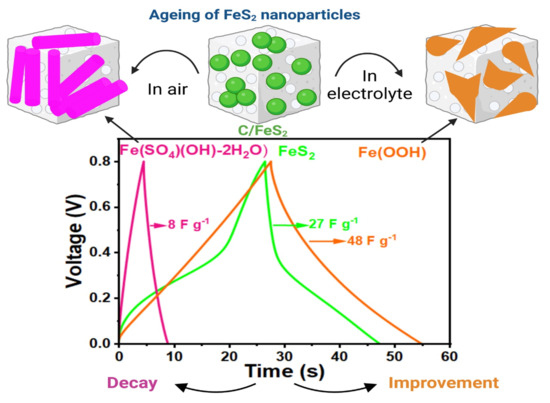
FeS2 Nanoparticles in S-Doped Carbon: Ageing Effects on Performance as a Supercapacitor Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of C/FeS2 Composites
2.2. Physicochemical Characterisation
2.3. Electrochemical Studies
3. Results
3.1. Carbon/FeS2 Formation
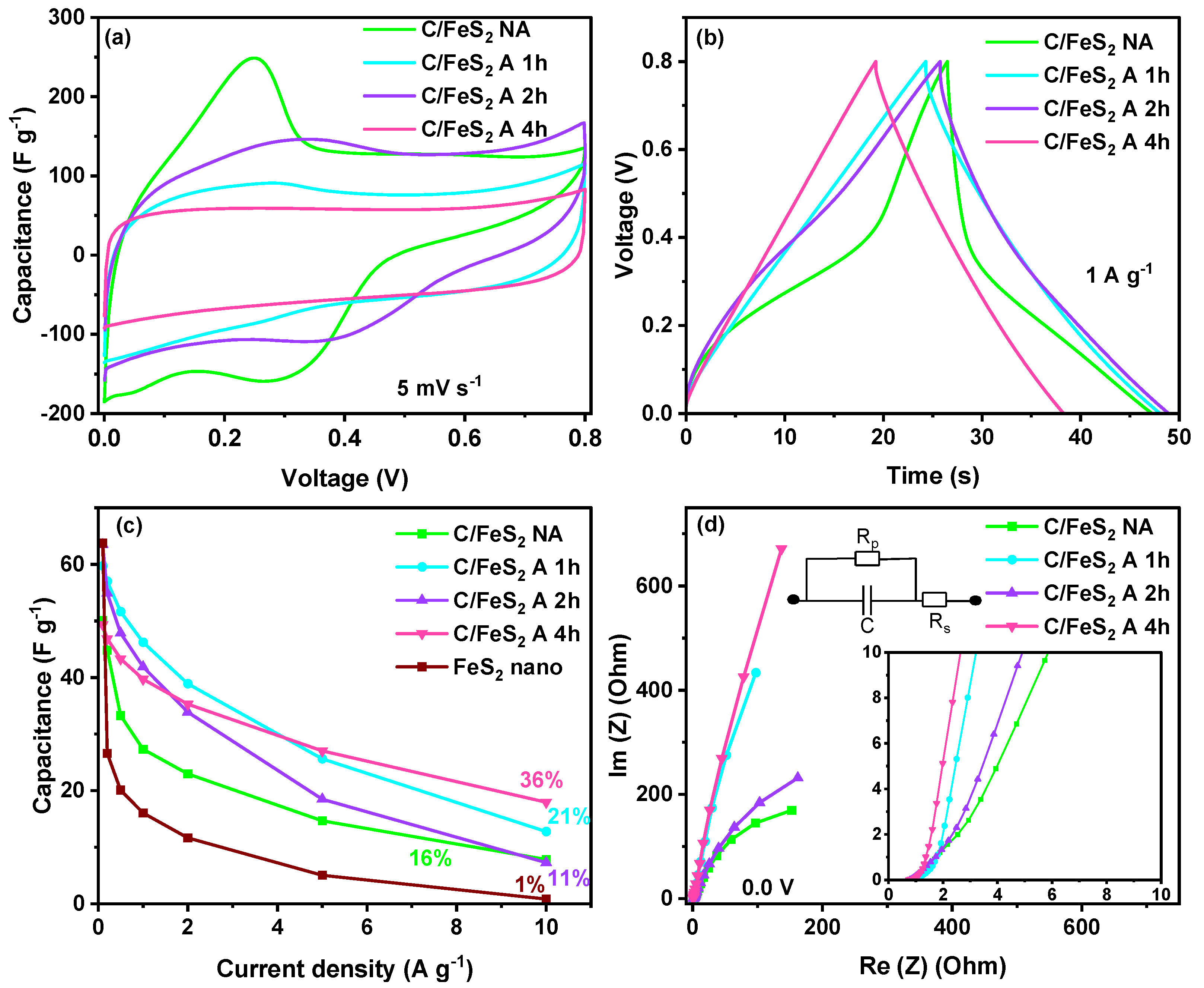
3.2. Electrochemical Characterisation of Activated C/FeS2 Materials
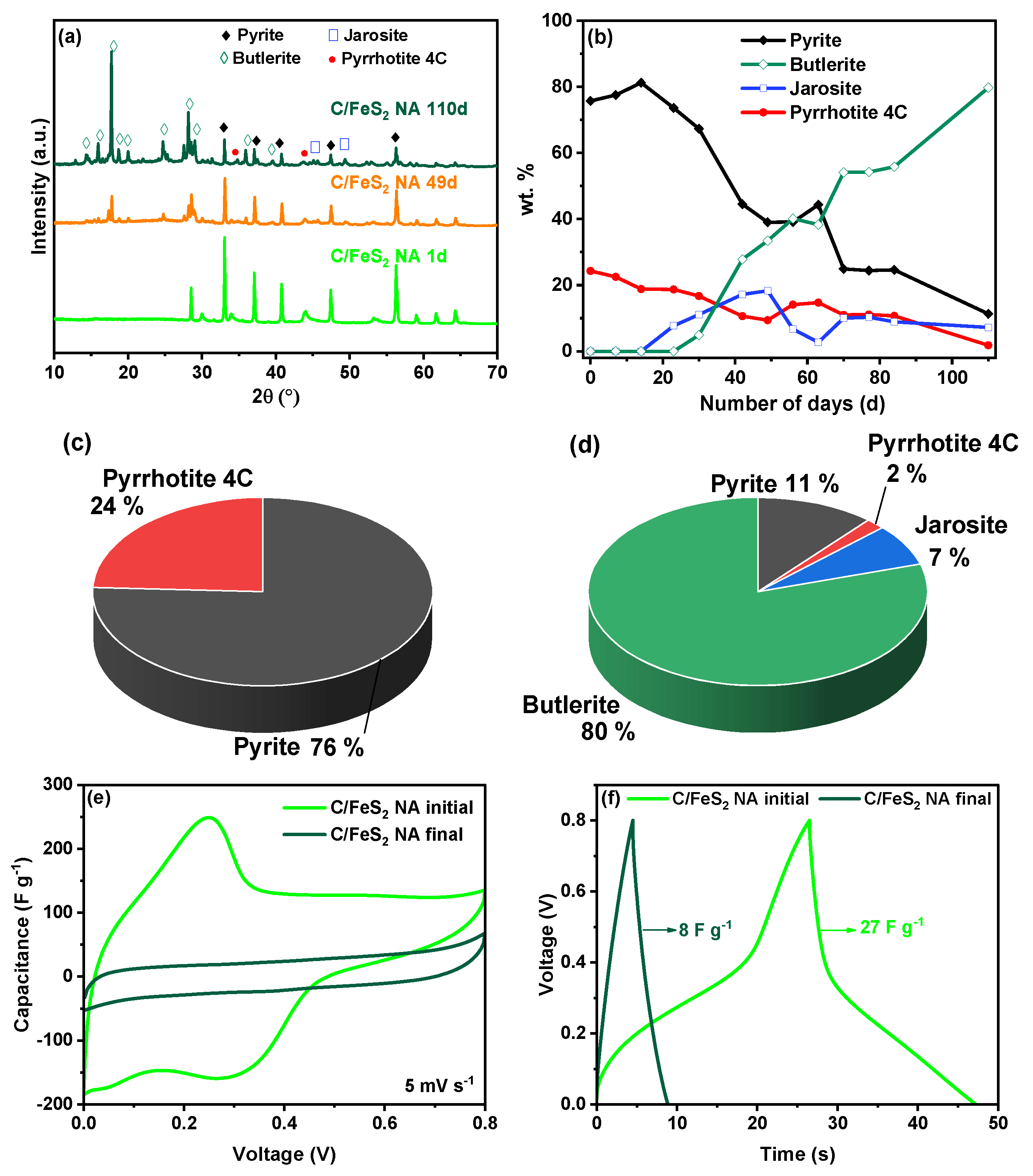
3.3. Ageing of C/FeS2 over Time
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frąckowiak, E.; Płatek-Mielczarek, A.; Piwek, J.; Fic, K. Advanced Characterization Techniques for Electrochemical Capacitors. Adv. Inorg. Chem. 2022, 79, 151–207. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal Oxide-Based Supercapacitors: Progress and Prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, L. Review on Recent Advances in Nanostructured Transition-Metal-Sulfide-Based Electrode Materials for Cathode Materials of Asymmetric Supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Yu, S.; Ng, V.M.H.; Wang, F.; Xiao, Z.; Li, C.; Kong, L.B.; Que, W.; Zhou, K. Synthesis and Application of Iron-Based Nanomaterials as Anodes of Lithium-Ion Batteries and Supercapacitors. J. Mater. Chem. A 2018, 6, 9332–9367. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, H.; Bao, S.; Yin, Y.; Zhang, Y.; Lu, J. Hollow FeS2 Nanospheres Encapsulated in N/S Co-Doped Carbon Nanofibers as Electrode Material for Electrochemical Energy Storage. J. Alloys Compd. 2022, 905, 164184. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Balasingam, S.K.; Sivalingam Nallathambi, K.; Ramadoss, A.; Kundu, M.; Bak, J.S.; Cho, I.H.; Kandasamy, P.; Jun, Y.; Kim, H.-J. Facile Synthesis of Pristine FeS2 Microflowers and Hybrid rGO-FeS2 Microsphere Electrode Materials for High Performance Symmetric Capacitors. J. Ind. Eng. Chem. 2019, 71, 191–200. [Google Scholar] [CrossRef]
- Pei, L.; Yang, Y.; Chu, H.; Shen, J.; Ye, M. Self-Assembled Flower-like FeS2/Graphene Aerogel Composite with Enhanced Electrochemical Properties. Ceram. Int. 2016, 42, 5053–5061. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lu, M.-Y.; Chen, L.-J. Metal Sulfide Nanostructures: Synthesis, Properties and Applications in Energy Conversion and Storage. J. Mater. Chem. 2012, 22, 19–30. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Goban Kumar, P.; Kollu, P.; Jeong, S.K.; Grace, A.N. Solvothermal Synthesis and Electrochemical Properties of Phase Pure Pyrite FeS2 for Supercapacitor Applications. Electrochim. Acta 2018, 290, 378–389. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Ashiq, M.N.; Zhang, M. Design of Metals Sulfides with Carbon Materials for Supercapacitor Applications: A Review. Energy Technol. 2021, 9, 2000987. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Ma, T.; Pan, D.; Li, Y.; Xie, J.; Shao, S. A High-Performance Flexible Supercapacitor Electrode Material Based on Nano-Flowers-like FeS2/NSG Hybrid Nanocomposites. Mater. Lett. 2018, 218, 10–13. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Carbon Nanofiber Linked FeS2 Mesoporous Nano-Alloys as High Capacity Anodes for Lithium-Ion Batteries and Supercapacitors. J. Alloys Compd. 2018, 732, 799–805. [Google Scholar] [CrossRef]
- Liu, X.; Deng, W.; Liu, L.; Wang, Y.; Huang, C.; Wang, Z. Passion Fruit-like Microspheres of FeS2 Wrapped with Carbon as an Excellent Fast Charging Material for Supercapacitors. New J. Chem. 2022, 46, 11212–11219. [Google Scholar] [CrossRef]
- Wang, F.; Li, G.; Zhou, Q.; Zheng, J.; Yang, C.; Wang, Q. One-Step Hydrothermal Synthesis of Sandwich-Type NiCo2S4@reduced Graphene Oxide Composite as Active Electrode Material for Supercapacitors. Appl. Surf. Sci. 2017, 425, 180–187. [Google Scholar] [CrossRef]
- Zallouz, S.; Réty, B.; Vidal, L.; Le Meins, J.-M.; Matei Ghimbeu, C. Co3O4 Nanoparticles Embedded in Mesoporous Carbon for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 5022–5037. [Google Scholar] [CrossRef]
- Jahel, A.; Ghimbeu, C.M.; Monconduit, L.; Vix-Guterl, C. Confined Ultrasmall SnO2 Particles in Micro/Mesoporous Carbon as an Extremely Long Cycle-Life Anode Material for Li-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400025. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Nithyadharseni, P.; Lee, S.J.; Durai, G.; Kuppusami, P.; Madhavan, J.; Choi, M.Y. Recent Progress and Emerging Challenges of Transition Metal Sulfides Based Composite Electrodes for Electrochemical Supercapacitive Energy Storage. Ceram. Int. 2020, 46, 14317–14345. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H.; Ashtiani, M.M.; Sarparast, M. Designing an Asymmetric Device Based on Graphene Wrapped Yolk–Double Shell NiGa2S4 Hollow Microspheres and Graphene Wrapped FeS2–FeSe2 Core–Shell Cratered Spheres with Outstanding Energy Density. J. Mater. Chem. A 2019, 7, 10282–10292. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Shi, J.-H.; Hsu, Y.-K. Multifunctional FeS2 in Binder-Independent Configuration as High-Performance Supercapacitor Electrode and Non-Enzymatic H2O2 Detector. Appl. Surf. Sci. 2020, 503, 144304. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.; Wang, Y.; Ning, Q.; Wang, B.; Zeng, G. Aging of Zero-Valent Iron-Based Nanoparticles in Aqueous Environment and the Consequent Effects on Their Reactivity and Toxicity. Water Environ. Res. 2020, 92, 646–661. [Google Scholar] [CrossRef]
- Zallouz, S.; Meins, J.-M.L.; Ghimbeu, C.M. Alkaline Hydrogel Electrolyte from Biosourced Chitosan to Enhance the Rate Capability and Energy Density of Carbon-Based Supercapacitors. Energy Adv. 2022, 1, 1051–1064. [Google Scholar] [CrossRef]
- Beda, A.; Villevieille, C.; Taberna, P.-L.; Simon, P.; Ghimbeu, C.M. Self-Supported Binder-Free Hard Carbon Electrodes for Sodium-Ion Batteries: Insights into Their Sodium Storage Mechanisms. J. Mater. Chem. A 2020, 8, 5558–5571. [Google Scholar] [CrossRef]
- Bujewska, P.; Gorska, B.; Fic, K. Redox Activity of Selenocyanate Anion in Electrochemical Capacitor Application. Synth. Met. 2019, 253, 62–72. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Perera, V.S.; Ralph, J.M.; Huang, S.D.; Jaroniec, M. Cysteine-Assisted Tailoring of Adsorption Properties and Particle Size of Polymer and Carbon Spheres. Langmuir 2013, 29, 4032–4038. [Google Scholar] [CrossRef] [PubMed]
- Ghimbeu, C.M.; Vidal, L.; Delmotte, L.; Meins, J.-M.L.; Vix-Guterl, C. Catalyst-Free Soft-Template Synthesis of Ordered Mesoporous Carbon Tailored Using Phloroglucinol/Glyoxylic Acid Environmentally Friendly Precursors. Green Chem. 2014, 16, 3079–3088. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Sopronyi, M.; Sima, F.; Delmotte, L.; Vaulot, C.; Zlotea, C.; Paul-Boncour, V.; Meins, J.-M.L. One-Pot Laser-Assisted Synthesis of Porous Carbon with Embedded Magnetic Cobalt Nanoparticles. Nanoscale 2015, 7, 10111–10122. [Google Scholar] [CrossRef]
- Sun, S.; Matei Ghimbeu, C.; Janot, R.; Le Meins, J.-M.; Cassel, A.; Davoisne, C.; Masquelier, C.; Vix-Guterl, C. One-Pot Synthesis of LiFePO4–Carbon Mesoporous Composites for Li-Ion Batteries. Microporous Mesoporous Mater. 2014, 198, 175–184. [Google Scholar] [CrossRef]
- Kiener, J.; Girleanu, M.; Ersen, O.; Parmentier, J. Direct Insight into the Confinement Effect of WS2 Nanostructures in an Ordered Carbon Matrix. Cryst. Growth Des. 2020, 20, 2004–2013. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Elliot, A.D. Structure of Pyrrhotite 5C (Fe9S10). Acta Cryst. B 2010, 66, 271–279. [Google Scholar] [CrossRef]
- Li, Y.; van Santen, R.A.; Weber, T. High-Temperature FeS–FeS2 Solid-State Transitions: Reactions of Solid Mackinawite with Gaseous H2S. J. Solid State Chem. 2008, 181, 3151–3162. [Google Scholar] [CrossRef]
- Grønvold, F.; Westrum, E.F. Heat Capacities of Iron Disulfides Thermodynamics of Marcasite from 5 to 700 K, Pyrite from 300 to 780 K, and the Transformation of Marcasite to Pyrite. J. Chem. Thermodyn. 1976, 8, 1039–1048. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of Activated Carbon from Forest and Agricultural Residues through CO2 Activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- De Groote, A.M.; Froment, G.F.; Kobylinski, T. Synthesis Gas Production from Natural Gas in a Fixed Bed Reactor with Reversed Flow. Can. J. Chem. Eng. 1996, 74, 735–742. [Google Scholar] [CrossRef]
- Huang, Y.; Bao, S.; Yin, Y.; Lu, J. Three-Dimensional Porous Carbon Decorated with FeS2 Nanospheres as Electrode Material for Electrochemical Energy Storage. Appl. Surf. Sci. 2021, 565, 150538. [Google Scholar] [CrossRef]
- Tung Pham, D.; Paul Baboo, J.; Song, J.; Kim, S.; Jo, J.; Mathew, V.; Hilmy Alfaruqi, M.; Sambandam, B.; Kim, J. Facile Synthesis of Pyrite (FeS2/C) Nanoparticles as an Electrode Material for Non-Aqueous Hybrid Electrochemical Capacitors. Nanoscale 2018, 10, 5938–5949. [Google Scholar] [CrossRef]
- Jahel, A.; Ghimbeu, C.M.; Darwiche, A.; Vidal, L.; Hajjar-Garreau, S.; Vix-Guterl, C.; Monconduit, L. Exceptionally Highly Performing Na-Ion Battery Anode Using Crystalline SnO2 Nanoparticles Confined in Mesoporous Carbon. J. Mater. Chem. A 2015, 3, 11960–11969. [Google Scholar] [CrossRef]
- Voorhees, P.W. The Theory of Ostwald Ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Chmiola, J. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Li, G.; Zhang, J.; Guo, Y. NO Removal Activity and Surface Characterization of Activated Carbon with Oxidation Modification. J. Energy Inst. 2017, 90, 813–823. [Google Scholar] [CrossRef]
- Shaheen Shah, S.; Abu Nayem, S.M.; Sultana, N.; Saleh Ahammad, A.J.; Abdul Aziz, M. Preparation of Sulfur-Doped Carbon for Supercapacitor Applications: A Review. ChemSusChem 2022, 15, e202101282. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Moon, J.H. Monodispersed N-Doped Carbon Nanospheres for Supercapacitor Application. ACS Appl. Mater. Interfaces 2014, 6, 13968–13976. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Wang, P.; Mo, Y.; Lai, W.; Li, Z.; Yu, R.; Du, Y.; Zhang, X.; Chen, Y. Effect of the Oxygen Functional Groups of Activated Carbon on Its Electrochemical Performance for Supercapacitors. New Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Moussa, G.; Hajjar-Garreau, S.; Taberna, P.-L.; Simon, P.; Matei Ghimbeu, C. Eco-Friendly Synthesis of Nitrogen-Doped Mesoporous Carbon for Supercapacitor Application. C—J. Carbon Res. 2018, 4, 20. [Google Scholar] [CrossRef]
- Kiciński, W.; Dziura, A. Heteroatom-Doped Carbon Gels from Phenols and Heterocyclic Aldehydes: Sulfur-Doped Carbon Xerogels. Carbon 2014, 75, 56–67. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Zhou, S.; Wong, C.-P. Pyrite FeS2 Nanobelts as High-Performance Anode Material for Aqueous Pseudocapacitor. Electrochim. Acta 2016, 222, 172–176. [Google Scholar] [CrossRef]
- Zhao, S.; Song, Z.; Qing, L.; Zhou, J.; Qiao, C. Surface Wettability Effect on Energy Density and Power Density of Supercapacitors. J. Phys. Chem. C 2022, 126, 9248–9256. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Nita, C.; Zhang, B.; Dentzer, J.; Matei Ghimbeu, C. Hard Carbon Derived from Coconut Shells, Walnut Shells, and Corn Silk Biomass Waste Exhibiting High Capacity for Na-Ion Batteries. J. Energy Chem. 2021, 58, 207–218. [Google Scholar] [CrossRef]
- Sun, Z.; Li, F.; Ma, Z.; Wang, Q.; Qu, F. Battery-Type Phosphorus Doped FeS2 Grown on Graphene as Anode for Hybrid Supercapacitor with Enhanced Specific Capacity. J. Alloys Compd. 2021, 854, 157114. [Google Scholar] [CrossRef]
- Come, J.; Augustyn, V.; Kim, J.W.; Rozier, P.; Taberna, P.-L.; Gogotsi, P.; Long, J.W.; Dunn, B.; Simon, P. Electrochemical Kinetics of Nanostructured Nb2O5 Electrodes. J. Electrochem. Soc. 2014, 161, A718. [Google Scholar] [CrossRef]
- Diard, J.-P.; Le Gorrec, B.; Montella, C. Linear Diffusion Impedance. General Expression and Applications. J. Electroanal. Chem. 1999, 471, 126–131. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Ni, Y.; Yang, Y.; Zhu, X.; Song, Y. N-Doped FeS2 Achieved by Thermal Annealing of Anodized Fe in Ammonia and Sulfur Atmosphere: Applications for Supercapacitors. J. Electrochem. Soc. 2021, 168, 080522. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Zhang, Y.; Zhang, T. One-Step Microwave-Hydrothermal Preparation of NiS/rGO Hybrid for High-Performance Symmetric Solid-State Supercapacitor. Appl. Surf. Sci. 2020, 514, 146080. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, H.; Yang, J.; Qin, Q.; Fan, H.; Wei, C.; Zheng, W. Solvothermal Synthesis of Three-Dimensional Hierarchical CuS Microspheres from a Cu-Based Ionic Liquid Precursor for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 21735–21744. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Sreemany, M.; Mahanty, S. Carbon Doped MnCo2S4 Microcubes Grown on Ni Foam as High Energy Density Faradaic Electrode. Electrochim. Acta 2016, 213, 672–679. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary Transition Metal Sulfides Embedded in Graphene Nanosheets as Both the Anode and Cathode for High-Performance Asymmetric Supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, Y.; Exstrom, C.L.; Darveau, S.A.; Huang, J. Air Stable, Photosensitive, Phase Pure Iron Pyrite Nanocrystal Thin Films for Photovoltaic Application. Available online: https://pubs.acs.org/doi/pdf/10.1021/nl202902z (accessed on 24 September 2022).
- Fan, D.; Lan, Y.; Tratnyek, P.G.; Johnson, R.L.; Filip, J.; O’Carroll, D.M.; Nunez Garcia, A.; Agrawal, A. Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ. Sci. Technol. 2017, 51, 13070–13085. [Google Scholar] [CrossRef] [PubMed]
- Wiese, R.G.; Powell, M.A.; Fyfe, W.S. Spontaneous Formation of Hydrated Iron Sulfates on Laboratory Samples of Pyrite- and Marcasite-Bearing Coals. Chem. Geol. 1987, 63, 29–38. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; de Mendonça Silva, J.C.; Duarte, H.A. Pyrite Oxidation Mechanism by Oxygen in Aqueous Medium. J. Phys. Chem. C 2016, 120, 2760–2768. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Huang, N.M.; Lim, H.N.; Chia, C.H.; Harrison, I.; Muhamad, M.R. One-Pot Hydrothermal Synthesis and Characterization of FeS2 (Pyrite)/Graphene Nanocomposite. Chem. Eng. J. 2013, 218, 276–284. [Google Scholar] [CrossRef]
- Turmuzi, M.; Daud, W.R.W.; Tasirin, S.M.; Takriff, M.S.; Iyuke, S.E. Production of Activated Carbon from Candlenut Shell by CO2 Activation. Carbon 2004, 42, 453–455. [Google Scholar] [CrossRef]
- Zallouz, S.; Pronkin, S.N.; Le Meins, J.-M.; Pham-Huu, C.; Matei Ghimbeu, C. Chapter 9—New Development in Carbon-Based Electrodes and Electrolytes for Enhancement of Supercapacitor Performance and Safety. In Renewable Energy Production and Distribution; Advances in Renewable Energy Technologies; Jeguirim, M., Dutournié, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 2, pp. 353–408. ISBN 978-0-443-18439-0. [Google Scholar]
- Barbieri, O.; Hahn, M.; Herzog, A.; Kötz, R. Capacitance Limits of High Surface Area Activated Carbons for Double Layer Capacitors. Carbon 2005, 43, 1303–1310. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M.d.F. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Platek, A.; Nita, C.; Ghimbeu, C.M.; Frąckowiak, E.; Fic, K. Electrochemical Capacitors Operating in Aqueous Electrolyte with Volumetric Characteristics Improved by Sustainable Templating of Electrode Materials. Electrochim. Acta 2020, 338, 135788. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-Crystalline Iron Oxide Hydroxide Nanoparticle Anode for High-Performance Supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef]



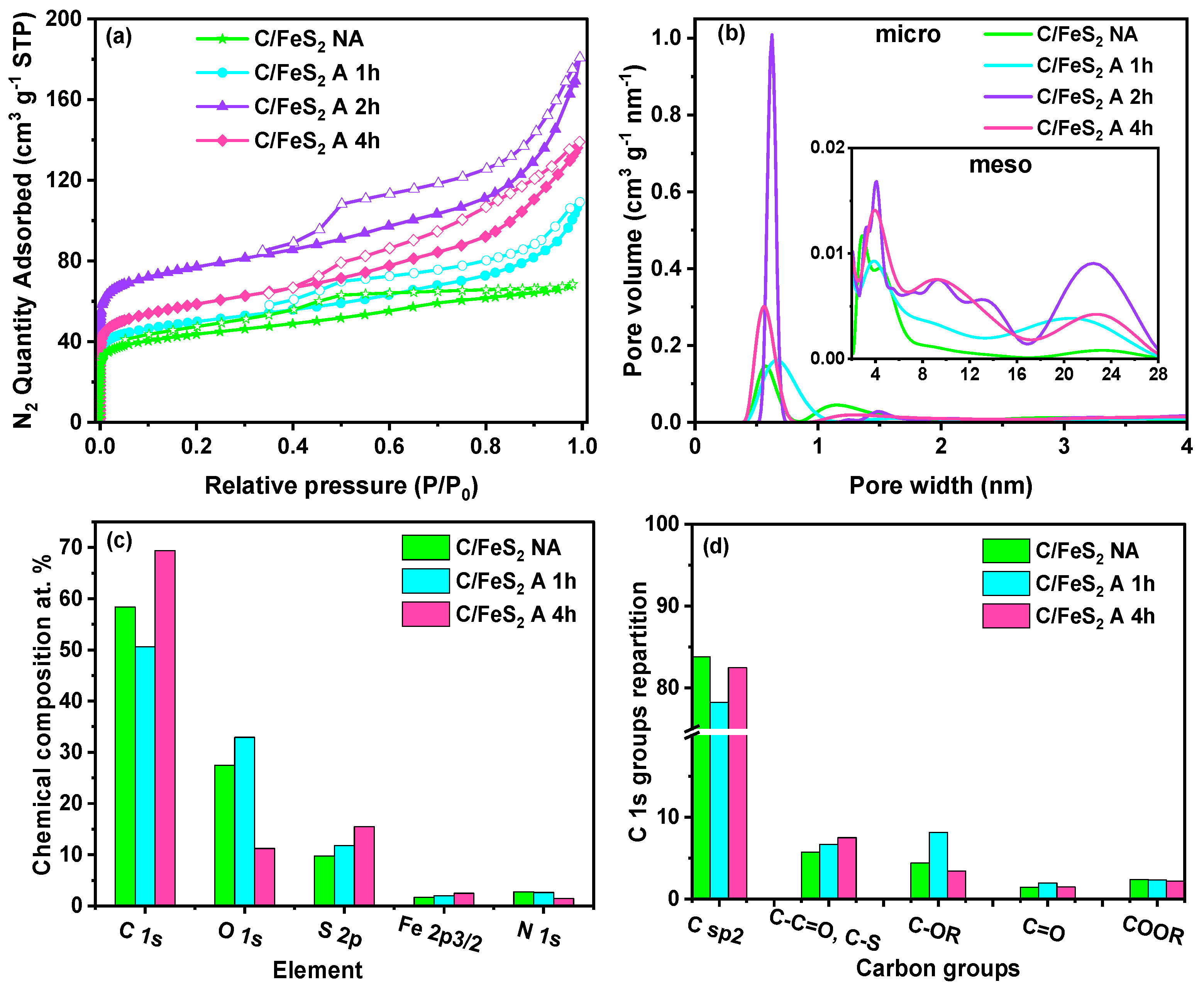


| Material | SBET (m² g−1) | VT (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | d STEM (nm) | % FeS2 TGA (wt%) |
|---|---|---|---|---|---|---|
| C/FeS2 NA | 161 | 0.10 | 0.06 | 0.054 | 7.00 | 15 |
| C/FeS2 A 1 h | 185 | 0.17 | 0.07 | 0.10 | 12.50 | 18 |
| C/FeS2 A 2 h | 296 | 0.28 | 0.11 | 0.17 | 14.50 | 22 |
| C/FeS2 A 4 h | 214 | 0.20 | 0.08 | 0.13 | 17.00 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zallouz, S.; Réty, B.; Le Meins, J.-M.; Ndiaye, M.Y.; Fioux, P.; Matei Ghimbeu, C. FeS2 Nanoparticles in S-Doped Carbon: Ageing Effects on Performance as a Supercapacitor Electrode. C 2023, 9, 112. https://doi.org/10.3390/c9040112
Zallouz S, Réty B, Le Meins J-M, Ndiaye MY, Fioux P, Matei Ghimbeu C. FeS2 Nanoparticles in S-Doped Carbon: Ageing Effects on Performance as a Supercapacitor Electrode. C. 2023; 9(4):112. https://doi.org/10.3390/c9040112
Chicago/Turabian StyleZallouz, Sirine, Bénédicte Réty, Jean-Marc Le Meins, Mame Youssou Ndiaye, Philippe Fioux, and Camélia Matei Ghimbeu. 2023. "FeS2 Nanoparticles in S-Doped Carbon: Ageing Effects on Performance as a Supercapacitor Electrode" C 9, no. 4: 112. https://doi.org/10.3390/c9040112
APA StyleZallouz, S., Réty, B., Le Meins, J.-M., Ndiaye, M. Y., Fioux, P., & Matei Ghimbeu, C. (2023). FeS2 Nanoparticles in S-Doped Carbon: Ageing Effects on Performance as a Supercapacitor Electrode. C, 9(4), 112. https://doi.org/10.3390/c9040112