Lactic Acid Bacteria-Fermented Diet Containing Bacterial Extracellular Vesicles Inhibited Pathogenic Bacteria in Striped Beakfish (Oplegnathus fasciatus)
Abstract
1. Introduction
2. Materials andMethods
2.1. Cultivation of LAB and Preparation of Fermented Dietary Feed
2.2. Fish and Husbandry Conditions
2.3. Feeding Trial
2.4. Assays for Superoxide Anion Production and Phagocytotic Activity of Leucocytes Isolated from O. fasciatus
2.5. Assays for Serum Bactericidal and Lysozyme Activity
2.6. Assay for Microbiota
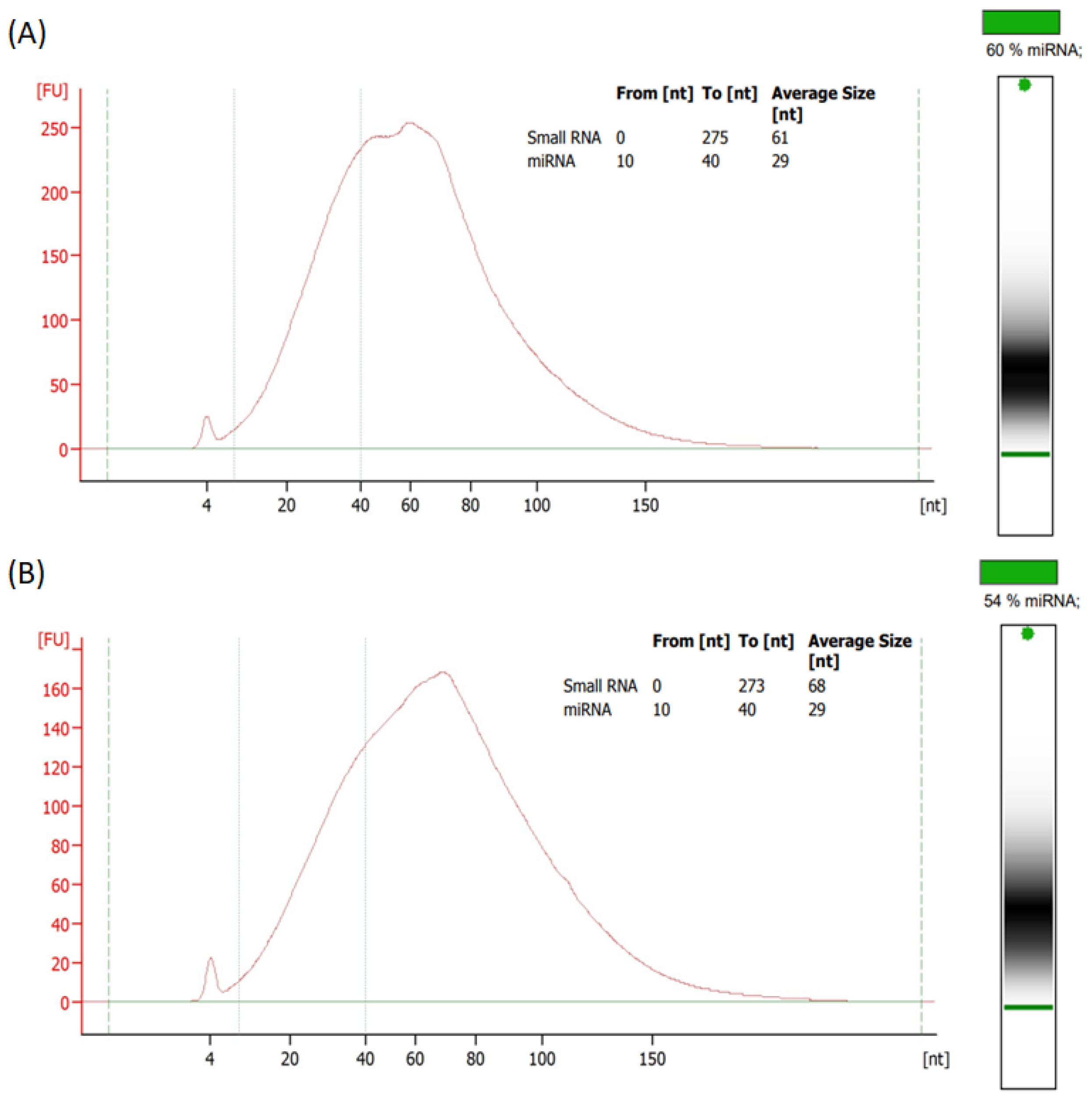
2.7. Isolation of Extracellular Vesicles (EVs)
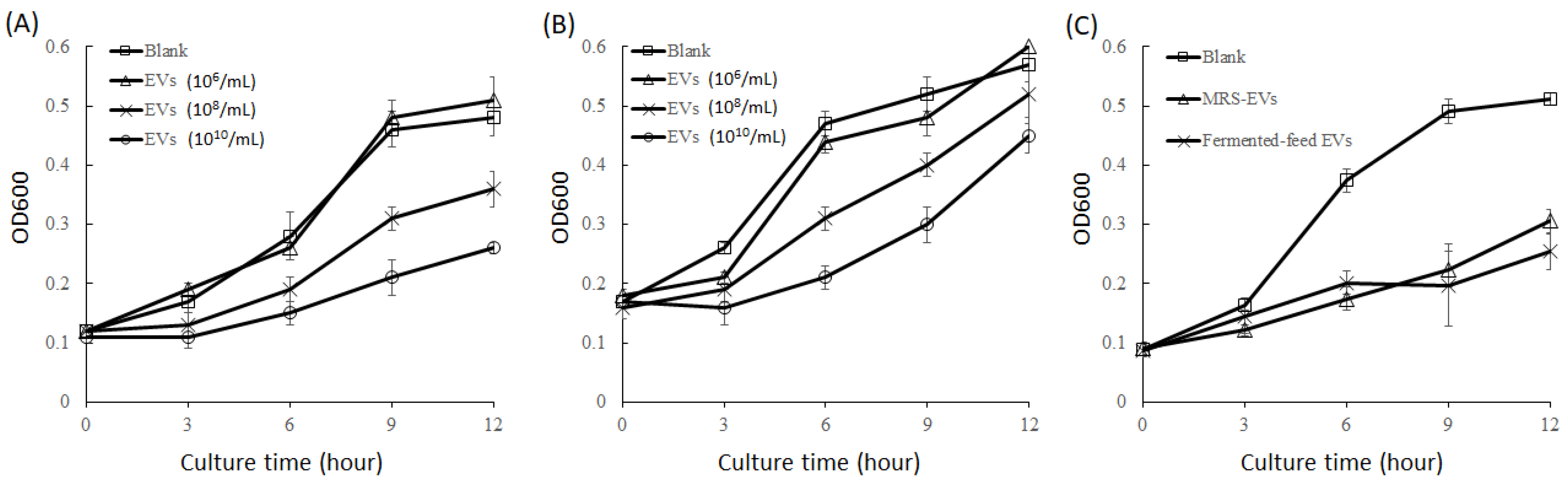
2.8. Inhibition of Pathogenic Bacteria
2.9. Statistical Analysis
3. Results
3.1. Feeding Fermentation with LAB
3.2. Effects of LAB-Fermented Feed for Fish Growth
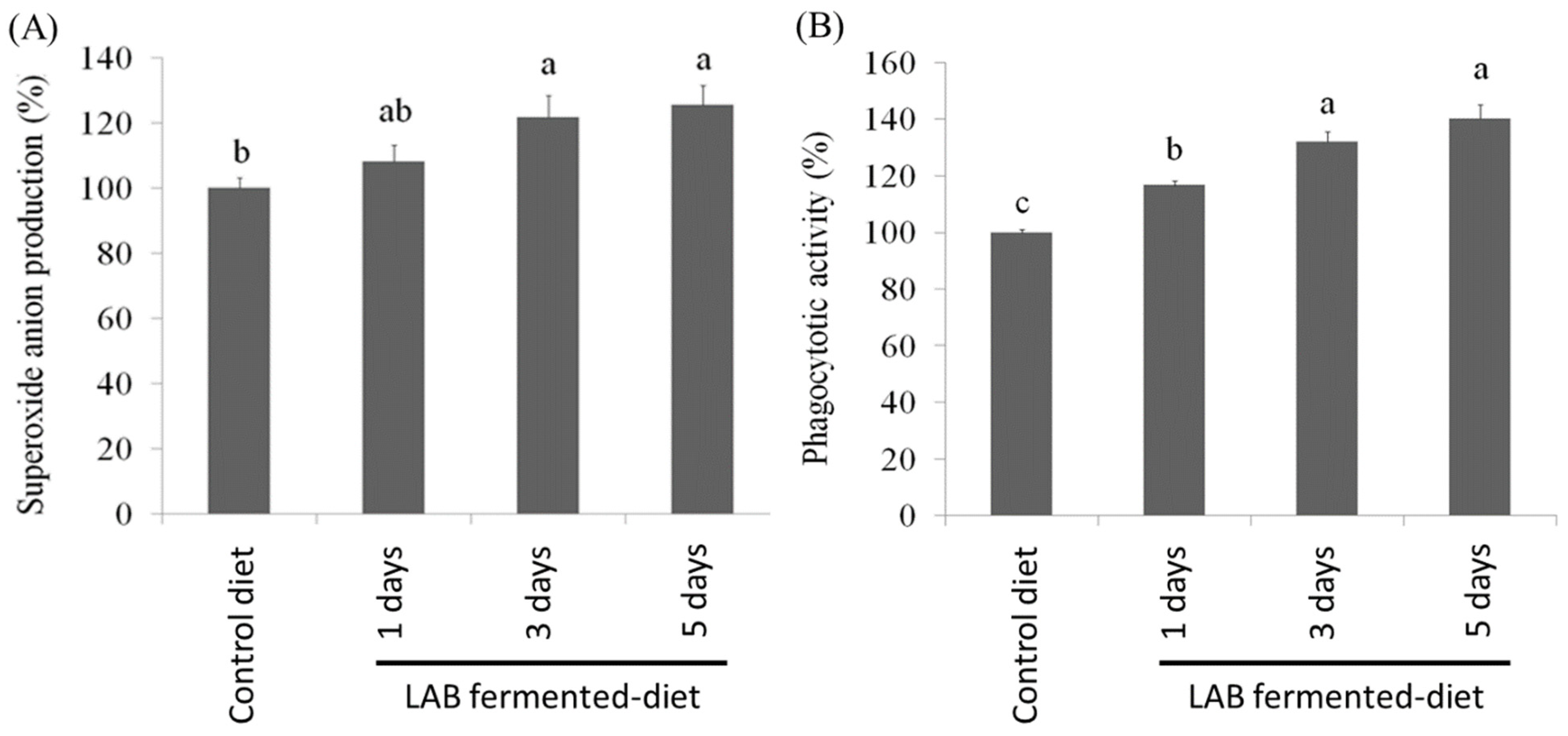
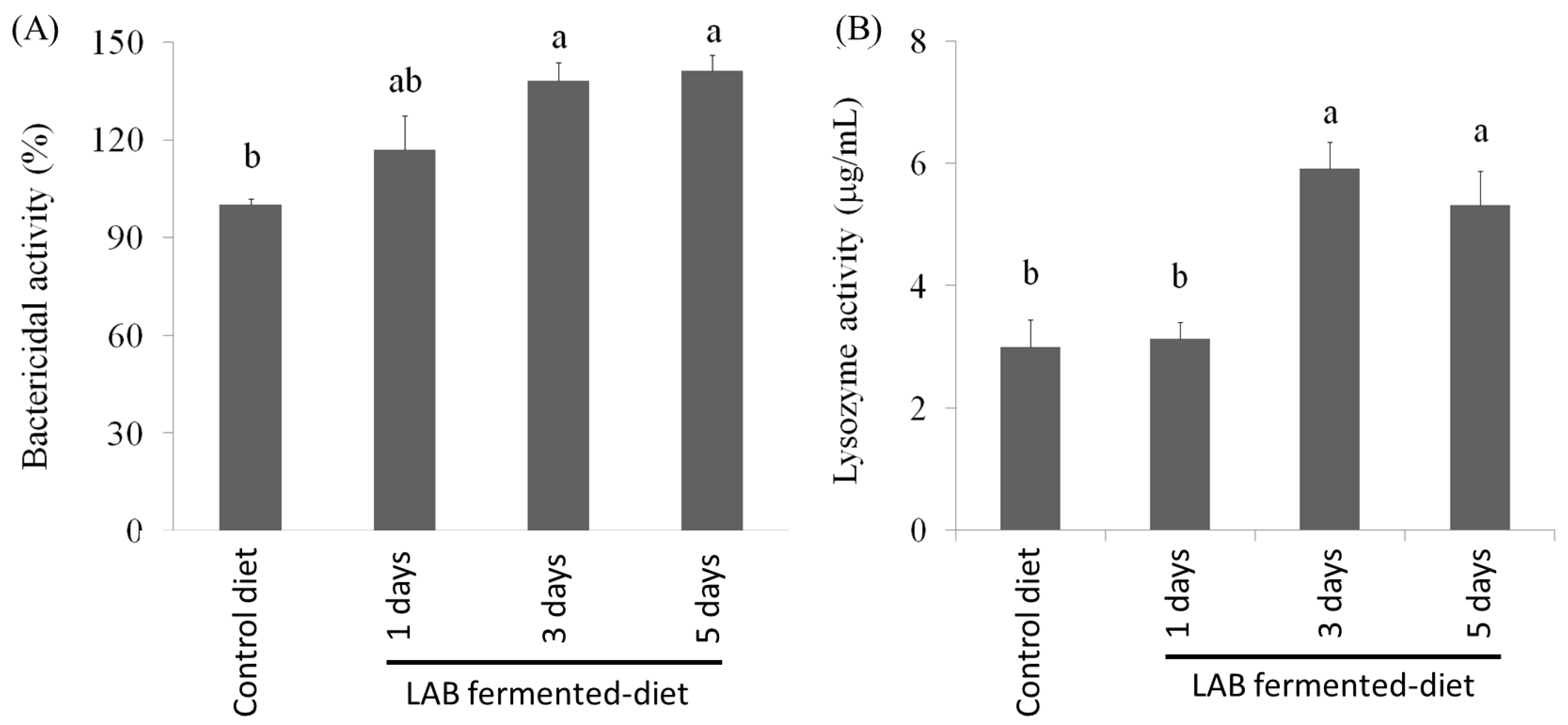
3.3. Effect of LAB-Fermented Feed on Immune Activity of O. fasciatus
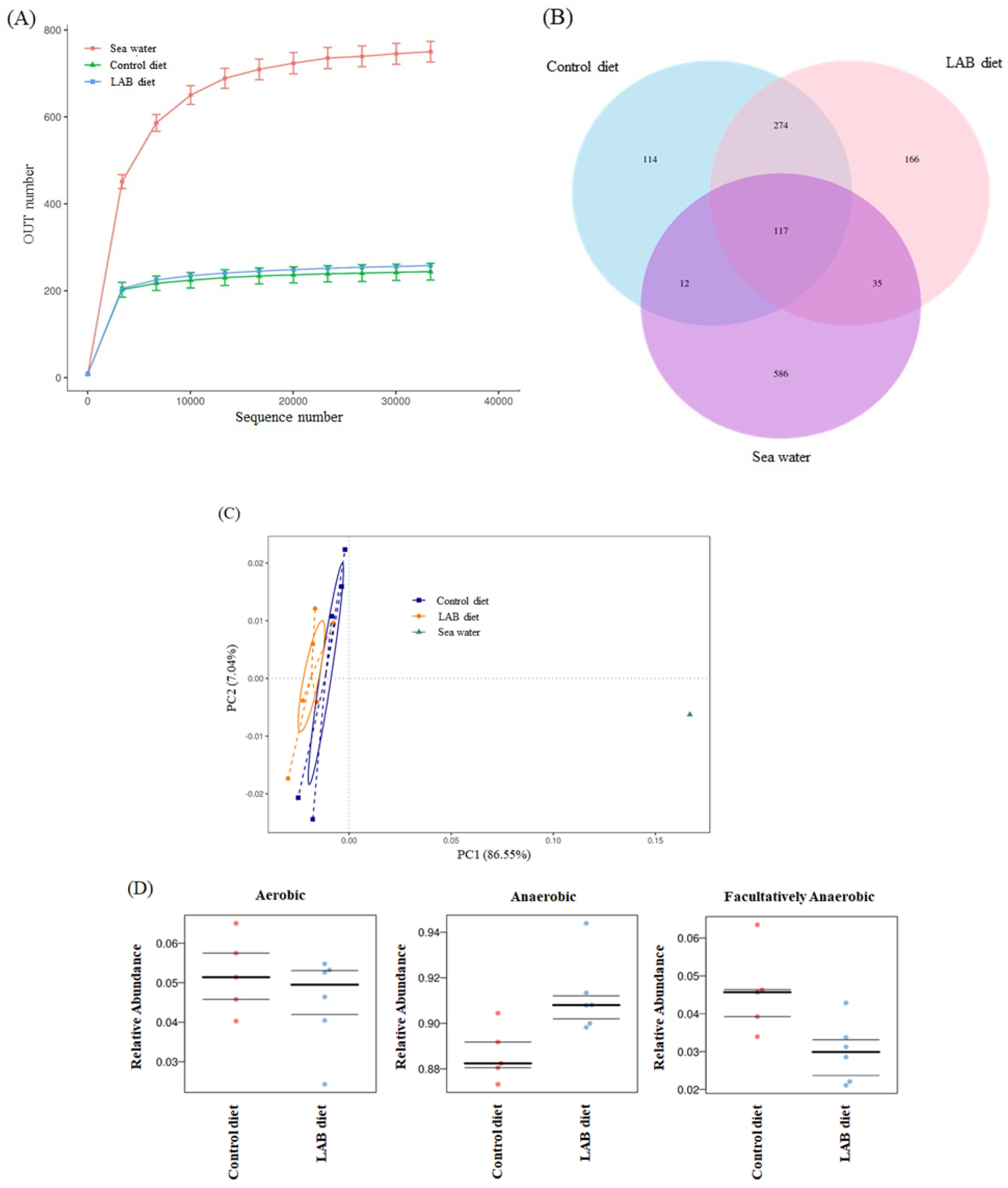
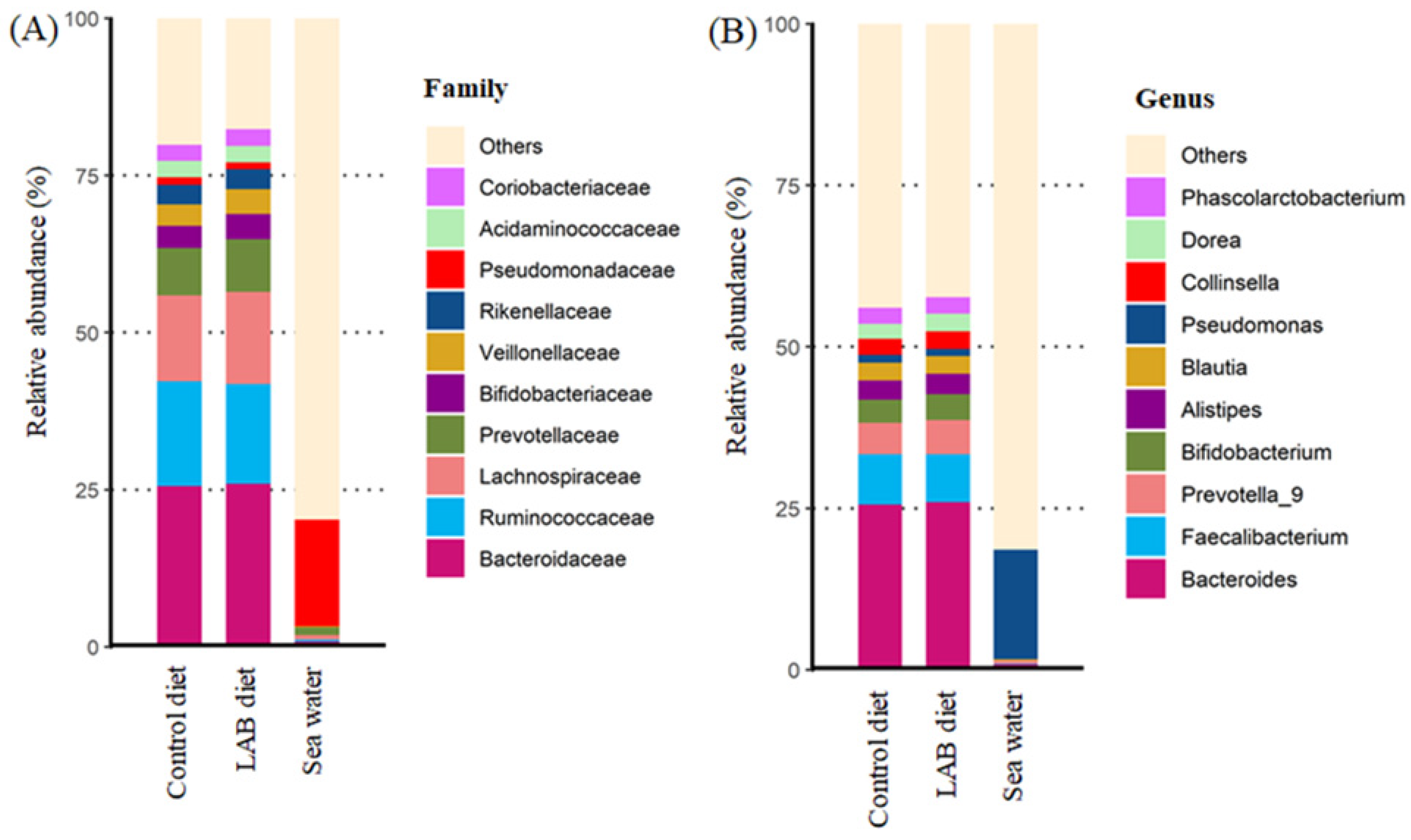
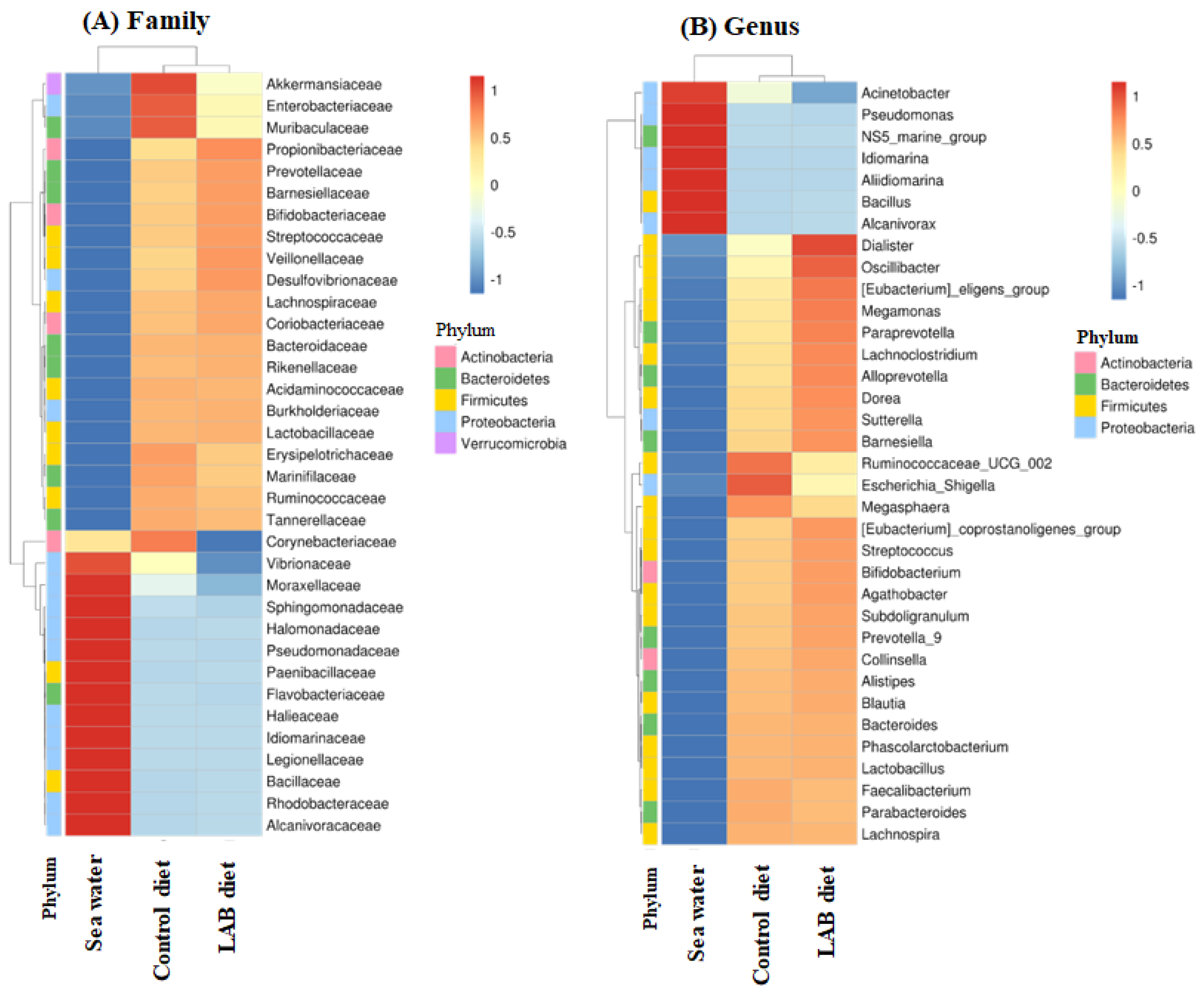
3.4. Effect of LAB-Fermented Feed on Intestinal Microbiota in Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luan, Y.; Li, M.; Zhou, W.; Yao, Y.; Yang, Y.; Zhang, Z.; Ringo, E.; Olsen, R.E.; Clarke, J.L.; Xie, S.; et al. The fish microbiota: Research progress and potential applications. Engineering 2023. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; He, Q.; Lin, F.; Wang, Q.; Xiao, S.; Dai, Y.; Zhang, Y.; Yang, H.; Zhao, H. Effect of starvation and refeeding on growth, gut microbiota and non-specific immunity in hybrid grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂. Fish Shell. Immunol. 2020, 97, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Rasheeda, M.K.; Rangamaran, V.R.; Srinivasan, S.; Ramaiah, S.K.; Gunasekaran, R.; Jaypal, S.; Gopal, D.; Ramalingam, K. Comparative profiling of microbial community of three economically important fishes reared in sea cages under tropical offshore environment. Mar. Genom. 2017, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Dhler, C.E.; Secombes, C.J.; Martin, S.A. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Solmo salar L.). Aquaculture 2017, 467, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, F.E.; Nicolas, D. Vertically and horizontally transmitted microbial symbionts shape the gut microbiota ontogenesis of a skin-mucus feeding discus fish progeny. Sci. Rep. 2017, 7, 5263. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Rahimnejad, S.; Wang, L.; Song, K.; Lu, K.; Zhang, C. Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibro parahaemolyticus. Fish Shell. Immunol. 2017, 70, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Liu, M.; Wang, B.; Jiang, K.; Qi, C.; Wang, L. Bacterial population in intestines of Litopenaeus vannamei fed different probiotics or probiotic supernatant. J. Microbiol. Biotechnol. 2016, 26, 1736–1745. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El-Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shell. Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef]
- Ramos, M.A.; Weber, B.; Concalves, J.F.; Santos, G.A.; Rema, P.; Ozorio, R.O.A. Dietary probiotic supplementation modulated gut microbiota and improved growth of juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A 2013, 166, 302–307. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, Z.; Wang, Y.; Li, S.; Ran, C.; Hu, J.; Xie, Y.; Li, W.; Zhou, Z. EPSP of L. casei BL23 protected against the infection caused by Aeromonas veronii via enhancement of immune response in zebrafish. Front. Microbiol. 2017, 8, 2406. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Wu, S.C.; Shen, T.L.; Hsu, Y.Y.; Chen, C.H.; Hsu, W.H. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem. 2021, 340, 128104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Lee, B.H.; Hou, C.Y.; Hsu, W.H.; Tai, C.J. Probiotics-derived extracellular vesicles protect oxidative stress against H2O2 induction in placental cells. Fermentation 2022, 8, 74. [Google Scholar] [CrossRef]
- Lee, B.H.; Chen, Y.Z.; Shen, T.L.; Pan, T.M.; Hsu, W.H. Proteomic characterization of extracellular vesicles derived from lactic acid bacteria. Food. Chem. 2023, 427, 136685. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Yano, T. Effect of sodium alginate on the non-specific defense system of the common carp (Cyprinuscarpio L.). Fish Shell. Immunol. 1997, 7, 417–427. [Google Scholar] [CrossRef]
- Iida, T.; Takahashi, T.; Wakabayashi, H. Decrease in the bactericidal activity of normal serum during the spawning period of rainbow trout. Nippon SuisanGakkaishi 1989, 55, 463–465. [Google Scholar] [CrossRef]
- Chen, H.C.; Huang, Y.R.; Hsu, H.H.; Lin, C.S.; Chen, W.C.; Lin, C.M. Determination of histamine and biogenic amines in fish cubes (Tetrapturus angustirostris) implicated in a food borne poisoning. Food Control 2010, 21, 13–18. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The host shapes the gut microbiota via fecal microRNA. Cell Host Microb. 2016, 19, 32–43. [Google Scholar] [CrossRef]
- Behera, B.K.; Paria, P.; Das, A.; Bhowmick, S.; Sahoo, A.K.; Das, B.K. Molecular characterization and pathogenicity of a virulent Acinetobacter baumannii associated with mortality of farmed Indian major carp Labeo rohita (Hamilton 1822). Aquaculture 2017, 471, 157–162. [Google Scholar] [CrossRef]
- Villegas-Plazas, M.; Villamil, L.; Martinez-Silva, M.A.; Gonzalez-Jimenez, T.; Salazar, M.; Guiza, L.; Mendoza, M.; Junca, H. Microbiome composition and autochthonous probiotics from contrasting probiosis/dysbiosis states in cobia (Rachycentron canadum) fish epitheliocystis. Access Microbiol. 2022, 4, acmi000405. [Google Scholar] [CrossRef]
- Aly, S.M.; Mohamed, M.F.; John, G. Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquac. Res. 2008, 39, 647–656. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Sen, S.S.; Jena, P.K. Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeorohita. Aquac. Nutr. 2014, 20, 163–171. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 2015, 442, 29–36. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Yang, H.L.; Ma, R.L.; Song, K.; Li, J.S. Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquac. Nutr. 2012, 18, 281–289. [Google Scholar] [CrossRef]
- Beck, B.R.; Kim, D.; Jeon, J.; Lee, S.M.; Kim, H.K.; Kim, O.J.; Lee, J.I.; Suh, B.S.; Do, H.K.; Lee, K.H.; et al. The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shell. Immunol. 2015, 42, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ljuboratovic, U.; Kosanovic, D.; Vukotic, G.; Molnar, Z.; Stanisavljevic, N.; Ristovic, T.; Peter, G.; Lukic, J.; Jeney, G. Supplementation of lactobacilli improves growth, regulates microbiota composition and suppresses skeletal anomalies in juvenile pike-perch (Sander lucioperca) reared in recirculating aquaculture system (RAS): A pilot study. Res. Vet. Sci. 2017, 115, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Hakima, Y.; Unib, Z.; Hulataa, G.; Harpaz, S. Relationship between intestinal brush border enzymatic activity and growth rate in tilapias fed diets containing 30% or 48% protein. Aquaculture 2006, 257, 420–428. [Google Scholar] [CrossRef]
- Furne, M.; Hidalgo, M.C.; Lopez, A.; García-Gallegoa, M.; Moralesa, A.E.; Domezainb, A.; Domezaine, J.; Sanza, A. Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 2005, 250, 391–398. [Google Scholar] [CrossRef]
- Xia, Y.; Lu, M.; Chen, G.; Gao, J.; Gao, F.; Wang, M.; Liu, Z.; Zhang, D.; Zhu, H.; Yi, M. Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia Oreochromis niloticus. Fish Shell. Immunol. 2018, 76, 368–379. [Google Scholar] [CrossRef]
- Geng, X.; Dong, X.H.; Tan, B.P.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y.; Liu, X.Q. Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac. Nutr. 2012, 18, 46–55. [Google Scholar] [CrossRef]
- Panigrahi, A.; Kiron, V.; Kobayashi, T.; Puangkaew, J.; Satoh, S.; Sugita, H. Immuneresponses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet. Immunol. Immunopathol. 2004, 102, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Li, E.; Li, T.; Jia, Y.; Qin, J.G.; Gu, Z.; Chen, L. Response of gut health and microbiota to sulfide exposure in Pacific white shrimp Litopenaeus vannamei. Fish Shell. Immunol. 2017, 63, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Chen, Y.Z.; Hsu, K.T.; Pan, T.M. Limosilactobacillus fermentum SWP-AFFS-2 improves the growth and survival rate of white shrimp via regulating immunity and intestinal microbiota. Fermentation 2021, 7, 179. [Google Scholar] [CrossRef]
- Wu, Y.S.; Chu, Y.T.; Chen, Y.Y.; Chang, C.S.; Lee, B.H.; Nan, F.H. Effects of dietary Lactobacillus reuteri and Pediococcus acidilactici on the cultured water qualities, the growth and non-specific immune responses of Penaeus vannamei. Fish Shell. Immunol. 2022, 127, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Hu, Y.F.; Lee, B.H.; Huang, C.Y.; Lin, Y.R.; Huang, S.N.; Chen, Y.Y.; Chang, J.J.; Nan, F.H. Dietary of Lactobacillus paracasei and Bifidobacterium longum improve nonspecific immune responses, growth performance, and resistance against Vibrio parahaemolyticus in Penaeus vannamei. Fish Shell. Immunol. 2022, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ott, L. Adhesion properties of toxigenic corynebacterial. AIMS Microbiol. 2018, 4, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Puliti, M.; von Hunolstein, C.; Marangi, M.; Bistoni, F.; Tissi, L. Experimental model of infection with non-toxigenic strains of Corynebacterium diphtheriae and development of septic arthritis. J. Med. Microbiol. 2006, 55, 229–235. [Google Scholar] [CrossRef]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzon, D.; Blokesch, M.; et al. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar]
- Anderson, M.; Sansonetti, P.J.; Marteyn, B.S. Shigella diversity and changing landscape: Insights for the twenty-first century. Front. Cell. Infect. Microbiol. 2016, 6, 45. [Google Scholar] [CrossRef]
- Srinivasan, S.; Beamer, M.A.; Fiedler, T.L.; Austin, M.N.; Sizova, M.V.; Strenk, S.M.; Agnew, K.J.; Nagana Gowda, G.A.; Raftery, D.; Epstein, S.S.; et al. Megasphaera lornae sp. nov., Megasphaera hutchinsoni sp. nov., and Megasphaera vaginalis sp. nov.: Novel bacteria isolated from the female genital tract. Int. J. Syst. Evol. Microbiol. 2021, 71, 004702. [Google Scholar] [CrossRef]
- Petrina, M.A.B.; Cosentino, L.A.; Rabe, L.K.; Hillier, S.L. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe 2017, 47, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Woo, K.J.; Jung, W.J.; Kim, M.J.; Sukumaran, V.; et al. Effects of dietary Lactiplantibacillus plantarum subsp. plantarum L7, alone or in combination with Limosilactobacillus reuteri P16, on growth, mucosal immune responses, and disease resistance of Cyprinus carpio. Probiotics Antimicrob. Proteins 2021, 13, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Nasir, A.; Sattar, F.; Ashfaq, I.; Chen, M.H.; Hayat, A.; Ur Rehman, M.; Zhao, S.; Khaliq, S.; Ghauri, M.A.; et al. Production of biomodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022, 67, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ghori, I.; Tubassam, M.; Ahmad, T.; Zuberi, A.; Imran, M. Gut microbiome modulation mediated by probiotics: Positive impact on growth and health status of Labeo rohita. Front. Physiol. 2022, 13, 949559. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Duan, Y.; Li, X.; Hu, Y.; Mo, Z.; Dan, X.; Li, Y. Effects of Cryptocaryon irritans infection on the histopathology, oxidative stress, immune response, and intestinal microbiota in the orange-spotted grouper Epinephelus coioides. Fish Shell. Imunol. 2023, 133, 108562. [Google Scholar] [CrossRef]
- Kewcharoen, W.; Srisapoome, O. Probiotic effects of Bacillus spp. From pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shell. Immunol. 2019, 94, 175–189. [Google Scholar] [CrossRef]
- Lee, J.W.; Chiu, S.T.; Wang, S.T.; Liao, Y.C.; Chang, H.T.; Ballantyne, R.; Lin, J.S.; Liu, C.H. Dietary SYNSEA probiotic improves the growth of white shrimp, Litopenaeus vannamei and reduces the risk of Vibrio infection via improving immunity and intestinal microbiota of shrimp. Fish Shell. Immunol. 2022, 127, 482–491. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microb. 2018, 24, 637–652. [Google Scholar] [CrossRef]



| Groups | Initial Weight (g) | Final Weight (g) | Weight Gain (%) |
|---|---|---|---|
| Control | 74.8 ± 2.61 | 152.3 ± 1.6 b | 103.7 ± 5.7 b |
| 1 day | 74.87 ± 6.38 | 153.9 ± 4.4 b | 106.9 ± 12.5 b |
| 3 days | 74.67 ± 1.15 | 205.1 ± 4.1 a | 174.8 ± 9.4 a |
| 5 days | 78.83 ± 5.03 | 188.1 ± 4.3 a | 139.5 ± 20.3 ab |
| Groups | Feed Intake (g) | Body Weight (g) | FCR |
|---|---|---|---|
| Control | 476.5 ± 0.3 b | 77.5 ± 1.7 c | 6.15 ± 0.14 a |
| 1 day | 471.4 ± 7.2 b | 79.1 ± 10.8 c | 6.05 ± 0.9 a |
| 3 days | 550.6 ± 20.6 a | 130.5 ± 5.2 a | 4.23 ± 0.28 b |
| 5 days | 547.2 ± 16.6 a | 109.3 ± 9.3 b | 5.03 ± 0.36 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Hu, Y.-F.; Chu, Y.-T.; Wu, Y.-S.; Hsu, W.-H.; Nan, F.-H. Lactic Acid Bacteria-Fermented Diet Containing Bacterial Extracellular Vesicles Inhibited Pathogenic Bacteria in Striped Beakfish (Oplegnathus fasciatus). Fermentation 2024, 10, 49. https://doi.org/10.3390/fermentation10010049
Lee B-H, Hu Y-F, Chu Y-T, Wu Y-S, Hsu W-H, Nan F-H. Lactic Acid Bacteria-Fermented Diet Containing Bacterial Extracellular Vesicles Inhibited Pathogenic Bacteria in Striped Beakfish (Oplegnathus fasciatus). Fermentation. 2024; 10(1):49. https://doi.org/10.3390/fermentation10010049
Chicago/Turabian StyleLee, Bao-Hong, Yeh-Fang Hu, Yu-Ting Chu, Yu-Sheng Wu, Wei-Hsuan Hsu, and Fan-Hua Nan. 2024. "Lactic Acid Bacteria-Fermented Diet Containing Bacterial Extracellular Vesicles Inhibited Pathogenic Bacteria in Striped Beakfish (Oplegnathus fasciatus)" Fermentation 10, no. 1: 49. https://doi.org/10.3390/fermentation10010049
APA StyleLee, B.-H., Hu, Y.-F., Chu, Y.-T., Wu, Y.-S., Hsu, W.-H., & Nan, F.-H. (2024). Lactic Acid Bacteria-Fermented Diet Containing Bacterial Extracellular Vesicles Inhibited Pathogenic Bacteria in Striped Beakfish (Oplegnathus fasciatus). Fermentation, 10(1), 49. https://doi.org/10.3390/fermentation10010049








