Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Bacterial Strain and Culture Conditions
- Natural fermentation with the olives’ endogenous microflora (control)
- Fermentation with the addition of Lacticaseibacillus rhamnosus GG ATCC53103 starter culture
- Fermentation with the addition of Levilactobacillus brevis ATCC8287 starter culture
- Fermentation with the addition of Lactiplantibacillus plantarum ATCC14917 starter culture
2.3. Microbiological Analysis
2.4. pH and Salt Measurement
2.5. Instrumentation and Analytical Conditions
2.5.1. Chromatographic Analysis (HPLC-UV/DAD)
2.5.2. 16S rRNA Sequencing with Nanopore MinION™
2.6. Statistics and Multivariate Analysis
2.7. Sensory Evaluation
3. Results and Discussion
3.1. Microbial and Physicochemical Quality of Fermented Table Olives
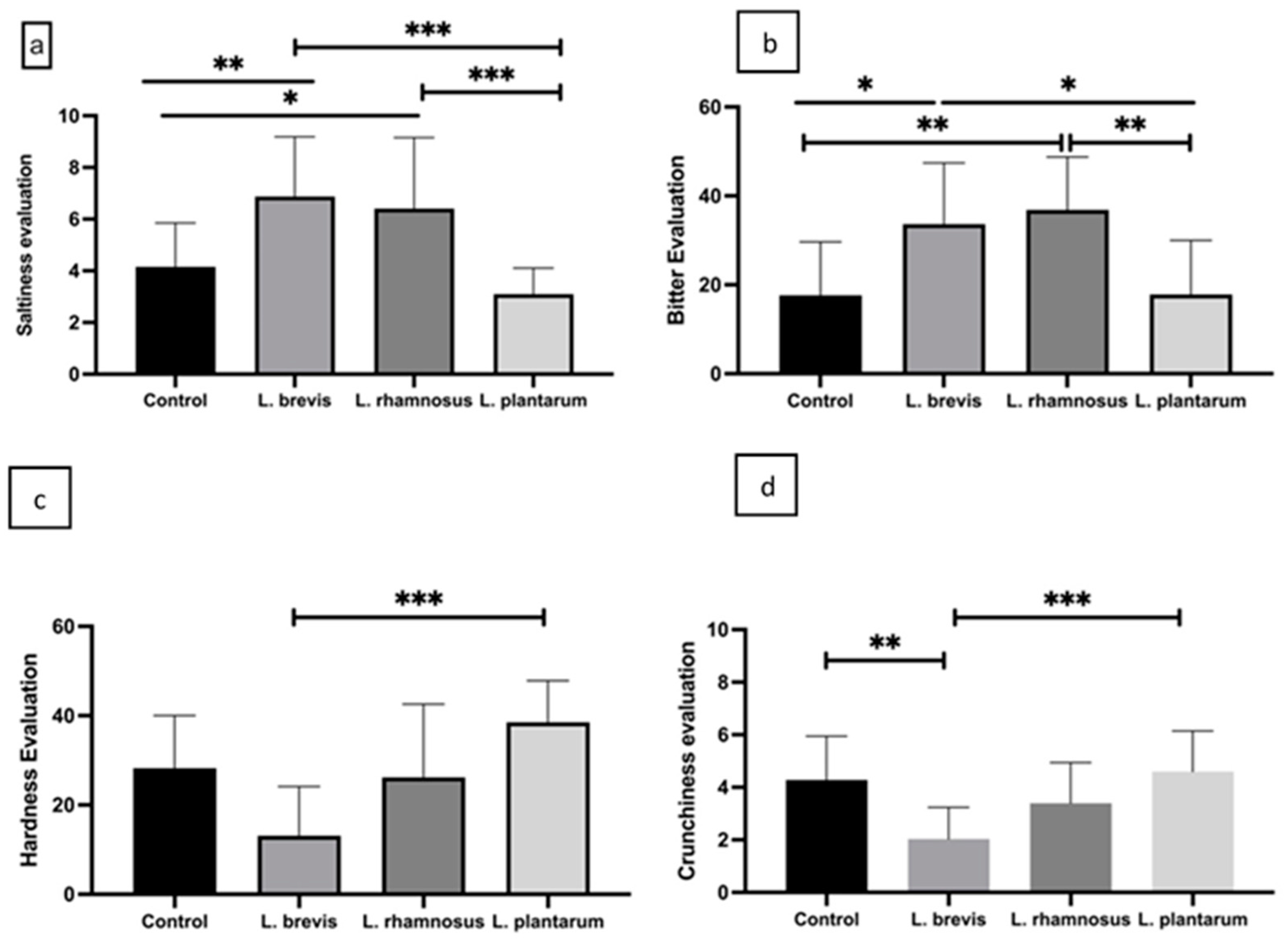
3.2. Sensory Evaluation of Fermented Kalamata Table Olives
3.3. Phenolic Content
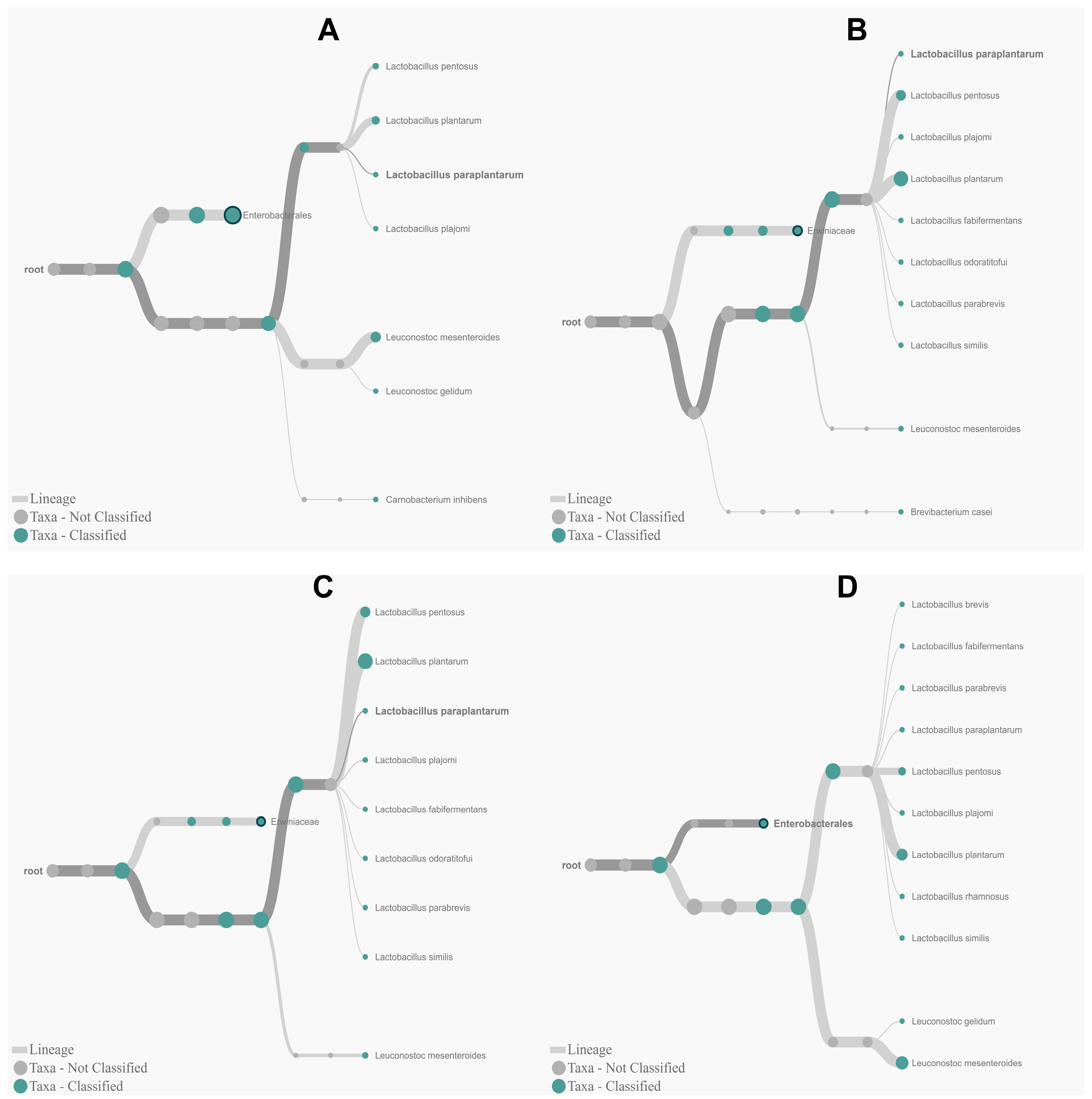
3.4. Determination of Olive Microbiota Using 16S rRNA Sequencing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Phenolic Compounds in Olives. Analyst 1998, 123, 31–44. [Google Scholar] [CrossRef]
- DOEPEL, Interprofessional Association for Table Olives. 2020. Available online: https://olivetreeroute.gr/wp-content/uploads/Studies_Publications_017a.pdf (accessed on 25 November 2023).
- Kazou, M.; Tzamourani, A.; Panagou, E.Z.; Tsakalidou, E. Unraveling the Microbiota of Natural Black Cv. Kalamata Fermented Olives through 16S and ITS Metataxonomic Analysis. Microorganisms 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, M.; Paramithiotis, S.; Panagou, E.Z. Evolution of yeast consortia du ring the fermentation of Kalamata natural black olives upon two initial acidification treatments. Front. Microbiol. 2018, 8, 2673. [Google Scholar] [CrossRef]
- Grounta, A.; Doulgeraki, A.I.; Nychas, G.J.E.; Panagou, E.Z. Biofilm formation on Conservolea natural black olives during single and combined inoculation with a functional Lactobacillus pentosus starter culture. Food Microbiol. 2016, 56, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Grounta, A.; Tassou, C.C.; Panagou, E.Z. Greek-style table olives and their functional value. In Olives and Olive Oil as Functional Foods; Kiritsakis, A., Shahidi, F., Eds.; Wiley: Oxford, UK, 2017; pp. 325–342. [Google Scholar]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of Starter Cultures to Table Olive Fermentation: An Overview on the Experimental Studies. Front. Microbiol. 2012, 3, 248. [Google Scholar] [CrossRef]
- Argyri, K.; Doulgeraki, A.I.; Manthou, E.; Grounta, A.; Argyri, A.A.; Nychas, G.J.E.; Tassou, C.C. Microbial Diversity of Fermented Greek Table Olives of Halkidiki and Konservolia Varieties from Different Regions as Revealed by Metagenomic Analysis. Microorganisms 2020, 8, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sisto, A.; Lavermicocca, P. Suitability of a Lactobacillus paracasei strain as a starter culture in olive fermentation and development of the innovative patented product “probiotic table olives”. Front. Microbiol. 2012, 3, 174. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A Review on Adventitious Lactic Acid Bacteria from Table Olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Argyri, A.A.; Nisiotou, A.A.; Mallouchos, A.; Panagou, E.Z.; Tassou, C.C. Performance of two potential probiotic Lactobacillus strains from the olive microbiota as starters in the fermentation of heat shocked green olives. Int. J. Food Microbiol. 2014, 171, 68–76. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Panagou, E.Z.; Tassou, C.C.; Katsaboxakis, K.Z. Induced lactic acid fermentation of untreated green olives of the Conservolea cultivar by Lactobacillus pentosus. J. Sci. Food Agric. 2003, 83, 667–674. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, A.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- De Bellis, P.; Valerio, F.; Sisto, A.; Lonigro, S.L.; Lavermicocca, P. Probiotic table olives: Microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int. J. Food Microbiol. 2010, 140, 6–13. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Azizi, F.; Habibi Najafi, M.B.; Edalatian Dovom, M.R. The Biodiversity of Lactobacillus Spp. from Iranian Raw Milk Motal Cheese and Antibacterial Evaluation Based on Bacteriocin-Encoding Genes. AMB Express 2017, 7, 176. [Google Scholar] [CrossRef]
- Blana, V.A.; Polymeneas, N.; Tassou, C.C.; Panagou, E.Z. Survival of potential probiotic lactic acid bacteria on fermented green table olives during packaging in polyethylene pouches at 4 and 20 °C. Food Microbiol. 2016, 53, 71–75. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Hondrodimou, O.; Iliopoulos, V.; Panagou, E.Z. Lactic acid bacteria and yeast heterogeneity during aerobic and modified atmosphere packaging storage of natural black Conservolea olives in polyethylene pouches. Food Control 2012, 26, 49–57. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Tsironi, T.; Tsantes, A.G.; Tsakali, E.; Van Impe, J.F.M.; Houhoula, D. Probiotic Properties and Antioxidant Activity In Vitro of Lactic Acid Bacteria. Microorganisms 2023, 11, 1264. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Tsironi, T.; Papaparaskevas, J.; Halvatsiotis, P.; Houhoula, D. Characterization of Lacticaseibacillus rhamnosus, Levilactobacillus brevis and Lactiplantibacillus plantarum Metabolites and Evaluation of Their Antimicrobial Activity against Food Pathogens. Appl. Sci. 2022, 12, 660. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Loka, K.; Tsakni, A.; Houhoula, D. Characterization of Metabolites Production by Lactobacillus gasseri ATCC 33323 and Antioxidant Activity. Nutr. Food Sci. Int. J. 2022, 11, 555815. [Google Scholar] [CrossRef]
- Medina, E.; Ruiz-Bellido, M.A.; Romero-Gil, V.; Rodríguez-Gómez, F.; Montes-Borrego, M.; Landa, B.B.; Arroyo-López, F.N. Assessment of the bacterial community in directly brined Aloreña de Málaga table olive fermentations by metagenetic analysis. Int. J. Food Microbiol. 2016, 236, 47–55. [Google Scholar] [CrossRef]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure–activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- De Castro, A.; Sánchez, A.; López-López, A.; Cortés-Delgado, A.; Medina, E.; Montaño, A. Microbiota and Metabolite Profiling of Spoiled Spanish-Style Green Table Olives. Metabolites 2018, 8, 73. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Todaro, A.; Pino, A.; Pitino, I.; Corona, O.; Caggia, C. Microbiota and metabolome during controlled and spontaneous fermentation of Nocellara Etnea table olives. Food Microbiol. 2017, 65, 136–148. [Google Scholar] [CrossRef]
- Cocolin, L.; Ercolini, D. Zooming into food-associated microbial consortia: A ‘cultural’ evolution. Curr. Opin. Food Sci. 2015, 2, 43–50. [Google Scholar] [CrossRef]
- Lucena-Padrós, H.; Caballero-Guerrero, B.; Maldonado-Barragán, A.; Ruiz-Barba, J.L. Microbial diversity and dynamics of Spanish-style green table-olive fermentations in large manufacturing companies through culture-dependent techniques. Food Microbiol. 2014, 42, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Padrós, H.; Ruiz-Barba, J.L. Microbial biogeography of Spanish-style green olive fermentations in the province of Seville, Spain. Food Microbiol. 2019, 82, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Padrós, H.; Jiménez, E.; Maldonado-Barragán, A.; Rodríguez, J.M.; Ruiz-Barba, J.L. PCR-DGGE assessment of the bacterial diversity in Spanish-style green table-olive fermentations. Int. J. Food Microbiol. 2015, 205, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, C.L.; Russo, N.; Pino, A.; Mazzaglia, A.; Ferrante, M.; Conti, G.O.; Caggia, C. Effects of selected bacterial cultures on safety and sensory traits of Nocellara Etnea olives produced at large factory scale. Food Chem. Toxicol. 2018, 115, 491–498. [Google Scholar] [CrossRef]
- Ghabbour, N.; Lamzira, Z.; Thonart, P.; Cidalia, P.; Markaoui, M.; Asehraou, A. Selection of oleuropein-degrading lactic acid bacteria strains isolated from fermenting Moroccan green olives. Grasas Aceites 2011, 62, 84–89. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Restuccia, C.; Romano, A.D.; Caggia, C. Lactobacillus casei, dominant species in naturally fermented Sicilian green olives. Int. J. Food Microbiol. 2004, 90, 9–14. [Google Scholar] [CrossRef]

| Name of Standard | Class | Control (ppm) | Lactiplantibacillus plantarum (ppm) | Lacticaseibacillus rhamnosus (ppm) | Levilactobacillus brevis (ppm) |
|---|---|---|---|---|---|
| (A) 4-Hydroxybenzoic acid | Phenolic acid | - | - | - | - |
| (Β) DL-p-Hydroxyphenyllactic acid | Phenolic acid | 573.5 | 1194.7 | 761.7 | 426.8 |
| (C) Phenyllactic acid | Phenolic acid | - | - | - | - |
| (D) 3-(4-Hydroxyphenyl) propionic acid | Phenolic acid | - | - | - | - |
| (E) Hydrocinnamic acid | Phenolic acid | - | - | - | - |
| (F) Methylcinnamic acid | Phenolic acid | 182.7 | 288.43 | 350.4 | 316.6 |
| (G) Salicylic acid | Phenolic acid | 35.9 | 46.3 | 65.0 | 58.31 |
| (H) 1,2-Dihydroxybenzene | Benzenediols | 20.2 | 19.6 | 19.5 | 20.8 |
| (I) 3,4-Dihydrocinnamic acid | Phenolic acid | - | - | - | - |
| (J) Vanillic acid | Μonohydroxy-benzoic acid | - | 18.8 | - | 20.0 |
| (K) 3,4-Dihydroxyhydrocinnamic acid | Phenolic acid | - | - | - | - |
| (L) Ferulic acid | Phenolic acid | 27.2 | 68.7 | 69.4 | 66.6 |
| (M) Benzoic acid | Phenolic acid | 63.5 | 150.6 | 146.5 | 145.5 |
| (O) 4-Hydrocinnamic acid | Phenolic acid | - | 82.2 | - | - |
| Taxon | MinION Reads Total |
|---|---|
| Lactiplantibacillus plantarum | 42,451 |
| Lactiplantibacillus pentosus | 18,122 |
| Lactiplantibacillus plajomi | 921 |
| Lactiplantibacillus paraplantarum | 817 |
| Lacticaseibacillus rhamnosus | 559 |
| Lactiplantibacillus fabifermentans | 284 |
| Levilactobacillus brevis | 190 |
| Levilactobacillus parabrevis | 147 |
| Latilactobacillus graminis | 105 |
| Secundilactobacillus odoratitofui DSM 19909 = JCM 15043 | 99 |
| Fructilactobacillus fructivorans | 8 |
| Lactiplantibacillus modestisalitolerans | 72 |
| Lacticaseibacillus paracasei | 71 |
| Paucilactobacillus suebicus | 71 |
| Secundilactobacillus paracollinoides | 69 |
| Companilactobacillus tucceti | 64 |
| Lactiplantibacillus argentoratensis | 57 |
| Lentilactobacillus rapi | 54 |
| Levilactobacillus hammesii | 51 |
| Taxon | MinION Reads Total |
|---|---|
| Lactiplantibacillus plantarum | 45,886 |
| Lactiplantibacillus pentosus | 23,335 |
| Lactiplantibacillus plajomi | 878 |
| Lactiplantibacillus paraplantarum | 593 |
| Lactiplantibacillus fabifermentans | 283 |
| Levilactobacillus parabrevis | 178 |
| Levilactobacillus fructivorans | 96 |
| Lactobacillus plantarum subsp. Argentoratensis | 93 |
| Levilactobacillus koreensis JCM 16448 | 92 |
| Secundilactobacillus paracollinoides | 91 |
| Paucilactobacillus suebicus | 87 |
| Companilactobacillus versmoldensis | 67 |
| Lactiplantibacillus modestisalitolerans | 60 |
| Latilactobacillus graminis | 57 |
| Lentilactobacillus rapi | 49 |
| Companilactobacillus tucceti | 48 |
| Paucilactobacillus vaccinostercus | 39 |
| Secundilactobacillus mixtipabuli | 37 |
| Taxon | MinION Reads Total |
|---|---|
| Lactiplantibacillus plantarum | 68,410 |
| Lactiplantibacillus pentosus | 34,893 |
| Lactiplantibacillus plajomi | 1,291 |
| Lactiplantibacillus paraplantarum | 908 |
| Lactiplantibacillus fabifermentans | 385 |
| Levilactobacillus parabrevis | 292 |
| Lactobacillus plantarum subsp. Argentoratensis | 155 |
| Levilactobacillus fructivorans | 134 |
| Secundilactobacillus paracollinoides | 131 |
| Paucilactobacillus suebicus | 131 |
| Levilactobacillus koreensis JCM 16448 | 130 |
| Lactiplantibacillus modestisalitolerans | 86 |
| Latilactobacillus graminis | 83 |
| Companilactobacillus versmoldensis | 73 |
| Lentilactobacillus rapi | 68 |
| Companilactobacillus tucceti | 58 |
| Companilactobacillus musae | 52 |
| Companilactobacillus furfuricola | 51 |
| Taxon | MinION Reads Total |
|---|---|
| Lactiplantibacillus plantarum | 45,218 |
| Lactiplantibacillus pentosus | 16,695 |
| Lactiplantibacillus plajomi | 744 |
| Lactiplantibacillus paraplantarum | 693 |
| Lactiplantibacillus fabifermentans | 294 |
| Levilactobacillus parabrevis | 141 |
| Lactobacillus plantarum subsp. argentoratensis | 111 |
| Latilactobacillus graminis | 94 |
| Levilactobacillus koreensis JCM 16448 | 85 |
| Paucilactobacillus suebicus | 85 |
| Fructilactobacillus fructivorans | 74 |
| Secundilactobacillus paracollinoides | 72 |
| Lentilactobacillus rapi | 61 |
| Lactiplantibacillus modestisalitolerans | 56 |
| Paucilactobacillus vaccinostercus | 54 |
| Companilactobacillus tucceti | 45 |
| Companilactobacillus versmoldensis | 35 |
| Lactiplantibacillus xiangfangensis | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vougiouklaki, D.; Letsiou, S.; Mavrokefalidou, I.; Tsakali, E.; Akkermans, S.; Van Impe, J.F.M.; Houhoula, D. Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters. Fermentation 2024, 10, 53. https://doi.org/10.3390/fermentation10010053
Vougiouklaki D, Letsiou S, Mavrokefalidou I, Tsakali E, Akkermans S, Van Impe JFM, Houhoula D. Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters. Fermentation. 2024; 10(1):53. https://doi.org/10.3390/fermentation10010053
Chicago/Turabian StyleVougiouklaki, Despina, Sophia Letsiou, Iliana Mavrokefalidou, Efstathia Tsakali, Simen Akkermans, Jan F. M. Van Impe, and Dimitra Houhoula. 2024. "Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters" Fermentation 10, no. 1: 53. https://doi.org/10.3390/fermentation10010053
APA StyleVougiouklaki, D., Letsiou, S., Mavrokefalidou, I., Tsakali, E., Akkermans, S., Van Impe, J. F. M., & Houhoula, D. (2024). Fermentation of Kalamata Natural Black Olives Using Selected Lactic Acid Bacteria as Starters. Fermentation, 10(1), 53. https://doi.org/10.3390/fermentation10010053







