Abstract
Previous research has indicated that Pseudomonas aurantiaca ST-TJ4 possesses a notable antagonistic impact on Phytophthora cinnamomi and holds promising potential for biocontrol. In this study, a combination of a single-factor experiment, a Plackett–Burman design and a response surface approach was employed to investigate the optimal formula of ST-TJ4 fermentation medium. Furthermore, the stability of ST-TJ4 fermentation filtrate and its biocontrol effect on Ph. cinnamomi in vivo were also evaluated. The results revealed that the optimal culture conditions for ST-TJ4 involved the use of 20.59 g/L of glucose and 18.76 g/L of yeast extract powder. Following optimization, the fermentation filtrate of ST-TJ4 exhibited an inhibition rate of 76.5%, representing a 15% increase compared to previous levels. Additionally, phzA, phzB, phzD, phzE, phzF and phzO genes involved in the synthesis of phenazine-1-carboxylic acid (PCA) and 2-hydroxyphenazine (2-OH-PHZ) were also upregulated. The ST-TJ4 fermentation filtrate demonstrated strong alkali resistance, weak acid resistance and favorable temperature and UV light stability. Furthermore, in vitro inoculation experiments confirmed that optimizing the fermentation medium reduced Ps. cinnamomi’s ability to infect the leaves of Rhododendron pulchrum.
1. Introduction
Pseudomonas is one of the most widespread Gram-negative bacterium in nature [1,2]. It is isolated from the center or leaf periphery of plants. Pseudomonas can promote plant growth by producing and secreting substances such as siderophores, phosphatases and plant hormones [3]. The inhibitory effect on pathogenic fungi is mainly caused by producing some antibiotic substances, such as phenazines and pyrrolnitrin, polyketides and peptides, etc. [4,5]. P. aurantiaca is a Pseudomonas strain with good biocontrol effect [6]. Previous studies have found that this species can produce a variety of antagonistic substances to inhibit the growth of plant pathogens [7,8]. Tagele et al. [9] found that P. aurantiaca KNU17Pc1 has antifungal activity against eight different phytopathogens and causes significant changes in the hyphal morphology. Hu et al. [10] screened a biocontrol bacterium P. aurantiaca Pcho10 from multiple wheat-related strains. The experiments in growth chamber conditions and field experiments showed that Pcho10 could colonize wheat well and effectively control the diseases caused by Fusarium graminearum. However, the current inhibition rate of strain fermentation broth using the traditional medium is relatively low, posing a challenge to the advancement of biological vaccines for addressing plant diseases.
The optimization of fermentation media can often promote the accumulation of target secondary metabolites [11,12,13]. Therefore, adding appropriate exogenous nutrients and/or growth factors (such as precursors, surfactants and metal ions) to the fermentation medium is one of the most effective methods to improve the yield of secondary metabolites. Zhang et al. [14] improved the nematicidal activity of Trichoderma longibrachiatum by optimizing the fermentation medium. The biocontrol efficacy of the T. longibrachiatum culture filtrate was 83.88% on the 70th day after sowing wheat seeds in a greenhouse experiment. By uniformly optimizing the fermentation conditions of Bacillus circulus FA13, Zhou et al. [15] increased the inhibitory effect of 20-fold diluted B. circulus FA13 fermentation filtrate on Aspergillus parasiticus from 23.1% to 100%. Furthermore, the active compounds in the best protocol’s cell-free supernatant are very stable and resistant to high temperature, high pressure, acid-alkali and zymolysis.
The Plackett–Burman design (PBD) is an effective method to evaluate the relative importance of medium components for secondary metabolites, which greatly reduces the total number of experiments [16]. However, PBD only considers the main effects and ignores the interactions between variables, which is why it is generally used in combination with response surface methodology (RSM) [17]. RSM is considered to be one of the preferred methods for determining the best culture conditions and the most effective components of the culture medium with a minimum number of experiments [18]. The combination of PBD and RSM overcome the shortcomings of traditional methods and have been widely used to optimize reaction conditions and processing parameters [19,20].
In our previous work, we found that P. chlororaphis subsp. aurantiaca ST-TJ4 has a significant antagonistic effect on Phytophthora cinnamomi, which has great potential for biological control [21]. However, the biocontrol effect of ST-TJ4 culture filtrate against P. cinnamomi varied greatly depending on the fermentation medium and conditions used. Therefore, there is an urgent need to develop optimal fermentation conditions under which antagonism against P. cinnamomi can be actively quantitatively evaluated. A stepwise optimization was performed that included (1) selecting the best carbon, nitrogen and microelement sources in single variable experiments; (2) combining PBD with RSM to optimize key media composition factors; (3) subsequently, the stability of the fermentation filtrate of strain ST-TJ4 and the in vivo biocontrol effect of P. aurantiaca ST-TJ4 on P. cinnamomi were evaluated.
2. Materials and Methods
2.1. Bacterial and Fungal Strains
P. chlororaphis subsp. aurantiaca ST-TJ4 was isolated from the rhizosphere of poplar in Tianjin, China, in 2018 [22] and is now preserved in the China Center for Type Culture Collection (CCTCC; No. M2020435). Strain ST-TJ4 was stored at −80 °C in Luria–Bertani (LB) medium containing 50% (v/v) glycerol for long-term use. The target pathogen P. cinnamomi was generously provided by the Forest Protection Department of Nanjing Forestry University and cultured on V8 agar medium at 25 °C.
2.2. Preparation of Fermentation Filtrate and Determination of Antagonistic Activity
To assess the inhibitory effect of the fermentation filtrate, single colonies of ST-TJ4 were inoculated into 20 mL King’s B (KB) medium and grown overnight. After that, 200 mL of fresh KB medium was used to reinoculate the overnight culture to a concentration of 5%. It was then incubated for 3 days at 28 °C with 200 rpm shaking. The fermentation broth was centrifuged at 4 °C for 15 min at 8000 rpm, and the supernatant was filtered through a 0.22 μm syringe filter [23].
The antagonistic activity of the sterile fermentation filtrate of ST-TJ4 was measured using the mycelial growth rate method. Specifically, this means the following: mix the prepared ST-TJ4 sterile fermentation filtrate with the V8 medium heated and cooled to 70 °C at a ratio of 1:10 (v/v) and pour it into the plate. The control group consists of the V8 medium without any addition. Then, fresh phytophthora plugs were added to the plates with different treatments and incubated at 25 °C for 7 days. The diameter of the pathogenic fungi colony was measured via the cross method, and the inhibition rate was calculated [24]. The experiment was repeated twice, and each treatment was conducted in triplicate.
2.3. Optimization of Fermentation Medium Components by Single-Factor Experiments
We performed a single-factor screening test for ST-TJ4 on the KB medium with different carbon sources, nitrogen sources and inorganic salts. We measured the effects of these nutrient conditions on the antagonistic activity of ST-TJ4. The carbon sources were glucose, sucrose, mannitol, soluble starch and glycerol; the nitrogen sources were ammonium chloride, corn flour, yeast extract, beef extract and peptone; the carbon–nitrogen ratios were (4:1), (2:1), (1:1), (1:2) and (1:4); the inorganic salts were manganese sulfate, ferrous sulfate, zinc sulfate, sodium chloride, magnesium sulfate and dipotassium hydrogen phosphate. The medium was sterilized at 121 °C for 20 min. All the above treatments were repeated three times.
2.4. Screening Significant Fermentation Medium Components via the Plackett–Burman Design
PBD was applied to evaluate the relative importance of different parameters with a minimum number of experiments [25,26]. Based on the initial shake flask fermentation experiment, the six components of the fermentation medium optimized in the previous experiment, namely glucose, glycerol, yeast extract, peptone, MgSO4∙7H2O and K2HPO4, were the components of the 12 experiments of PB. Two levels were selected for each factor: the low level was the medium concentration level after initial screening and the high level was 1.3 to 2 times the low level. See Table 1 for the selection of factor codes and high and low levels in PB design. The response value was the inhibition rate of the fermentation filtrate of ST-TJ4, and each experiment was repeated three times.

Table 1.
Factors and levels of the Plackett–Burman design.
2.5. Path of Steepest Ascent Method Test
The response surface fitting equation can only be established when the factors approach the optimal level. According to the PBD results, the slope direction and step length of the steepest ascent method are determined through the positive and negative effects of key factors.
2.6. Box–Behnken Design Central Combination Test
The Box–Behnken method (Design Expert software, version 12, Stat-Ease Inc., Minneapolis, MN, USA) is a comprehensive technique for optimizing experimental conditions. When optimizing the fermentation medium for biocontrol bacteria, a more reasonable systematic study can be carried out, the optimization process can be simplified, and the optimization range can be calculated step by step with an accurate statistical system. According to the PBD test results, we determined the two most important factors and levels for the Box–Behnken design experiment.
2.7. RNA Extraction and RT-qPCR Analysis
To investigate the impact of an optimized culture medium on the differential expression of genes associated with phenazine synthesis, the ST-TJ4 bacterial suspension was inoculated into a 250 mL Erlenmeyer flask containing 100 mL of either optimized culture medium or a basic medium and agitated for 24 h at 28 °C and 200 rpm. The bacteria were then harvested through centrifugation at 8000 rpm and 4 °C for 15 min. Total RNA extraction was carried out using a Trazol Up Plus RNA Kit (Takara Biotech., Beijing, China). Following DNaseI treatment, 2 μg of RNA was utilized in a 20 μL reaction system for the synthesis of first-strand cDNA through reverse transcription, as per the manufacturer’s instructions. RT-qPCR was conducted using Hieff RT-qPCR SYBR Green Master Mix (CAT: 11202ES08; Yeasen, Shanghai, China) under specific conditions: an initial denaturation step of 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s, with the entire reaction system performed on ABI 7500 (Applied Biosystems, Waltham, MA, USA). Six genes associated with phenazine synthesis, namely phzA, phzB, phzD, phzE, phzF and phzO, were chosen for RT-qPCR analysis (Table S1). The relative expression levels of these genes were determined using the 2−∆∆CT method, with the 16S rRNA gene serving as the reference gene.
2.8. Stability of the Active Metabolites in the Fermentation Filtrate
The study assessed the impact of temperature, pH and UV light on the stability of antagonistic active compounds present in the optimized fermentation filtrate. Thermal stability was evaluated by subjecting aliquots of the fermentation filtrate to temperatures of 20 °C, 60 °C, 80 °C and 100 °C for 30 min, as well as autoclaving at 121 °C for 30 min, followed by natural cooling to room temperature. The effect of acid–alkali treatment was examined by adjusting the pH of the cell-free supernatants to 2, 4, 8, 10 and 12 using 1 mol/L NaOH or 1 mol/L HCl, and then the pH was readjusted to approximately 7.0 after a 6 h incubation period at room temperature. UV light stability was assessed by exposing the fermentation filtrate to UV light at a distance of 30 cm from the UV lamp for 6, 12, 18 and 24 h, with untreated fermentation filtrate serving as the control. The antagonistic activity of the ST-TJ4 fermentation filtrate was determined using the same method as described above. Antifungal activity was tested for all treatments and the control, with each treatment being replicated three times.
2.9. Pathogenicity Assays on Detached Leaves of Rhododendron pulchrum
Healthy leaves of R. pulchrum were selected and rinsed with sterile water. The surface was then sterilized using cotton soaked in 75% ethanol, followed by 3 washes with sterile water and air drying. Subsequently, the leaves of R. pulchrum were immersed in the ST-TJ4 fermentation filtrate for 15 s. As a control, leaves were also submerged in sterile water for the same duration.
Each undamaged leaf was punctured at its center using a sterilized prunus needle. The experimental group received a 5 mm diameter mycelial plug at the site of the leaf wound, while the control group received a sterile agar block of the same size at the wound. Five leaves from each group were inoculated and then placed in a constant-light incubator (MLR-352H-PC, Panasonic, Kadoma, Japan) at 75% relative humidity. After 5 days of growth with alternating 12 h light and 12 h darkness cycles at 25 °C, the lesion areas of the leaves were quantified. The relative control effect was calculated using the formula: (control lesion areas—fermentation filtrate treatment lesion areas)/control lesion areas × 100%.
2.10. Detection of the Colonization of P. cinnamomi in Plant Leaves
The biomass of P. cinnamomi in R. pulchrum subjected to basic medium (KB) and optimized culture medium (OM) was assessed using RT-qPCR. DNA was extracted from the leaves utilizing a SteadyPure Plant Genomic DNA Extraction Kit (AG21011, Accurate Biotechnology Co., Ltd., Changsha, China) and was quantified via spectrophotometry. Subsequently, 20 ng of DNA from each sample was utilized for RT-qPCR. The methods and parameters utilized in RT-qPCR adhere to the specifications outlined in Section 2.7. The P. cinnamomi target gene Pcinn13739 was employed for the quantification of oomycetes colonization [27], while the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of R. pulchrum was used as the endogenous reference gene [28]. The primer details are provided in Table S2.
2.11. Statistical Analysis
The data were analyzed via analysis of variance and Duncan’s multiple comparison test with SPSS 22.0 software (IBM Inc., Armonk, NY, USA). Standard errors of all mean values were calculated at a significance level of p < 0.05. Graphs were generated using GraphPad Prism 8.0 (GraphPad Software Inc., Boston, MA, USA).
3. Results
3.1. Single-Factor Screening Test
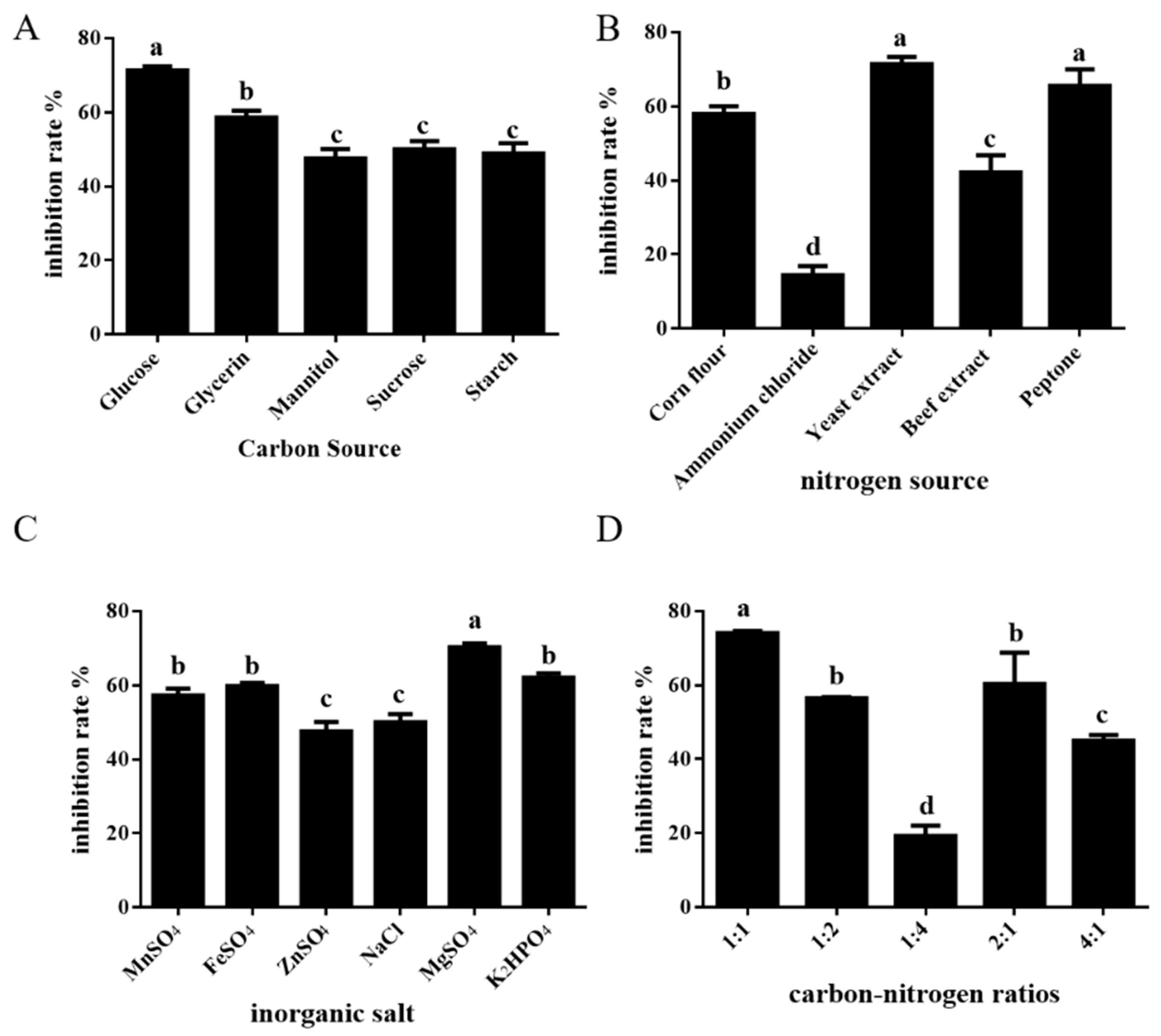
Microorganisms possess varying capacities for use and exhibit selectivity towards distinct carbon sources. Experiments revealed that the ST-TJ4 fermentation filtrate’s rate of inhibition against P. cinnamomi could be greatly increased by employing glucose as a carbon source (Figure 1A). The outcomes demonstrated that organic nitrogen sources were preferable to inorganic nitrogen sources and that ST-TJ4 exhibited great selectivity to nitrogen sources. The fermentation filtrate of ST-TJ4 exhibited the best rate of inhibition against P. cinnamomi when yeast extract powder was utilized as the nitrogen source (Figure 1B).
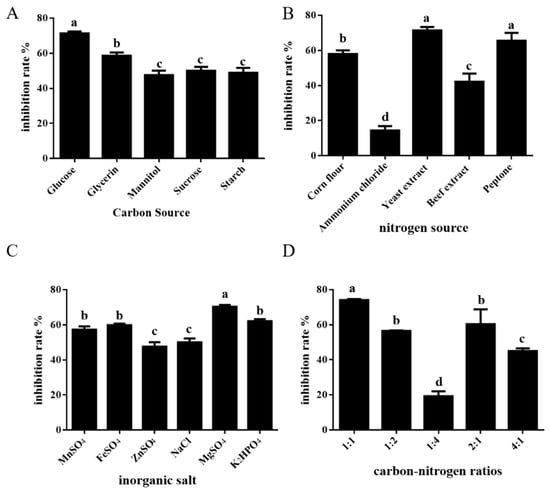
Figure 1.
Selection results of single-factor test of fermentation medium of ST-TJ4. (A) different carbon source; (B) different nitrogen source; (C) different inorganic salt different; (D) different carbon–nitrogen ratios. Different letters indicate statistically significant differences (p < 0.05) among treatments.
In addition to the optimal carbon and nitrogen sources, proper nutrient ratios can accelerate the growth rate of microorganisms and the accumulation of secondary metabolites. Through experiments, it was found that the optimal carbon–nitrogen ratio of ST-TJ4 fermentation was 1:1 (Figure 1D). Inorganic salts are essential nutrients for the growth of microorganisms, and they can participate in the composition of cellular structural substances and the physiological functions of cells. The test results indicated that the addition of manganese sulfate, ferrous sulfate, zinc sulfate and sodium chloride had no obvious effect on the inhibition rate, which may be because other components in the medium may contain trace elements required by ST-TJ4. The addition of magnesium sulfate and dipotassium hydrogen phosphate can improve the inhibition rate of ST-TJ4 fermentation filtrate against P. cinnamomi (Figure 1C).
3.2. Plackett–Burman Design Test
The Plackett–Burman experimental design is carried out using the ideal medium components that were selected by the single-factor experiment: the design factors and levels are shown in Table 1, the test results are shown in Table 2 and the effect evaluation results are shown in Table 3.

Table 2.
Plackett–Burman experimental design and results.

Table 3.
Regression analysis of experimental results based on the Plackett–Burman design.
The model is significant, as indicated by the model p-value of 0.0135. A regression equation, Y = 69.99 + 3.01A + 0.48B + 0.075C + 1.93D + 0.21E + 0.56F + 0.16G + 0.075H, describes the inhibition rate of the fermentation broth. This model’s complicated correlation coefficient, R2 = 0.9835, shows that it can account for 98.35% of the experimental data. Table 3 shows that both A glucose (p = 0.0016) and D yeast extract (p = 0.0059) have reliability levels higher than 95% (p < 0.05), suggesting that these two components are in charge of ST-TJ4 fermentation. As a result, two variables were chosen for the next test: glucose and yeast extract powder.
3.3. Path of Steepest Ascent Method Test
We then created the steepest climbing test path in order to bring each key factor closer to the maximum response value area for the response surface analysis that follows. The PBD analysis revealed that the glucose and yeast extract test steps with the steepest ascending were 3 g/L and 2 g/L, respectively.
The inhibition rate against P. cinnamomi reached its maximum when the fourth set of testing was conducted, i.e., when the glucose was 19 g/L and the yeast extract was 16 g/L. This was the region where the maximum response value of the two variables was found (Table 4). As a result, for additional response surface analysis, we used the level of each factor in the fourth set of experiments as the central value.

Table 4.
Experimental design and response of the steepest ascent design.
3.4. Optimizing Significant Fermentation Parameters via Box–Behnken Design
Based on the steepest climbing test, a response surface analysis test of two factors and two levels was performed on the fermentation medium of ST-TJ4 using the Box–Behnken design. The optimal value was obtained within a certain level range, with the experimental design and results shown in Table 5 and Table 6. The experimental data were then used to perform regression fitting using Design Expert 12, resulting in the quadratic regression equation of glucose (A) and yeast extract (B), with the inhibition rate (R) calculated as 75.84 + 0.94A − 0.11B − 0.22AB − 0.90A2 − 0.95B2. The model was found to be significant with p = 0.0015 and R2 = 0.9379, indicating a reliable experimental design with small error.

Table 5.
The design scheme and test results of the center combination based on the Box–Behnken design.

Table 6.
Regression analysis of experimental results based on the Box–Behnken design.
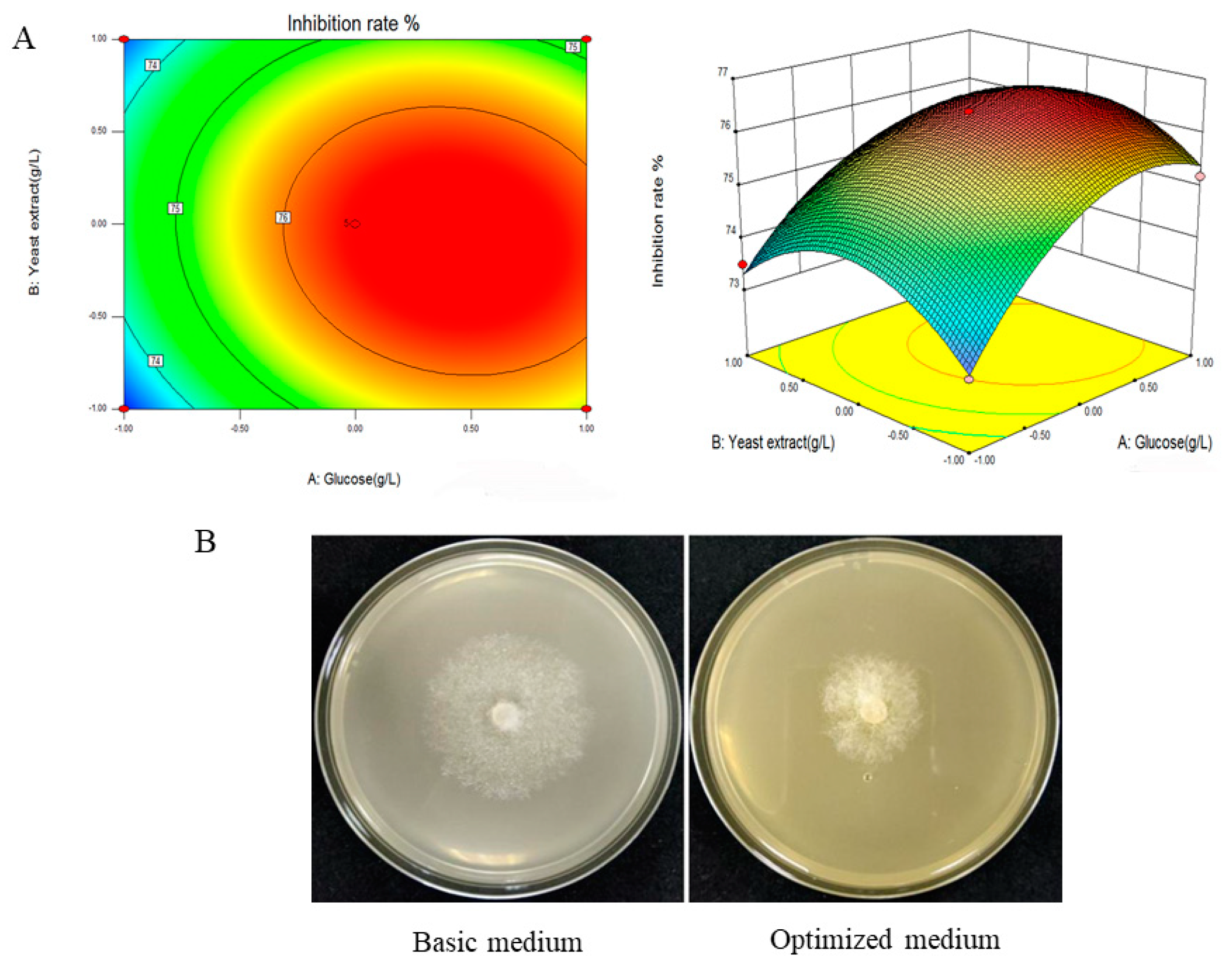
To further investigate the interaction between glucose and yeast extract and determine the optimal point, the response surface curve diagram was drawn using Design Expert 12 software (Figure 2A). Based on the analysis of the response surface plot, the maximum inhibition rate of 76.1% was predicted at the optimal value of 20.59 g/L and 18.76 g/L for the two main factors, glucose and yeast extract, respectively (Figure 2B).
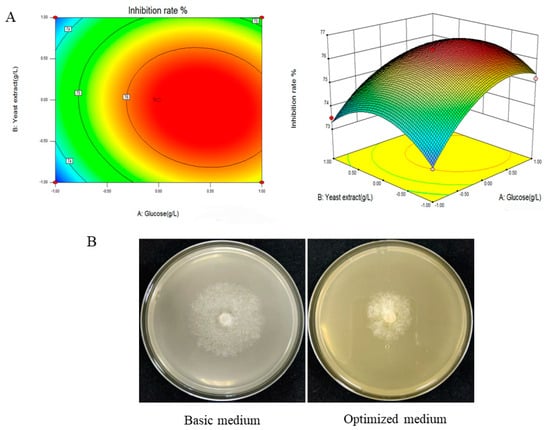
Figure 2.
Response surface plots of the effect of various factors on the inhibition rate of P. cinnamomi growth by fermentation filtrate of ST-TJ4 and validation of the optimal formulation. (A) Effect of interaction between glucose and yeast extract on antagonistic activity of the ST-TJ4 fermentation filtrate; (B) validation of the optimal formulation of fermentation medium for ST-TJ4.
Three verification experiments were conducted to verify the optimal fermentation medium formula of ST-TJ4 obtained via response surface analysis. The average fungal inhibition rate of the final fermentation filtrate was 76.5%, approximately 15% higher than before optimization (Figure 2B). The degree of fit between the experimental value and the predicted value is high, indicating that the established model is effective.
3.5. P. aurantiaca ST-TJ4 Phenazine-Related Gene Expression Analysis
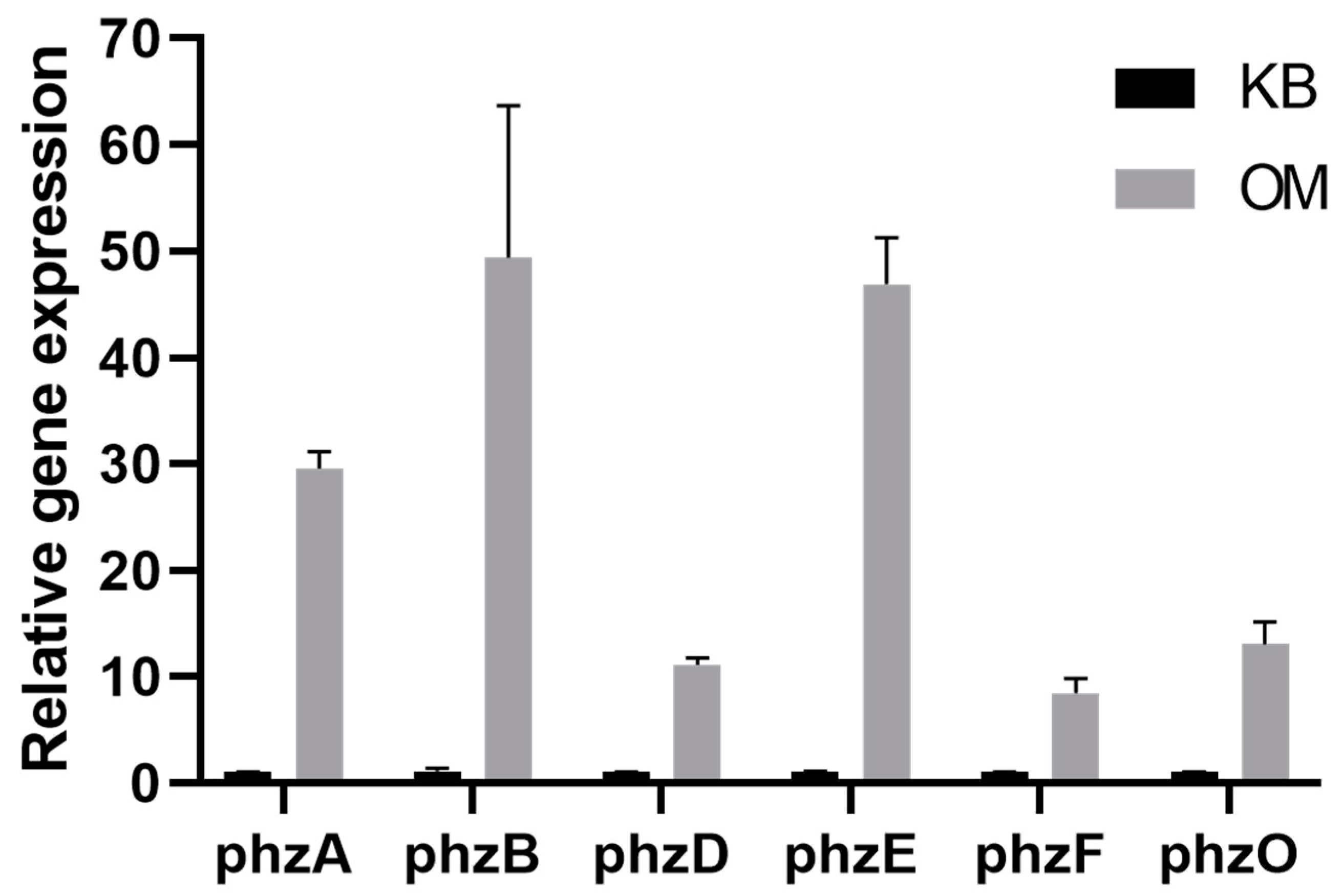
To determine the differential change in phenazine-related genes (before or after optimization), the expression levels of the genes phzA-B, phzD-F and phzO were measured. As shown in Figure 3, the expression level of phzA, phzB, phzD, phzE and phzF genes involved in phenazine−1-carboxylic acid (PCA) synthesis were upregulated by 29.54-, 49.35-, 11.11-, 46.89- and 8.42-fold, respectively. The phzO gene, which is responsible for the synthesis of 2-hydroxyphenazine (2-OH-PHZ), was upregulated 8.42-fold. These results indicated that the expression levels of phenazine-related genes were upregulated after optimization and the ability to form PCA and 2-OH-PHZ was improved.
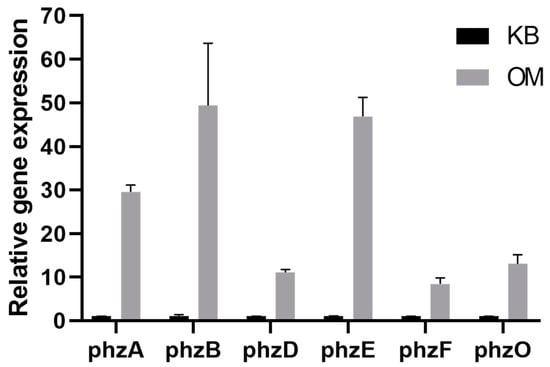
Figure 3.
Expression of the genes phzA, phzB, phzD, phzE, phzF and phzO related to PCA and 2-OH-PHZ formation were detected using qRT-PCR.
3.6. Stability of Fermentation Filtrate from ST-TJ4
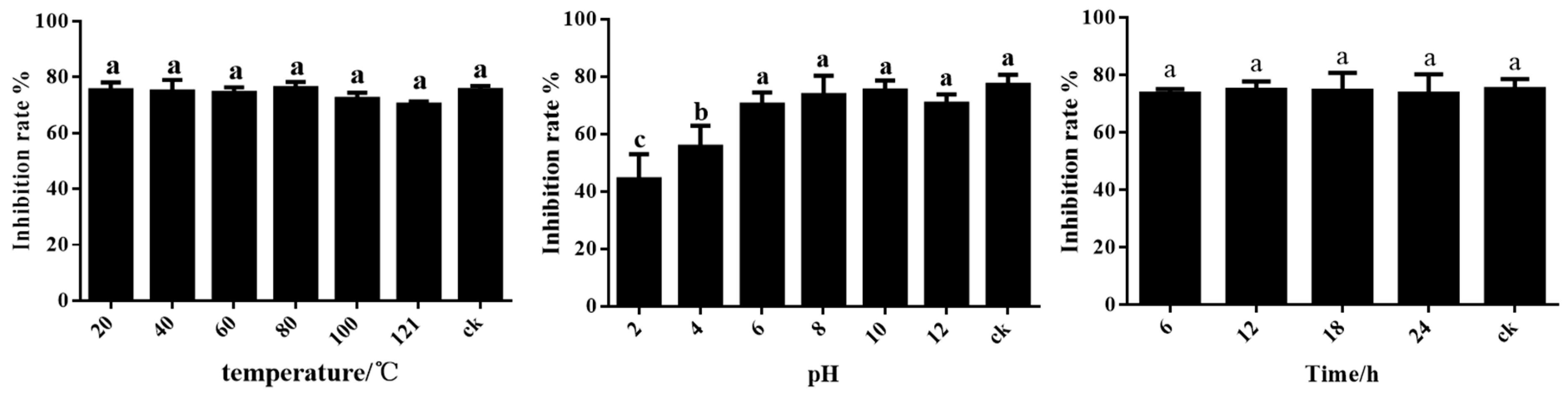
The results showed that the fermentation filtrate of ST-TJ4 was sensitive to acid–base conditions after treatment at pH 2–10. Under the alkaline condition, the antagonistic effect of the fermentation filtrate is stable, while the acidic condition weakens the antagonistic activity, suggesting that overly acidic conditions affect the stability of the active ingredients in the fermentation filtrate. Therefore, the pH values of the environment should be considered in practical applications. UV light and temperature treatments had no effect on the stability of the fermentation filtrate of ST-TJ4 (Figure 4).
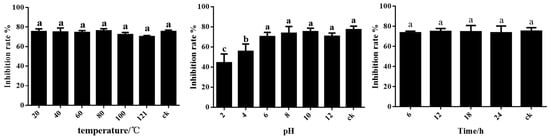
Figure 4.
Effect of temperature, pH and UV on inhibition activity of the ST-TJ4 fermentation filtrate. Different letters indicate statistically significant differences (p < 0.05) among treatments.
3.7. Control Effect of ST-TJ4 on R. pulchrum Spot Disease Caused by P. cinnamomi
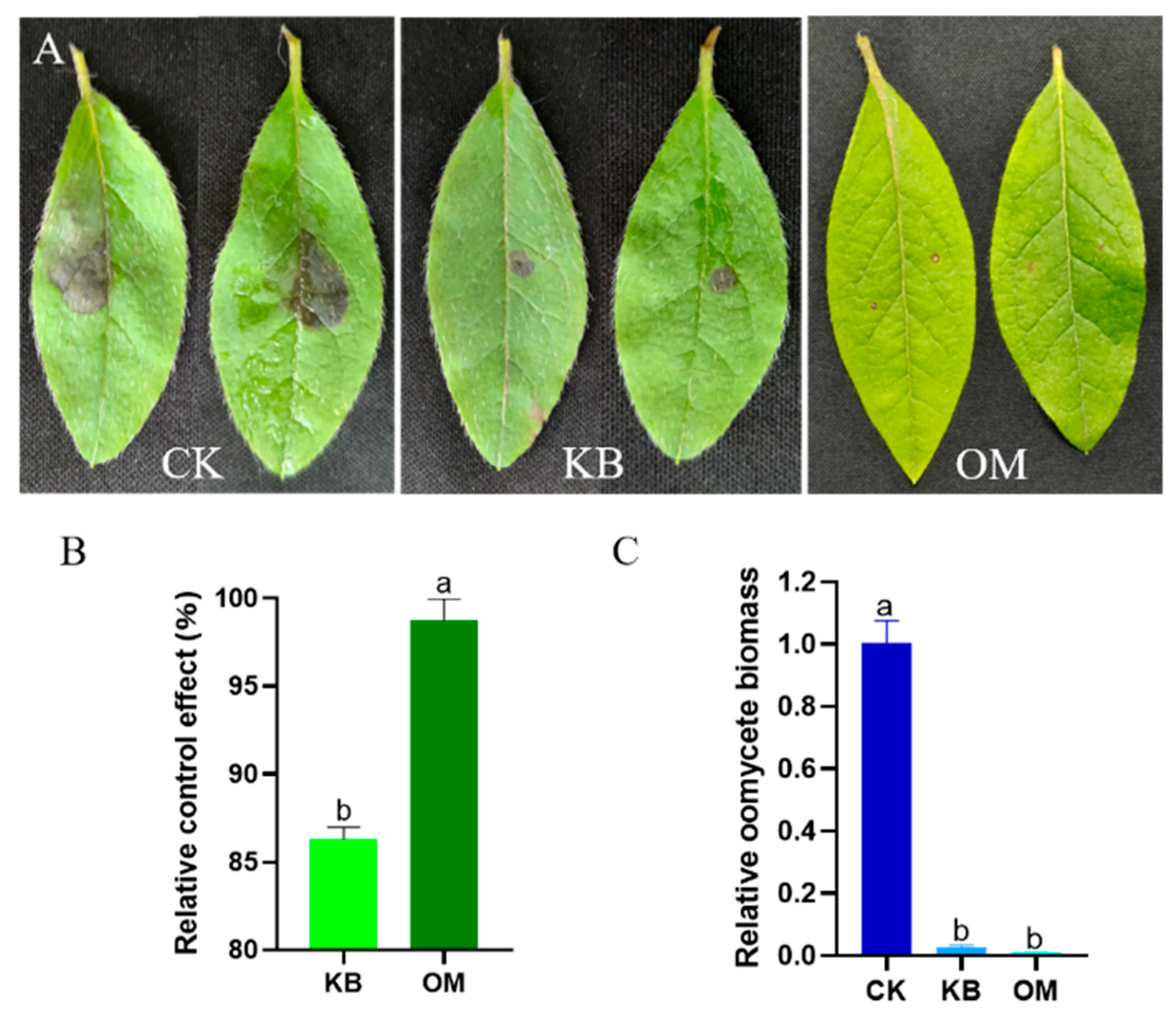
We further assessed the effect of ST-TJ4 on the biological control of diseases caused by P. cinnamomi on the leaves of R. pulchrum. Compared with the control, the fermentation filtrate of ST-TJ4 can reduce the lesions caused by P. cinnamomi, but the inhibitory effect is limited. The fermentation filtrate obtained by culturing ST-TJ4 in the optimized medium can further reduce the foliar disease spots caused by P. cinnamomic (Figure 5A). The relative control effect increased by 12.44% (Figure 5B). Furthermore, upfront filtrate treatment reduced the biomass of P. cinnamomi in plant leaves, although there is no significant difference before and after optimization (Figure 5C). Therefore, optimization of the fermentation medium improved the antagonistic effect of ST-TJ4 on P. cinnamomi and reduced its ability to infect the leaves of R. pulchrum.
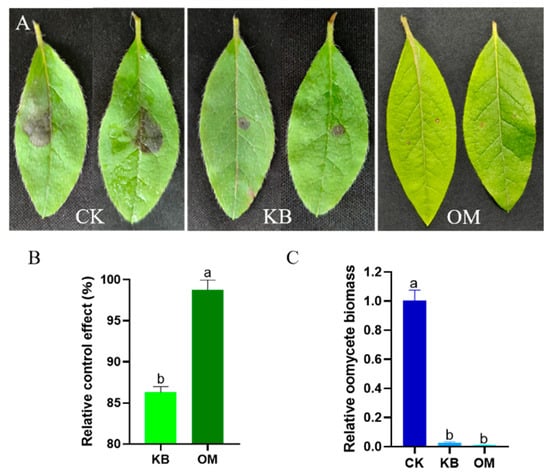
Figure 5.
The optimization of the fermentation medium improved the biocontrol effect of ST-TJ4 against P. cinnamomic. (A). Effect of different treatments on the P. cinnamomi infecting R. pulchrum leaves (CK: control; KB: basic medium; OM: optimized medium); (B). relative control effect; (C). detection of the development of P. cinnamomi biomass in plant leaves. Different letters indicate statistically significant differences (p < 0.05) among treatments.
4. Discussion
Pseudomonas bacteria commonly inhabit and proliferate in the rhizosphere soil of plants and are recognized as a species of plant growth-promoting bacteria with significant potential for biocontrol and practical application [29,30]. They possess the capability to directly impede the growth of pathogenic fungi by releasing antagonistic substances, especially those that are soil-borne [31,32]. In our laboratory, a biocontrol bacterium, P. chlororaphis subsp. aurantiaca ST-TJ4, exhibited antagonistic properties against various plant pathogens. To enhance the biocontrol efficacy of this strain, the fermentation medium was optimized in this study using the Plackett–Burman test, the steepest climb test and the Box–Behnken design.
Based on the KB medium, a single-factor screening experiment was conducted to identify the optimal combination of fermentation medium components. Subsequently, the significance of the factors identified in the single-factor experiment was assessed using the Plackett–Burman test to determine the optimal combination of the fermentation medium components. The results of the test indicated that glucose, as a carbon source, played a pivotal role in the antagonistic effect of ST-TJ4 fermentation filtrate. This finding is consistent with previous research, such as the study by Liu et al. [12], which demonstrated that the use of glucose as a carbon source by Lysobacter antibioticus 13–6 is a critical factor in increasing bacterial content and enhancing antifungal activity. Similarly, Zhang et al. [33] reported that the utilization of glucose as a carbon source can enhance the yield of antibiotics in a strain, thereby improving its antagonistic ability.
In addition to carbon sources, various nitrogen sources also affected the antagonistic effect of the ST-TJ4 fermentation filtrate. Studies have shown that organic nitrogen sources can notably enhance the yield of secondary metabolites compared to inorganic nitrogen sources [34]. Normally, in a fermentation test, yeast extract powder as a medium raw material exhibits superior production rate and yield of fermented product compared to other nitrogen sources [24]. However, the mechanism by which the addition of yeast extract enhances the antagonistic ability of ST-TJ4 against P. cinnamomi warrants further investigation. Moreover, inorganic salts are essential for the growth of biocontrol bacteria, and the PB test indicated that inorganic salts did not significantly impact the inhibition rate of the ST-TJ4 fermentation filtrate, possibly due to the presence of other medium components containing trace elements necessary for ST-TJ4.
The effectiveness of the biocontrol agent in field application is largely determined by its stability, which also serves as a key factor in determining its potential for successful application and development into a viable product. Yu et al. [35] discovered that the aseptic fermentation broth of Bacillus amyloliquefaciens DF-7 exhibited enhanced stability within the temperature range of 40–60 °C, at a pH level between 4 and 8 and when subjected to 90 min of UV irradiation. Additionally, the antagonistic protein 1–4-2F produced by B. velezensis NT35 demonstrated relatively stable characteristics and exhibited improved antifungal activity within the pH range of 4–10 and at temperatures between 20 and 100 °C [36]. The current study indicates that the fermentation filtrate of ST-TJ4 displays greater stability across a wider temperature range (20–121 °C), pH range (2–12) and UV irradiation time (24 h), suggesting its enhanced adaptability to complex field environments compared to Bacillus. Furthermore, the R. pulchrum leaf experiment provided confirmation that optimizing the fermentation medium reduced the susceptibility of P. cinnamomi to infect leaves.
Various strains exhibit distinct fermentation characteristics, making it imperative to identify the most suitable fermentation medium for each strain to ensure successful development and industrial-scale production. This investigation employed a single-factor experiment, the Plackett–Burman design and the Box–Behnken design to optimize the fermentation medium for the ST-TJ4 strain, focusing on the carbon source, nitrogen source and inorganic salt. The study determined the optimal medium composition, offering a foundational framework for the formulation of fermentation processes in future industrial production. Furthermore, given the substantial disparities between laboratory and industrial production environments, it is essential to conduct further assessments to ascertain the suitability of the fermentation conditions for large-scale industrial production.
5. Conclusions
In this study, the fermentation parameters for the production of inhibitory compounds by ST-TJ4 strain were optimized using a single-factor test in conjunction with response surface design. The findings revealed that a medium containing 20.59 g/L of glucose and 18.76 g/L of yeast extract powder was favorable for the generation of inhibitory substances by the ST-TJ4 strain. The antifungal efficacy of the ST-TJ4 strain against P. cinnamomi increased by 15% following the optimization process. Subsequent to optimization, the biocontrol properties were enhanced, and there was an upregulation in the expression levels of the phenazine synthesis genes phzA, phzB, phzD, phzE, phzF and phzO. Furthermore, the inhibitory compounds exhibited robust stability under high temperatures and UV light exposure and demonstrated increased tolerance to highly alkaline environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10010021/s1, Table S1. Primers used in RT-qPCR analysis; Table S2. Primers for measuring the relative oomycetes biomass in R. pulchrum.
Author Contributions
Data analysis and the first draft of the paper, W.-L.K. and Y.Z.; experimental research, W.-L.K. and Y.Z.; directed experimental design, data analysis, paper writing and revision, X.-Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFD0600104) and the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
All the data and materials have been provided in main manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Santoyo, G.; Orozco-Mosqueda, M.D.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of a Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Ni, H.; Kong, W.-L.; Zhang, Y.; Wu, X.-Q. Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae. J. Fungi 2022, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, O.V.; Walter, N.; Elateek, S.; Taylor, C.G.; Okubara, P.A. Suppression of Rhizoctonia and Pythium root rot of wheat by new strains of Pseudomonas. Biol. Control 2012, 62, 93–102. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; De Kock, M.J. Cyclic Lipopeptide Production by Plant-Associated Pseudomonas spp.: Diversity, Activity, Biosynthesis, and Regulation. Mol. Plant-Microbe Interact. 2006, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Almario, J.; Bruto, M.; Vacheron, J.; Prigent-Combaret, C.; Moenne-Loccoz, Y.; Muller, D. Distribution of 2,4-Diacetylphloroglucinol Biosynthetic Genes among the Pseudomonas spp. Reveals Unexpected Polyphyletism. Front. Microbiol. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Peix, A.; Valverde, A.; Rivas, R.; Igual, J.M.; Ramirez-Bahena, M.H.; Mateos, P.F.; Santa-Regina, I.; Rodriguez-Barrueco, C.; Martinez-Molina, E.; Velazquez, E. Reclassification of Pseudomonas aurantiaca as a synonym of Pseudomonas chlororaphis and proposal of three subspecies, P. chlororaphis subsp. chlororaphis subsp. nov., P. chlororaphis subsp. aureofaciens subsp. nov., comb. nov. and P. chlororaphis subsp. aurantiaca subsp. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1286–1290. [Google Scholar] [PubMed]
- Shi, Y.; Zaleta-Pinet, D.A.; Clark, B.R. Isolation, Identification, and Decomposition of Antibacterial Dialkylresorcinols from a Chinese Pseudomonas aurantiaca Strain. J. Nat. Prod. 2020, 83, 194–201. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hardie, D.; Baig, D.N.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of antimicrobial metabolites of plant growth promoting Pseudomonas spp. isolated from different plant hosts. 3 Biotech 2021, 11, 48. [Google Scholar]
- Tagele, S.B.; Lee, H.G.; Kim, S.W.; Lee, Y.S. Phenazine and 1-Undecene Producing Pseudomonas chlororaphis subsp. aurantiaca Strain KNU17Pc1 for Growth Promotion and Disease Suppression in Korean Maize Cultivars. J. Microbiol. Biotechnol. 2019, 29, 66–78. [Google Scholar] [CrossRef]
- Hu, W.Q.; Gao, Q.X.; Hamada, M.S.; Dawood, D.H.; Zheng, J.W.; Chen, Y.; Ma, Z.H. Potential of Pseudomonas chlororaphis subsp. aurantiaca Strain Pcho10 as a Biocontrol Agent against Fusarium graminearum. Phytopathology 2014, 104, 1289–1297. [Google Scholar] [CrossRef]
- Lazazzara, V.; Perazzolli, M.; Pertot, I.; Biasioli, F.; Puopolo, G.; Cappellin, L. Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains. Microbiol. Res. 2017, 201, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.; Wang, X.; Wei, L.; Ji, G. Effect of culture medium optimization on the secondary metabolite activity of Lysobacter antibioticus 13-6. Prep. Biochem. Biotechnol. 2021, 51, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Y.H.; Li, M.; Zhu, M.L.; Wen, T.Y.; Wu, X.Q. Medium optimization to analyze the protein composition of Bacillus pumilus HR10 antagonizing Sphaeropsis sapinea. AMB Express 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Gan, Y.T.; Liu, J.; Zhou, J.J.; Xu, B.L. Optimization of the Fermentation Media and Parameters for the Bio-control Potential of Trichoderma longibrachiatum T6 Against Nematodes. Front. Microbiol. 2020, 11, 574601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.Y.; Gao, X.J.; Wang, K.; Wang, W.W.; Wang, Q.; Yan, P.S. Isolation of a novel deep-sea Bacillus circulus strain and uniform design for optimization of its anti-aflatoxigenic bioactive metabolites production. Bioengineered 2019, 10, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Z.; Zhang, Y.; Yang, H.; Qiu, G.; Wang, D.; Lian, Y. Improvement of tacrolimus production in Streptomyces tsukubaensis by mutagenesis and optimization of fermentation medium using Plackett-Burman design combined with response surface methodology. Biotechnol. Lett. 2021, 43, 1765–1778. [Google Scholar] [CrossRef]
- Shafi, J.; Sun, Z.; Ji, M.; Gu, Z.; Ahmad, W. ANN and RSM based modelling for optimization of cell dry mass of Bacillus sp. strain B67 and its antifungal activity against Botrytis cinerea. Biotechnol. Biotechnol. Equip. 2017, 32, 58–68. [Google Scholar] [CrossRef]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef]
- Yun, T.Y.; Feng, R.J.; Zhou, D.B.; Pan, Y.Y.; Chen, Y.F.; Wang, F.; Yin, L.Y.; Zhang, Y.D.; Xie, J.H. Optimization of fermentation conditions through response surface methodology for enhanced antibacterial metabolite production by Streptomyces sp. 1–14 from cassava rhizosphere. PLoS ONE 2018, 13, e0206497. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Y.R.; Tan, J.J.; Liu, F.; Hu, J.F. Optimization of Fermentation Conditions for Bacillus pumilus LYMC-3 to Antagonize Sphaeropsis sapinea. Fermentation 2023, 9, 482. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, W.L.; Wu, X.Q.; Li, P.S. Inhibitory effects of phenazine compounds and VOCs produced by Pseudomonas aurantiaca ST-TJ4 against Phytophthora cinnamomi. Phytopathology 2022, 112, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.L.; Li, P.S.; Wu, X.Q.; Wu, T.Y.; Sun, X.R. Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms 2020, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Wu, X.Q.; Wang, Y.H.; Dai, Y. Role of Biofilm Formation by Bacillus pumilus HR10 in Biocontrol against Pine Seedling Damping-Off Disease Caused by Rhizoctonia solani. Forests 2020, 11, 652. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, X.Q.; Wang, Y.H.; Zhu, M.L. Biocontrol potential of Bacillus pumilus HR10 against Sphaeropsis shoot blight disease of pine. Biol. Control 2021, 152, 104458. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Abdelhafez, A.A.; Ali, A.A.; Sand, H.M. Optimization of β-Carotene Production from Rhodotorula glutinis ATCC 4054 Growing on Agro-industrial Substrate Using Plackett–Burman Design. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1637–1646. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 Culture Medium by Response Surface Methodology for Low Cost Production of Antifungal Activity. Microorganisms 2022, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Jiao, B.B.; Zhou, J.; He, H.B.; Dai, T.T. Rapid detection of Phytophthora cinnamomi based on a new target gene Pcinn13739. Front. Cell. Infect. Microbiol. 2022, 12, 923700. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Y.; Zhang, M.; Du, G.; Wang, J. Selection and Evaluation of Candidate Reference Genes for Quantitative Real-Time PCR in Aboveground Tissues and Drought Conditions in Rhododendron delavayi. Front. Genet. 2022, 13, 876482. [Google Scholar] [CrossRef]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Stummer, B.E.; Guo, Q.Q.; Zhang, W.; Zhang, X.J.; Harvey Paul, R. Quantification of Pseudomonas protegens FD6 and Bacillus subtilis NCD-2 in soil and the wheat rhizosphere and suppression of root pathogenic Rhizoctonia solani AG-8. Biol. Control 2021, 154, 104504. [Google Scholar] [CrossRef]
- Mazurier, S.; Corberand, T.; Lemanceau, P.; Raaijmakers, J.M. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J. 2009, 3, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.P.; Liu, H.C.; Xian, M.; Huang, W. Biosynthetic Pathway Construction and Production Enhancement of 1-Hydroxyphenazine Derivatives in Pseudomonas chlororaphis H18. J. Agric. Food Chem. 2022, 70, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Wu, X.G.; Zhang, L.Q. Effect of carbon sources on production of 2,4-diacetylphoroglucinol in Pseudomonas fluorescens 2P24. Acta Microbiol. Sin. 2018, 28, 1202–1212. [Google Scholar]
- Yue, S.J.; Huang, P.; Li, S.; Jan Malik Hu, H.B.; Wang, W.; Zhang, X.H. Enhanced Production of 2-Hydroxyphenazine from Glycerol by a Two-Stage Fermentation Strategy in Pseudomonas chlororaphis GP72AN. J. Agric. Food Chem. 2020, 68, 561–566. [Google Scholar] [CrossRef]
- Yu, L.; Jing, A.; Li, Y.; Gao, X.M.; Liu, X.H. Optimization of Fluid Medium and Stability of Sterile Fermentation Fluid of Bacillus amyloliticus DF-7. Shandong Agric. Sci. 2021, 53, 149–154. [Google Scholar]
- Li, M.T.; Tang, H.; Li, Z.Y.; Song, Y.; Chen, L.; Ran, C.; Jiang, Y.; Chen, C.Q. Optimization of the Production and Characterization of an Antifungal Protein by Bacillus velezensis Strain NT35 and Its Antifungal Activity against Ilyonectria robusta Causing Ginseng Rusty Root Rot. Fermentation 2023, 9, 358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).