodes
2.5. Fabrication of ASSCs
2.6. Characterization
2.7. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Du, X.; Wang, J.; Ma, H.; Jing, X. Polypyrrole composites with carbon materials for supercapacitors. Chem. Pap. 2017, 71, 293. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189. [Google Scholar] [CrossRef]
- Lakshmi, K.C.S.; Vedhanarayanan, B. High-performance supercapacitors: A comprehensive review on paradigm shift of conventional energy storage devices. Batteries 2023, 9, 202. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Gholami, M.; Naseri, N. Inkjet printing of MnO2 nanoflowers on surface-modified A4 paper for flexible all-solid-state microsupercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 3894. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.G.; Yan, X.H.; Cao, Y.; Gao, H.L.; Luo, H.W.; Gao, K.Z.; Xue, S.C.; Jing, X. Recent advances and perspectives on graphene-based gels for superior flexible all-solid-state supercapacitors. J. Power Sources 2023, 565, 232916. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Hu, J.; Wei, L.; Liu, J.; Zheng, M. Facile synthesis of MnO2 grown on nitrogen-doped carbon nanotubes for asymmetric supercapacitors with enhanced electrochemical performance. J. Power Sources 2018, 393, 135. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wu, H.B.; Lin, J.; Shen, Z.; Lou, X.W. High-performance flexible asymmetric supercapacitors based on a new graphene foam/carbon nanotube hybrid film. Energy Environ. Sci. 2014, 7, 3709. [Google Scholar] [CrossRef]
- Ning, P.; Duan, X.; Ju, X.; Lin, X.; Tong, X.; Pan, X.; Wang, T.; Li, Q. Facile synthesis of carbon nanofibers/MnO2 nanosheets as high-performance electrodes for asymmetric supercapacitors. Electrochim. Acta 2016, 210, 754. [Google Scholar] [CrossRef]
- Ghosh, K.; Yue, C.Y.; Sk, M.M.; Jena, R.K. Development of 3D urchin-shaped coaxial manganese dioxide@polyaniline (MnO2@PANI) composite and self-assembled 3D pillared graphene foam for asymmetric all-solid-state flexible supercapacitor application. ACS Appl. Mater. Interfaces 2017, 9, 15350. [Google Scholar] [CrossRef]
- Kong, S.; Cheng, K.; Ouyang, T.; Gao, Y.; Ye, K.; Wang, G.; Cao, D. Facile dip coating processed 3D MnO2-graphene nanosheets/MWNT-Ni foam composites for electrochemical supercapacitors. Electrochim. Acta 2017, 226, 29. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.S.; Lu, X.; Lee, P.S. Dodecyl sulfate-induced fast faradic process in nickel cobalt oxide-reduced graphite oxide composite material and its application for asymmetric supercapacitor device. J. Mater. Chem. 2012, 22, 23114. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Wu, H.; Duan, J.; Zhang, Y. Hydrothermal self-assembly of manganese dioxide/manganese carbonate/reduced graphene oxide aerogel for asymmetric supercapacitors. Electrochim. Acta 2015, 164, 154. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Zhai, T.; Xie, S.; Ling, Y.; Tong, Y.; Li, Y. H-TiO2@MnO2//H-TiO2@C core-shell nanowires for high performance and flexible asymmetric supercapacitors. Adv. Mater. 2013, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 2017, 240, 53. [Google Scholar] [CrossRef]
- Tseng, L.H.; Hsiao, C.H.; Nguyen, D.D.; Hsieh, P.Y.; Lee, C.Y.; Tai, N.H. Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes. Electrochim. Acta 2018, 266, 284. [Google Scholar] [CrossRef]
- Niu, Z.; Dong, H.; Zhu, B.; Li, J.; Hng, H.H.; Zhou, W.; Chen, X.; Xie, S. Highly stretchable, integrated supercapacitors based on single-walled carbon nanotube films with continuous reticulate architecture. Adv. Mater. 2013, 25, 1058. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013, 13, 2628. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ding, T.; Yuan, L.; Shen, Y.; Zhong, Q.; Zhang, X.; Cao, Y.; Hu, B.; Zhai, T.; Gong, L. WO3-x/MoO3-x core/shell nanowires on carbon fabric as an anode for all-solid-state asymmetric supercapacitors. Adv. Energy Mater. 2012, 2, 1328. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, K.H.; Lin, M.C.; Wu, Y.T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006, 6, 2690. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly flexible and all-solid-state paper like polymer supercapacitors. Nano Lett. 2010, 10, 4025. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, X.; Fang, J.; Zhang, X.; Chen, S.; Li, W.; Feng, W.; Chen, Y.; Wang, W.; Zhang, Y. Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 5251. [Google Scholar] [CrossRef]
- Liu, M.; Gan, L.; Xiong, W.; Xu, Z.; Zhu, D.; Chen, L. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes. J. Mater. Chem. 2014, 2, 2555. [Google Scholar] [CrossRef]
- Mu, B.; Zhang, W.; Xu, W.; Wang, A. Hollowed-out tubular carbon@MnO2 hybrid composites with controlled morphology derived from kapok fibers for supercapacitor electrode materials. Electrochim. Acta 2015, 178, 709. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Zhan, J.; Sun, X.; Kang, L.; Jiang, F.; Zhang, X.; Shao, Q.; Dong, M.; Liu, H.; et al. Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 296, 907. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y.; et al. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771. [Google Scholar] [CrossRef]
- Wen, Y.; Ding, H.; Shan, Y. Preparation and visible light photocatalytic activity of Ag/TiO2/graphene nanocomposite. Nanoscale 2011, 3, 4411. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Guo, Y.; Wang, S.; Liu, Y. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 2013, 5, 2227. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Cao, C.B.; Tao, S.; Chu, W.S.; Wu, Z.Y.; Li, Y.D. Ultrathin nickel hydroxide and oxide nanosheets: Synthesis, characterizations and excellent supercapacitor performances. Sci. Rep. 2015, 4, 5787. [Google Scholar] [CrossRef]
- Zeiger, M.; Jackel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172. [Google Scholar] [CrossRef]
- Brousse, T.; Taberna, P.L.; Crosnier, O.; Dugas, R.; Guillemet, P.; Scudeller, Y.; Zhou, Y.; Favier, F.; Bélanger, D.; Simon, P. Long-term cycling behavior of asymmetric activated carbon/MnO2 aqueous electrochemical supercapacitor. J. Power Sources 2007, 173, 633. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Zainal, Z.; Huang, N.M.; Harrison, I.; Andou, Y. Flexible graphene-based supercapacitors: A review. J. Phys. Chem. C 2016, 120, 4153. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Cho, C.P. Mixed-phase MnO2/N-Containing graphene composites applied as electrode active materials for flexible asymmetric solid-state supercapacitors. Nanomaterials 2018, 8, 924. [Google Scholar] [CrossRef]
- Liu, L.; Su, L.; Lang, J.; Hu, B.; Xu, S.; Yan, X. Controllable synthesis of Mn3O4 nanodots@nitrogen-doped graphene and its application for high energy density supercapacitors. J. Mater. Chem. A 2017, 5, 5523. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, N.; Shi, C.; Liu, E.; He, C. Improve the supercapacity performance of MnO2-decorated graphene by controlling the oxidization extent of graphene. J. Phys. Chem. C 2012, 116, 25226. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wu, C.W.; Chen, I.W.P.; Chang, H.T.; Lin, C.H.; Huang, C.C. Carbon dot-mediated synthesis of manganese oxide decorated graphene nanosheets for supercapacitor application. ACS Sustain. Chem. Eng. 2016, 4, 3008. [Google Scholar] [CrossRef]
- Du, D.; Li, P.; Ouyang, J. Nitrogen-doped reduced graphene oxide prepared by simultaneous thermal reduction and nitrogen doping of graphene oxide in air and its application as an electrocatalyst. ACS Appl. Mater. Interfaces 2015, 7, 26952. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, B.; Liu, J.; Long, Y.; Li, N.; Wen, Z.; Jiang, Y. Graphene/ MnO2 composite prepared by a simple method for high performance supercapacitor. Mater. Res. Innov. 2016, 20, 92. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, W.; Yu, H.; Pu, Y.; Zhang, Y.; Hu, C. Template synthesis of hierarchical mesoporous δ-MnO2 hollow microspheres as electrode material for high-performance symmetric supercapacitor. Electrochim. Acta 2018, 264, 1. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. High-performance asymmetric supercapacitor based on graphene hydrogel and nanostructured MnO2. ACS Appl. Mater. Interfaces 2012, 4, 2801. [Google Scholar] [CrossRef]
- Yang, M.; Kim, D.S.; Hong, S.B.; Sim, J.W.; Kim, J.; Kim, S.S.; Choi, B.G. MnO2 nanowire/biomass-derived carbon from hemp stem for high-performance supercapacitors. Langmuir 2017, 33, 5140. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020. [Google Scholar] [CrossRef]
- Cheng, H.; Duon, H.M. Three dimensional manganese oxide on carbon nanotube hydrogels for asymmetric supercapacitors. RSC Adv. 2016, 6, 36954. [Google Scholar] [CrossRef]
- Śliwak, A.; Gryglewicz, G. High-voltage asymmetric supercapacitors based on carbon and manganese oxide/oxidized carbon nanofiber composite electrodes. Energy Technol. 2014, 2, 819. [Google Scholar] [CrossRef]
- Chou, T.C.; Doong, R.A.; Hu, C.C.; Zhang, B.; Su, D.S. Hierarchically porous carbon with manganese oxides as highly efficient electrode for asymmetric supercapacitors. ChemSusChem 2014, 7, 841. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability. J. Phys. Chem. C 2014, 118, 22865. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Zhang, H.; Varanasi, C.V.; Liu, J. Synergistic effects from graphene and carbon nanotubes enable flexible and robust electrodes for high-performance supercapacitors. Nano Lett. 2012, 12, 4206. [Google Scholar] [CrossRef]
- Khomenko, V.; Pinero, E.R.; Beguin, F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2V in aqueous medium. J. Power Sources 2006, 153, 183. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Yang, D.; Li, J. High performance MnO2 supercapacitor material prepared by modified electrodeposition method with different electrodeposition voltages. J. Energy Storage 2020, 29, 101363. [Google Scholar] [CrossRef]
- Wu, J.; Raza, W.; Wang, P.; Hussain, A.; Ding, Y.; Yu, J.; Wu, Y.; Zhao, J. Zn-doped MnO2 ultrathin nanosheets with rich defects for high performance aqueous supercapacitors. Electrochim. Acta 2022, 418, 140339. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on carbon fibers modified with carbon nanotubes for ultrafast flexible supercapacitors in ionic liquid electrolytes with wide voltage windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]

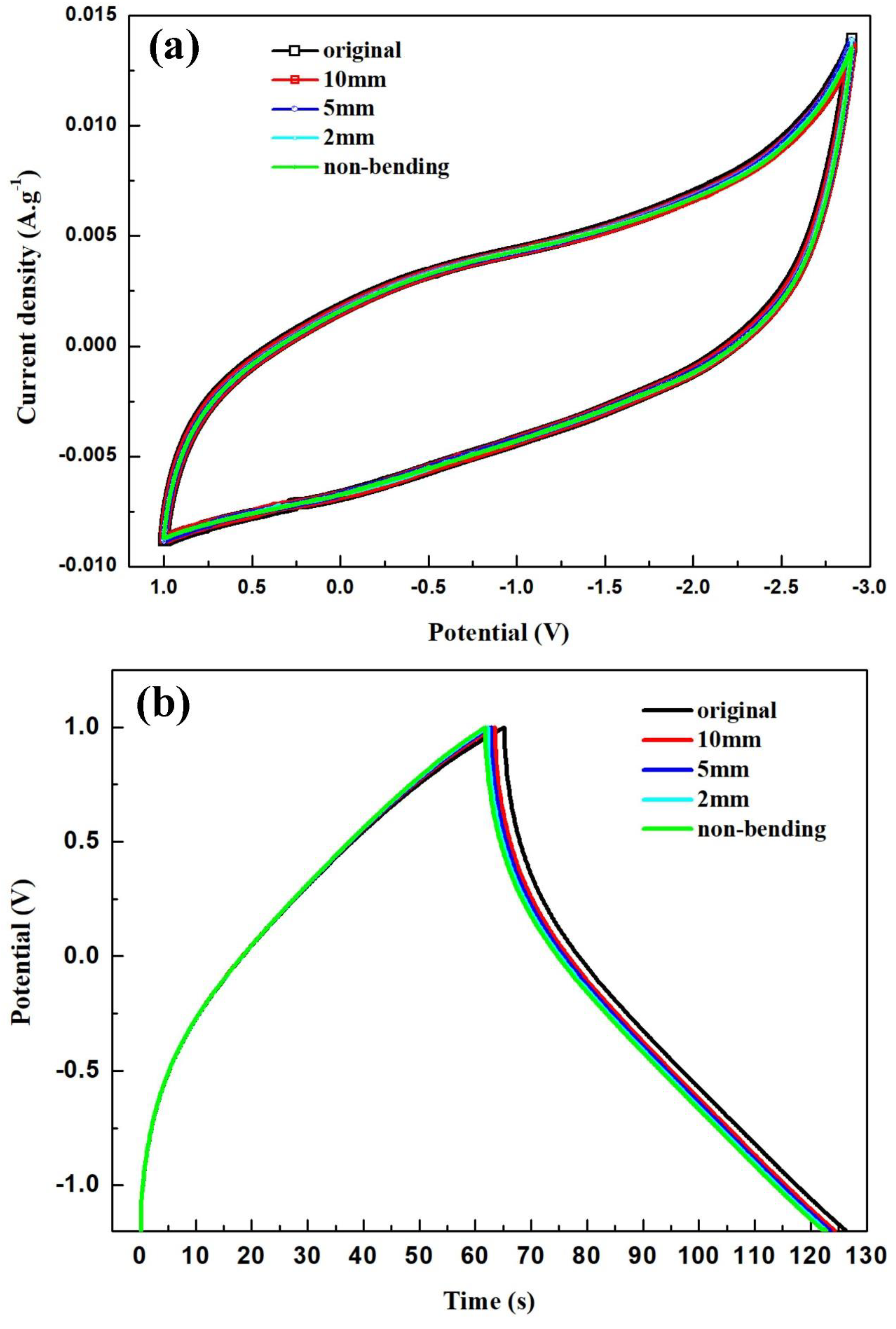
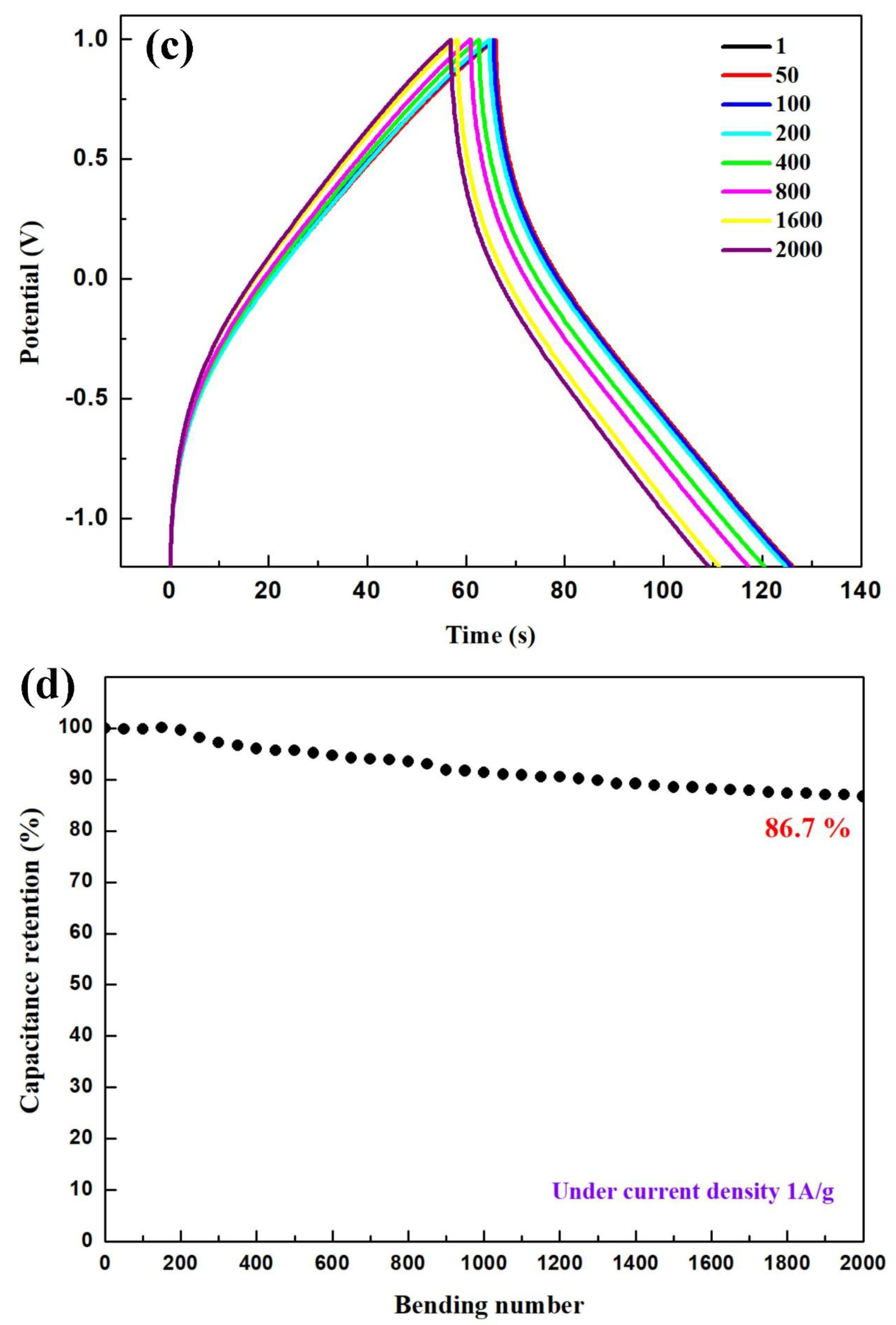
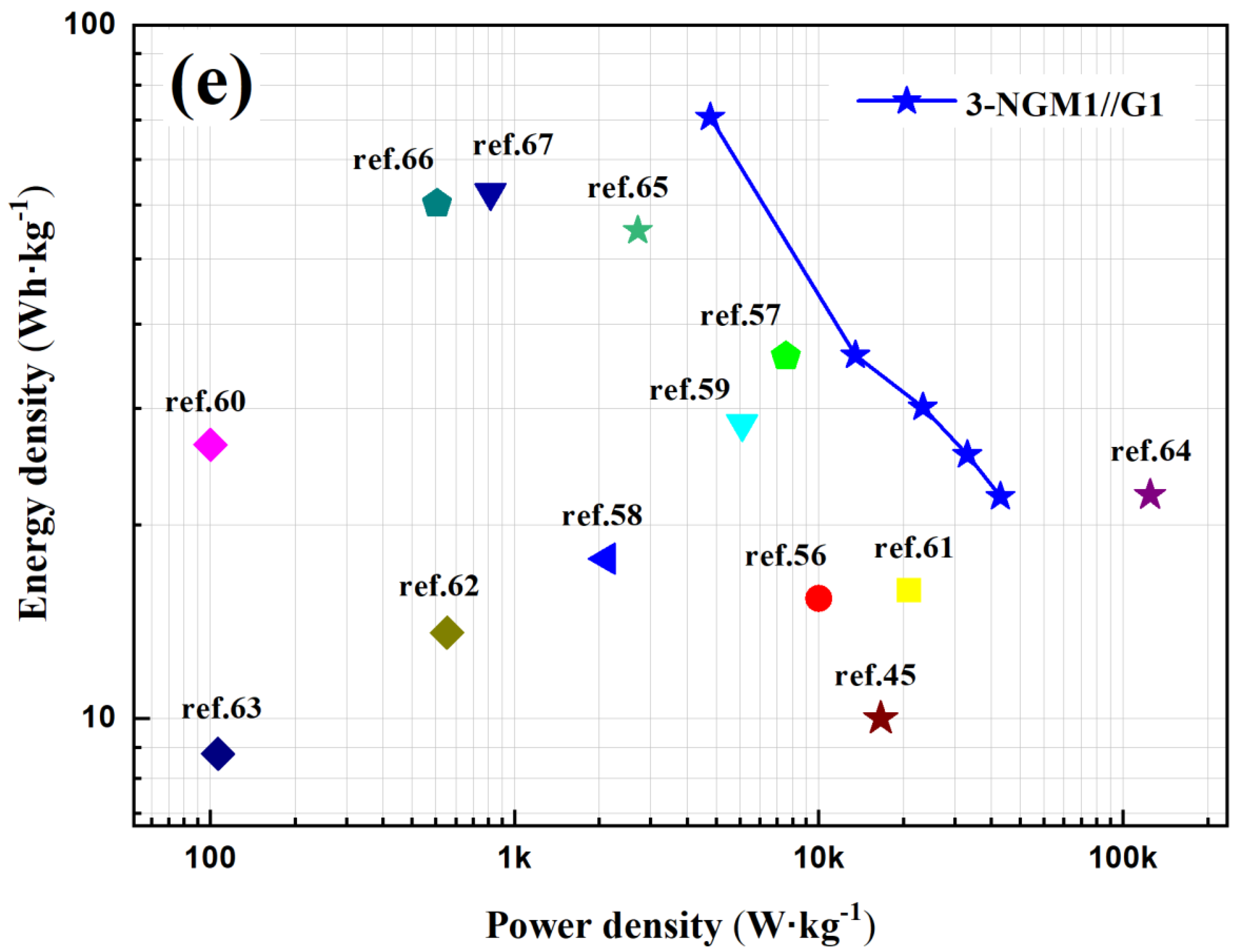
| Capacitor | RCT (Ω) | Capacitor | RCT (Ω) | Capacitor | RCT (Ω) |
|---|---|---|---|---|---|
| G1//G1 | 3.46 | 1-NGM2//G1 | 2.59 | 3-NGM3//G1 | 1.89 |
| G2//G1 | 3.74 | 1-NGM3//G1 | 8.97 | 4-NGM1//G1 | 9.14 |
| G3//G1 | 15.40 | 2-NGM1//G1 | 1.43 | 4-NGM2//G1 | 9.43 |
| NG1//G1 | 2.68 | 2-NGM2//G1 | 1.74 | 4-NGM3//G1 | 9.74 |
| NG2//G1 | 3.41 | 2-NGM3//G1 | 2.24 | 5-NGM1//G1 | 9.87 |
| NG3//G1 | 9.46 | 3-NGM1//G1 | 1.24 | 5-NGM2//G1 | 10.24 |
| 1-NGM1//G1 | 2.35 | 3-NGM2//G1 | 1.32 | 5-NGM3//G1 | 13.60 |
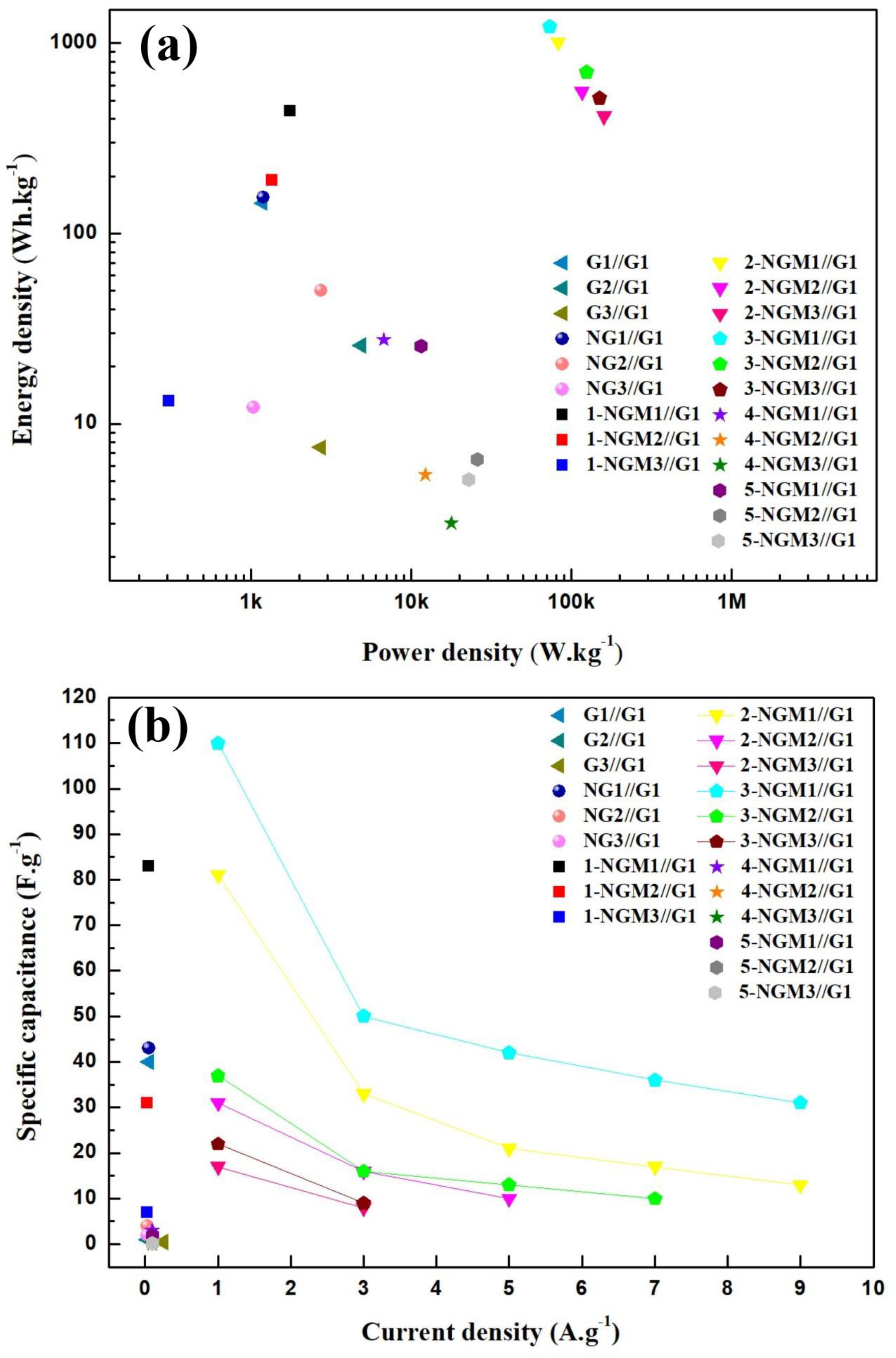
| Capacitor | Scan Rate (mV·s−1) | Specific Capacitance (F·g−1) | Energy Density (Wh·kg−1) | Power Density (W·kg−1) |
|---|---|---|---|---|
| G1//G1 | 100 | 72 | 144.6 | 1177.9 |
| G2//G1 | 100 | 12 | 25.8 | 4837.2 |
| G3//G1 | 100 | 4 | 7.5 | 2713.2 |
| NG1//G1 | 100 | 73 | 154.5 | 1185.6 |
| NG2//G1 | 100 | 26 | 50.2 | 2730.2 |
| NG3//G1 | 100 | 6 | 12.2 | 1034.8 |
| 1-NGM1//G1 | 100 | 209 | 441.9 | 1744.3 |
| 1-NGM2//G1 | 100 | 95 | 191.1 | 1346.1 |
| 1-NGM3//G1 | 100 | 7 | 13.2 | 305.7 |
| 2-NGM1//G1 | 100 | 480 | 1014.1 | 82,407.0 |
| 2-NGM2//G1 | 100 | 263 | 556.4 | 116,454.6 |
| 2-NGM3//G1 | 100 | 196 | 413.6 | 160,087.4 |
| 3-NGM1//G1 | 100 | 579 | 1223.3 | 73,153.6 |
| 3-NGM2//G1 | 100 | 334 | 705.9 | 123,958.1 |
| 3-NGM3//G1 | 100 | 243 | 513.4 | 150,269.1 |
| 4-NGM1//G1 | 100 | 13 | 27.7 | 6726.1 |
| 4-NGM2//G1 | 100 | 3 | 5.4 | 12,221.9 |
| 4-NGM3//G1 | 100 | 1 | 2.9 | 17,744.4 |
| 5-NGM1//G1 | 100 | 12 | 25.6 | 11,496.8 |
| 5-NGM2//G1 | 100 | 3 | 6.5 | 25,867.5 |
| 5-NGM3//G1 | 100 | 2 | 5.1 | 22,750.9 |
| Capacitor | Current Density (A·g−1) | Specific Capacitance (F·g−1) | Energy Density (Wh·kg−1) | Power Density (W·kg−1) |
|---|---|---|---|---|
| 2-NGM1//G1 | 1 | 81 | 54.1 | 4400.0 |
| 2-NGM2//G1 | 1 | 31 | 21.0 | 4400.0 |
| 2-NGM3//G1 | 1 | 17 | 11.4 | 4400.0 |
| 3-NGM1//G1 | 1 | 110 | 73.6 | 4400.0 |
| 3-NGM2//G1 | 1 | 37 | 25.0 | 4400.0 |
| 3-NGM3//G1 | 1 | 22 | 15.0 | 4400.0 |
| 2-NGM1//G1 | 3 | 33 | 22.0 | 13,200.0 |
| 2-NGM2//G1 | 3 | 16 | 10.6 | 13,200.0 |
| 2-NGM3//G1 | 3 | 8 | 5.1 | 13,200.0 |
| 3-NGM1//G1 | 3 | 50 | 33.4 | 13,200.0 |
| 3-NGM2//G1 | 3 | 16 | 11.0 | 13,200.0 |
| 3-NGM3//G1 | 3 | 9 | 6.2 | 13,200.0 |
| 2-NGM1//G1 | 5 | 21 | 14.1 | 22,000.0 |
| 2-NGM2//G1 | 5 | 10 | 6.7 | 22,000.0 |
| 3-NGM1//G1 | 5 | 42 | 28.1 | 22,000.0 |
| 3-NGM2//G1 | 5 | 13 | 8.6 | 22,000.0 |
| 2-NGM1//G1 | 7 | 17 | 11.1 | 30,800.0 |
| 3-NGM1//G1 | 7 | 36 | 24.0 | 30,800.0 |
| 3-NGM2//G1 | 7 | 10 | 6.8 | 30,800.0 |
| 2-NGM1//G1 | 9 | 13 | 8.8 | 39,600.0 |
| 3-NGM1//G1 | 9 | 31 | 20.9 | 39,600.0 |
| Bending Cycle | Current Density (A·g−1) | Specific Capacitance (F·g−1) | Energy Density (Wh·kg−1) | Power Density (W·kg−1) |
|---|---|---|---|---|
| 1 | 1 | 110 | 73.6 | 4400.0 |
| 50 | 1 | 109 | 73.5 | 4400.0 |
| 100 | 1 | 109 | 73.5 | 4400.0 |
| 200 | 1 | 109 | 73.3 | 4400.0 |
| 400 | 1 | 105 | 70.6 | 4400.0 |
| 800 | 1 | 102 | 68.8 | 4400.0 |
| 1600 | 1 | 97 | 64.9 | 4400.0 |
| 2000 | 1 | 95 | 63.8 | 4400.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-Y.; Cho, C.-P. Impacts of Mn Content and Mass Loading on the Performance of Flexible Asymmetric Solid-State Supercapacitors Using Mixed-Phase MnO2/N-Containing Graphene Composites as Cathode Materials. C 2023, 9, 88. https://doi.org/10.3390/c9030088
Chiu H-Y, Cho C-P. Impacts of Mn Content and Mass Loading on the Performance of Flexible Asymmetric Solid-State Supercapacitors Using Mixed-Phase MnO2/N-Containing Graphene Composites as Cathode Materials. C. 2023; 9(3):88. https://doi.org/10.3390/c9030088
Chicago/Turabian StyleChiu, Hsin-Ya, and Chun-Pei Cho. 2023. "Impacts of Mn Content and Mass Loading on the Performance of Flexible Asymmetric Solid-State Supercapacitors Using Mixed-Phase MnO2/N-Containing Graphene Composites as Cathode Materials" C 9, no. 3: 88. https://doi.org/10.3390/c9030088
APA StyleChiu, H.-Y., & Cho, C.-P. (2023). Impacts of Mn Content and Mass Loading on the Performance of Flexible Asymmetric Solid-State Supercapacitors Using Mixed-Phase MnO2/N-Containing Graphene Composites as Cathode Materials. C, 9(3), 88. https://doi.org/10.3390/c9030088







