Transcriptional Analysis of Antrodin C Synthesis in Taiwanofungus camphoratus (Syn. Antrodia camphorate, Antrodia cinnamomea) to Understand Its Biosynthetic Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Determination of Biomass
2.3. Determination of Antrodin C Yield with Different Concentrations of Inositol and Maleic Acid
2.4. Detection of Biomass in Fermentation Broth
2.5. Detection of Residual Sugars in Fermentation Broth
2.6. Extraction and Determination of Antrodin C Yield
2.7. Transcriptome Analysis
2.8. qPCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Biological Characterisation of T. camphoratus
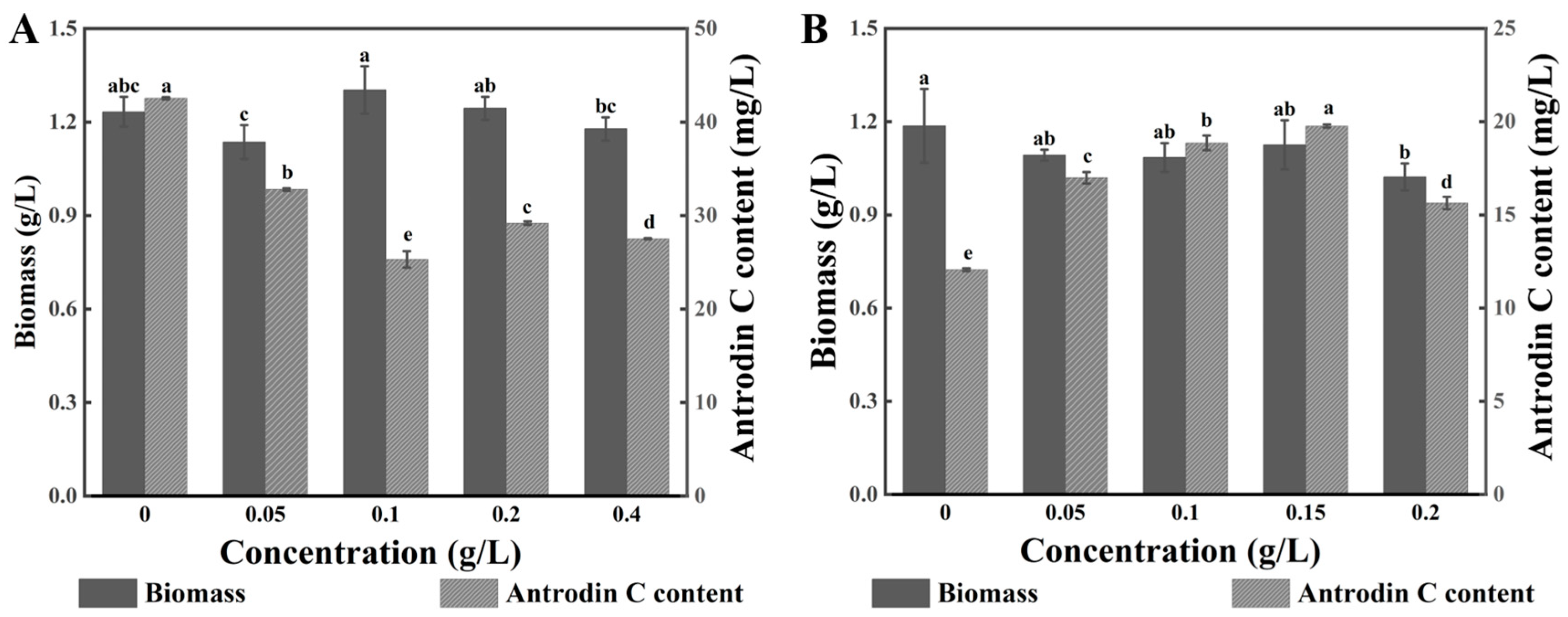
3.2. Effect of Different Additives on Biomass and Antrodin C Yield of T. camphoratus
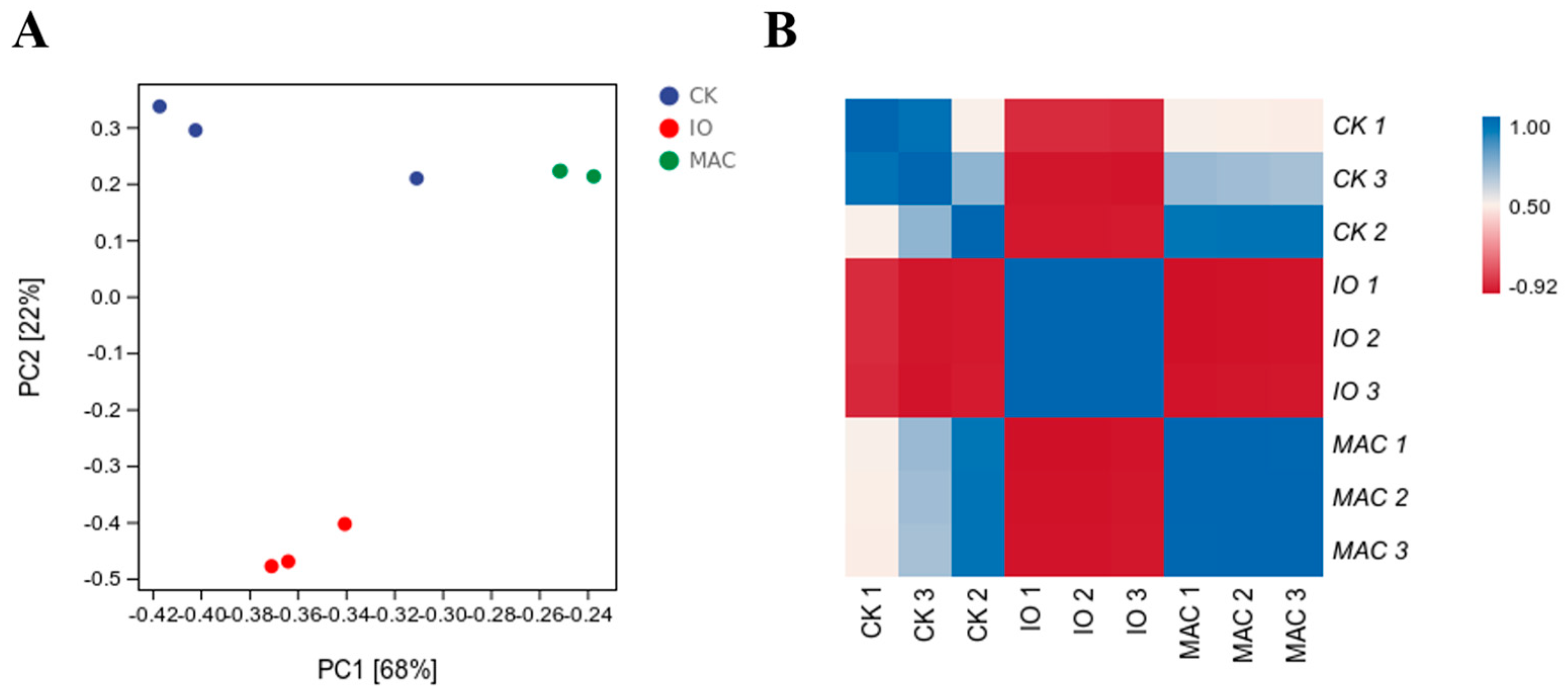
3.3. Descriptive Analysis of RNA Sequencing Data
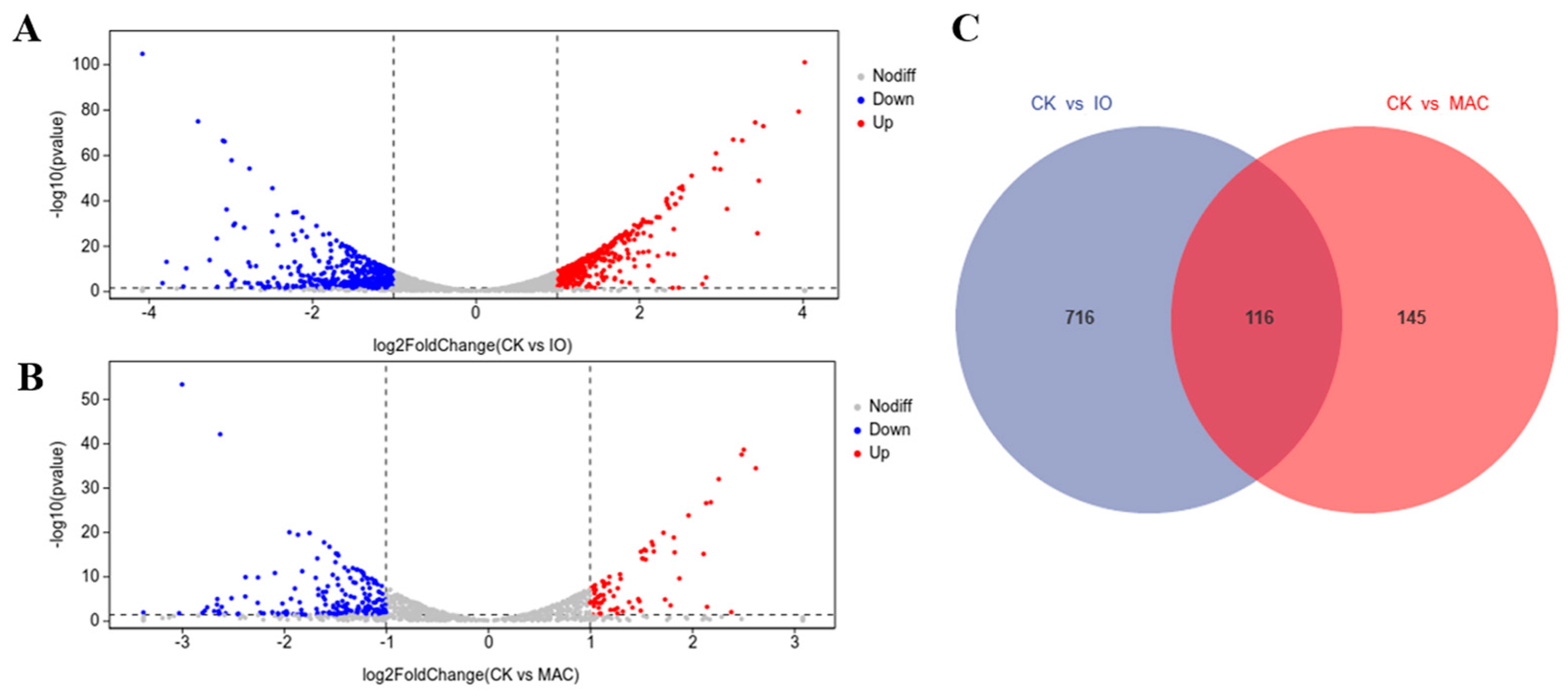
3.4. Sample Reproducibility and DEGs
3.5. Identification of DEGs
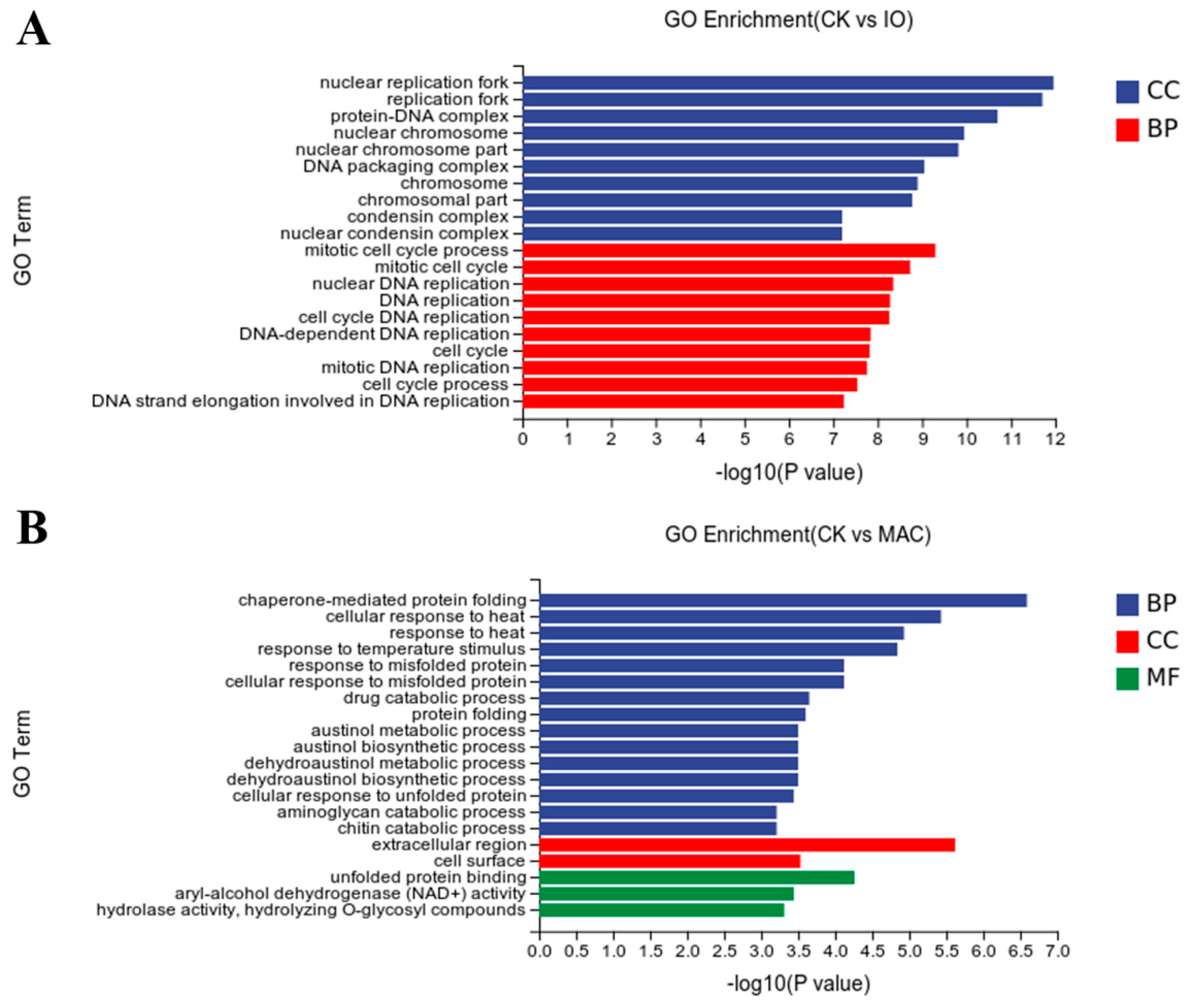
3.6. Functional Analysis of DEGs Based on GO Categories
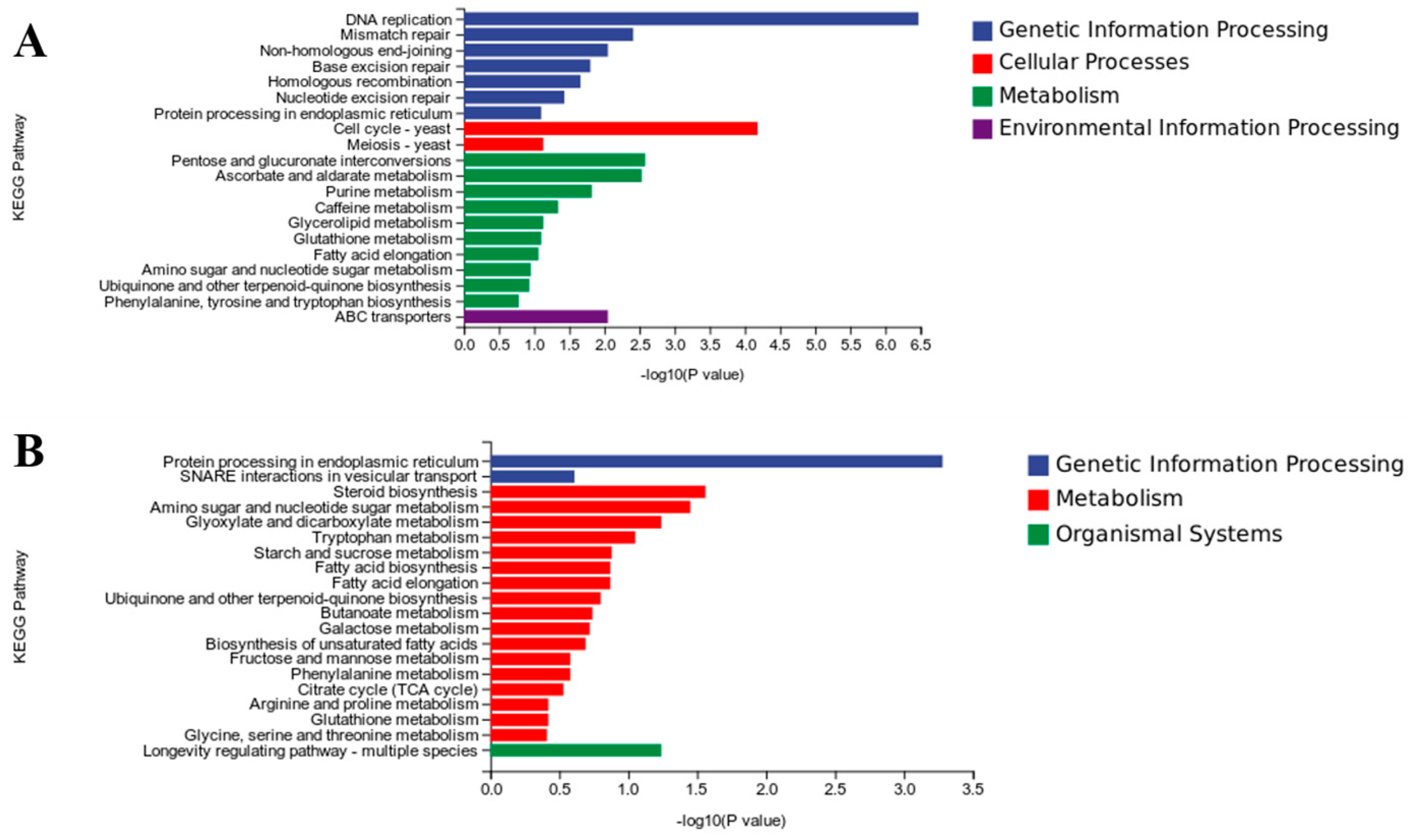
3.7. Functional Analysis of DEGs Based on KEGG Pathways
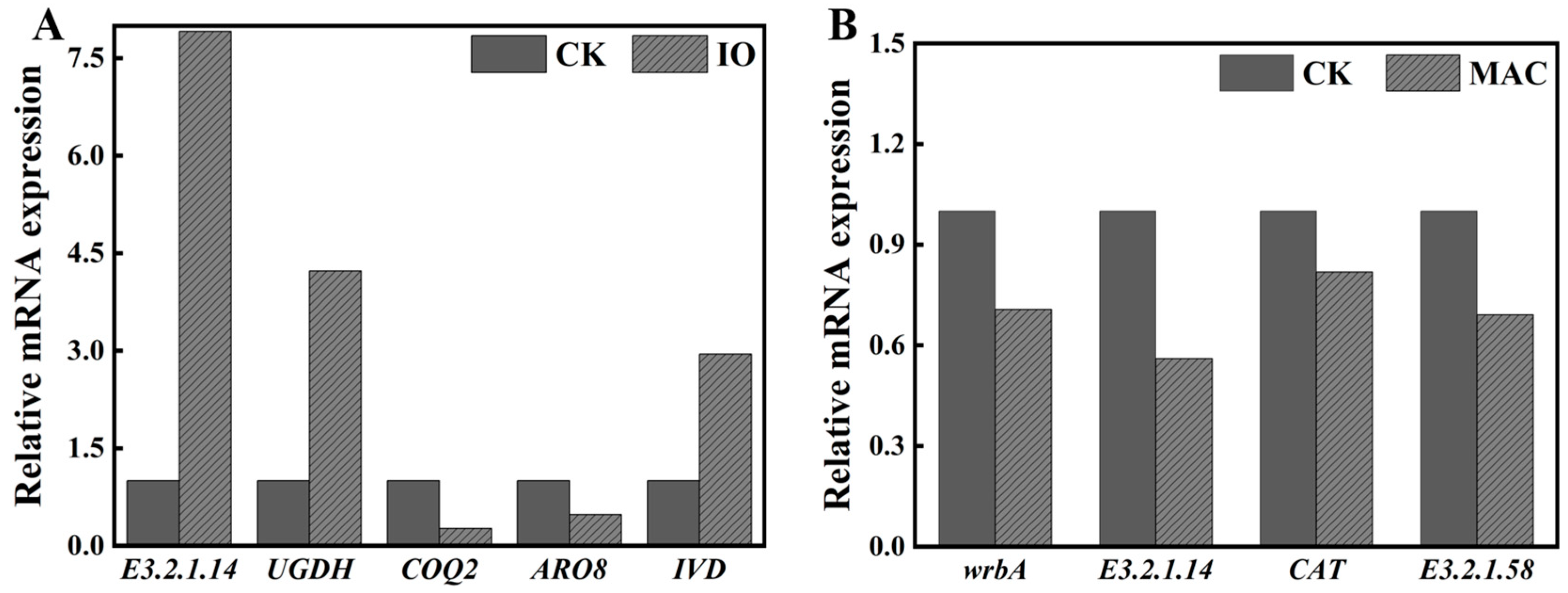
3.8. Validation of RNA-Seq Data Using qPCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.H.; Yu, Z.H.; Dai, Y.C.; Chen, C.; Su, C.H.; Chen, L.C.; Hsu, W.C.; Hwang, G.Y. Taiwanofungus, a polypore new genus. Fungal Sci. 2004, 19, 109–116. [Google Scholar]
- Cha, W.S.; Ding, J.L.; Choi, D. Comparative evaluation of antioxidant, nitrite scavenging, and antitumor effects of Antrodia camphorata extract. Biotechnol. Bioprocess Eng. 2009, 14, 232–237. [Google Scholar] [CrossRef]
- Zeng, W.W.; Chen, T.C.; Liu, C.H.; Wang, S.Y.; Shaw, J.F.; Chen, Y.T. Identification and isolation of an intermediate metabolite with dual antioxidant and anti-proliferative activity present in the fungus Antrodia cinnamomea cultured on an alternative medium with Cinnamomum kanehirai leaf extract. Plants 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.L.; Fu, X.Q.; Li, J.K.; Tse, A.K.W.; Guo, H.; Yin, C.L.; Chou, J.Y.; Wang, Y.P.; Liu, Y.X.; Chen, Y.J. Antrodia camphorata mycelia exert anti-liver cancer effects and inhibit STAT3 signaling in vitro and in vivo. Front. Pharmacol. 2018, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Tzang, B.S.; Chang, Y.Y.; Chiu, C.H.; Kang, W.Y.; Huang, C.H.; Chen, Y.C. Effects of Antrodia camphorata on alcohol clearance and antifibrosis in livers of rats continuously fed alcohol. J. Agric. Food Chem. 2011, 59, 4248–4254. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hirakawa, A.; Gao, J.J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.C.; Hattori, M. Five New Maleic and Succinic Acid Derivatives from the Mycelium of Antrodia camphorata and Their Cytotoxic Effects on LLC Tumor Cell Line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Vani, M.G.; Chueh, P.J.; Mau, J.L.; Wang, S.Y. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and β-catenin signaling pathways. PLoS ONE 2015, 10, e0117111. [Google Scholar] [CrossRef]
- Phuong, D.T.; Ma, C.M.; Hattori, M.; Jin, J.S. Inhibitory effects of antrodins A–E from Antrodia cinnamomea and their metabolites on hepatitis C virus protease. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 582–584. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, W.J. Research progress on submerged fermentation of macroscopic edible fungi. J. Food Saf. Qual. 2016, 7, 1240–1246. [Google Scholar]
- Li, H.X.; Lu, Z.M.; Geng, Y.; Gong, J.S.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Efficient production of bioactive metabolites from Antrodia camphorata ATCC 200183 by asexual reproduction-based repeated batch fermentation. Bioresour. Technol. 2015, 194, 334–343. [Google Scholar] [CrossRef]
- Xia, Y.J.; Zhang, X.F.; Xu, G.R. Using Response Surface Methodology Optimization of Medium Components for Antrodin C Production by Antrodia camphorata. Food Sci. 2012, 33, 185–189. [Google Scholar]
- Zhang, H.; Xia, Y.j.; Wang, Y.L.; Zhang, B.B.; Xu, G.R. Coupling use of surfactant and in situ extractant for enhanced production of Antrodin C by submerged fermentation of Antrodia camphorata. Biochem. Eng. J. 2013, 79, 194–199. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhang, Y.; Wang, Z.C.; Du, C.; Zou, L.J.; Liu, H.D.; Yu, C.W.; Ai, L.Z.; Xia, Y.J. High-yield production of Antrodin C and Antroquinonol from in situ extractive fermentation of Antrodia camphorata. Food Ferment. Ind. 2019, 45, 8–13. [Google Scholar]
- Hu, P.F.; Huang, J.; Chen, L.; Ding, Z.; Liu, L.; Molnár, I.; Zhang, B.-B. Oxidative stress induction is a rational strategy to enhance the productivity of Antrodia cinnamomea fermentations for the antioxidant secondary metabolite antrodin C. J. Agric. Food Chem. 2020, 68, 3995–4004. [Google Scholar] [CrossRef]
- Bai, X.; Wang, W.H.; Zhang, J.S.; Ju, J.W.; Zhang, S.B.; Ding, B.A.; Jia, W. Liquid Fermentation of Antrodin A and Antrodin C from Taiwanofungus camphoratus. Acta Edulis Fungi 2022, 29, 73–79. [Google Scholar]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, H.; Bai, X.; Zhang, H.; Zhang, J.; Wu, Y.; Tang, C.; Liu, Y.; Yang, Y.; Liu, Z.; Jia, W. Antrodin C, an NADPH dependent metabolism, encourages crosstalk between autophagy and apoptosis in lung carcinoma cells by use of an AMPK inhibition-independent blockade of the Akt/mTOR pathway. Molecules 2019, 24, 993. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.D.; Zhang, H.; Lu, R.Q.; Liao, X.R.; Zhang, B.B.; Xu, G.R. Enabling the biosynthesis of Antroquinonol in submerged fermentation of Antrodia camphorata. Biochem. Eng. J. 2014, 91, 157–162. [Google Scholar] [CrossRef]
- Geng, G.; Lv, C.; Stevanato, P.; Li, R.; Liu, H.; Yu, L.; Wang, Y. Transcriptome analysis of salt-sensitive and tolerant genotypes reveals salt-tolerance metabolic pathways in sugar beet. Int. J. Mol. Sci. 2019, 20, 5910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Jin, X.; Shi, X.; Xue, Y.; Jiang, J.; Yuan, C.; Du, Y.; Liu, X.; Xie, R.; Liu, X. Transcriptomic analysis reveals candidate genes responsive to Sclerotinia scleroterum and cloning of the Ss-inducible chitinase genes in Morus laevigata. Int. J. Mol. Sci. 2020, 21, 8358. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, K.; Gaur, R.; Gupta, V. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21. [Google Scholar]
- Klinghammer, M.; Tenhaken, R. Genome-wide analysis of the UDP-glucose dehydrogenase gene family in Arabidopsis, a key enzyme for matrix polysaccharides in cell walls. J. Exp. Bot. 2007, 58, 3609–3621. [Google Scholar] [CrossRef]
- Giner, R.M.; Ríos, J.L.; Máñez, S. Antioxidant activity of natural hydroquinones. Antioxidants 2022, 11, 343. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S. Plastoquinone and ubiquinone in plants: Biosynthesis, physiological function and metabolic engineering. Front. Plant Sci. 2016, 7, 1898. [Google Scholar] [CrossRef]
- Forsgren, M.; Attersand, A.; Lake, S.; Grünler, J.; Swiezewska, E.; Dallner, G.; Climent, I. Isolation and functional expression of human COQ2, a gene encoding a polyprenyl transferase involved in the synthesis of CoQ. Biochem. J. 2004, 382, 519–526. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E. Genomic and transcriptomic basis of Hanseniaspora vineae’s impact on flavor diversity and wine quality. Appl. Environ. Microbiol. 2019, 85, e01959-18. [Google Scholar] [CrossRef]
- Kutateladze, A.G.; Kuznetsov, D.M. Triquinanes and related sesquiterpenes revisited computationally: Structure corrections of hirsutanols B and D, hirsutenol E, cucumin B, antrodins C–E, chondroterpenes A and H, chondrosterins C and E, dichrocephone A, and pethybrene. J. Org. Chem. 2017, 82, 10795–10802. [Google Scholar] [CrossRef]

| Sample | Clean Reads | Clean Data (bp) | Q20 (%) | Q30 (%) | Top Mapped | Multiple Mapped | Uniquely Mapped |
|---|---|---|---|---|---|---|---|
| CK 1 | 44,798,630 | 6,760,298,404 | 97.76 | 93.82 | 43,882,934 (97.96%) | 1,755,369 (4.00%) | 42,127,565 (96.00%) |
| CK 2 | 40,799,084 | 6,153,300,013 | 98.09 | 94.5 | 40,129,578 (98.36%) | 2,245,947 (5.60%) | 37,883,631 (94.40%) |
| CK 3 | 41,295,462 | 6,226,806,032 | 97.87 | 94.15 | 40,531,757 (98.15%) | 1,833,723 (4.52%) | 38,698,034 (95.48%) |
| IO 1 | 46,860,468 | 7,069,852,321 | 97.63 | 93.57 | 45,980,182 (98.12%) | 1,767,746 (3.84%) | 44,212,436 (96.16%) |
| IO 2 | 45,405,964 | 6,851,073,561 | 98.08 | 94.61 | 44,576,558 (98.17%) | 1,706,534 (3.83%) | 42,870,024 (96.17%) |
| IO 3 | 46,122,616 | 6,959,518,905 | 97.74 | 93.81 | 45,247,681 (98.10%) | 1,781,445 (3.94%) | 43,466,236 (96.06%) |
| MAC 1 | 40,041,196 | 6,041,792,837 | 98.05 | 94.6 | 39,246,233 (98.01%) | 1,873,489 (4.77%) | 37,372,744 (95.23%) |
| MAC 2 | 42,802,110 | 6,458,223,523 | 97.82 | 93.98 | 41,937,635 (97.98%) | 2,024,335 (4.83%) | 39,913,300 (95.17%) |
| MAC 3 | 49,487,160 | 7,449,231,565 | 98.13 | 94.75 | 48,535,741 (98.08%) | 2,423,780 (4.99%) | 46,111,961 (95.01%) |
| Pathway | Gene | Gene Function |
|---|---|---|
| Amino sugar and nucleotide sugar metabolism | E3.2.1.14 | chitinase |
| UGDH | UDPglucose 6-dehydrogenase | |
| galK | galactokinase | |
| Glutathione metabolism | GST | glutathione S-transferase |
| HPGDS | glutathione transferase | |
| RRM2 | ribonucleoside-diphosphate reductase subunit M2 | |
| Pentose and glucuronate interconversions | UGDH | UDPglucose 6-dehydrogenase |
| E3.2.1.67 | galacturan 1,4-alpha-galacturonidase | |
| GAAA | D-galacturonate reductase | |
| adh | alcohol dehydrogenase (NADP+) | |
| Purine metabolism | RRM2 | ribonucleoside-diphosphate reductase subunit M2 |
| rdgB | XTP/dITP diphosphohydrolase | |
| add | adenosine deaminase | |
| ppnN | purine-5′-nucleotide nucleosidase | |
| uaZ | urate oxidase | |
| Ubiquinone and other terpenoid-quinone biosynthesis | COQ2 | 4-hydroxybenzoate polyprenyltransferase |
| ARO8 | aromatic amino acid aminotransferase I | |
| Starch and sucrose metabolism | E3.2.1.4 | endoglucanase |
| AMY | alpha-amylase | |
| E3.2.1.58 | glucan 1,3-beta-glucosidase | |
| E2.4.1.34 | 1,3-beta-glucan synthase | |
| Valine, leucine, and isoleucine degradation | IVD | isovaleryl-CoA dehydrogenase |
| ALDH | aldehyde dehydrogenase (NAD+) |
| Pathway | Gene | Gene Function |
|---|---|---|
| Steroid biosynthesis | ERG2 CYP51 | C-8 sterol isomerase sterol 14alpha-demethylase |
| Tryptophan metabolism | CAT | catalase |
| amiE | amidase | |
| Ubiquinone and other terpenoid-quinone biosynthesis | wrbA | NAD(P)H dehydrogenase (quinone) |
| Amino sugar and nucleotide sugar metabolism | E3.2.1.14 | chitinase |
| abfA | alpha-L-arabinofuranosidase | |
| Glyoxylate and dicarboxylate metabolism | CAT | catalase |
| Starch and sucrose metabolism | E3.2.1.58 | glucan 1,3-beta-glucosidase |
| malZ | alpha-glucosidase | |
| Fructose and mannose metabolism | MAN | mannan endo-1,4-beta-mannosidase |
| Gene Name | KO | Gene Function | log2FoldChange (IO/CK) | log2FoldChange (MAC/CK) | p-Value (IO/CK) | p-Value (MAC/CK) |
|---|---|---|---|---|---|---|
| wrbA | K03809 | NAD(P)H dehydrogenase (quinone) | - | −1.45603 | - | 0.01894 |
| E3.2.1.14 | K01183 | chitinase | 2.644593 | −1.66418 | 1.56 × 10−51 | 7.81 × 10−8 |
| CAT | K03781 | catalase | - | −1.2896 | - | - |
| E3.2.1.58 | K01210 | glucan 1,3-beta-glucosidase | −1.32575 | - | - | |
| UGDH | K00012 | UDPglucose 6-dehydrogenase | 1.537218 | - | 4.08 × 10−19 | - |
| COQ2 | K06125 | 4-hydroxybenzoate polyprenyltransferase | −1.91367 | - | 6.78 × 10−14 | - |
| ARO8 | K00838 | aromatic amino acid aminotransferase I | −1.28393 | - | 7.71 × 10−5 | - |
| IVD | K00253 | isovaleryl-CoA dehydrogenase | 1.009587 | - | 1.92 × 10−8 | - |
| AMY | K01176 | alpha-amylase | 1.765041 | - | 3.55 × 10−25 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Gai, S.-P.; Li, X.-H.; Zhang, J.-S.; Wang, W.-H. Transcriptional Analysis of Antrodin C Synthesis in Taiwanofungus camphoratus (Syn. Antrodia camphorate, Antrodia cinnamomea) to Understand Its Biosynthetic Mechanism. Fermentation 2024, 10, 28. https://doi.org/10.3390/fermentation10010028
Jia W, Gai S-P, Li X-H, Zhang J-S, Wang W-H. Transcriptional Analysis of Antrodin C Synthesis in Taiwanofungus camphoratus (Syn. Antrodia camphorate, Antrodia cinnamomea) to Understand Its Biosynthetic Mechanism. Fermentation. 2024; 10(1):28. https://doi.org/10.3390/fermentation10010028
Chicago/Turabian StyleJia, Wei, Shu-Ping Gai, Xiao-Hui Li, Jing-Song Zhang, and Wen-Han Wang. 2024. "Transcriptional Analysis of Antrodin C Synthesis in Taiwanofungus camphoratus (Syn. Antrodia camphorate, Antrodia cinnamomea) to Understand Its Biosynthetic Mechanism" Fermentation 10, no. 1: 28. https://doi.org/10.3390/fermentation10010028
APA StyleJia, W., Gai, S.-P., Li, X.-H., Zhang, J.-S., & Wang, W.-H. (2024). Transcriptional Analysis of Antrodin C Synthesis in Taiwanofungus camphoratus (Syn. Antrodia camphorate, Antrodia cinnamomea) to Understand Its Biosynthetic Mechanism. Fermentation, 10(1), 28. https://doi.org/10.3390/fermentation10010028





