Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Culture Medium and Conditions
2.3. Protein Expression and Purification
2.4. Homology Modeling and Molecular Docking
2.5. Site-Directed Saturation Mutagenesis
2.6. Enzyme Assay
2.7. Analytical Methods
3. Results and Discussion
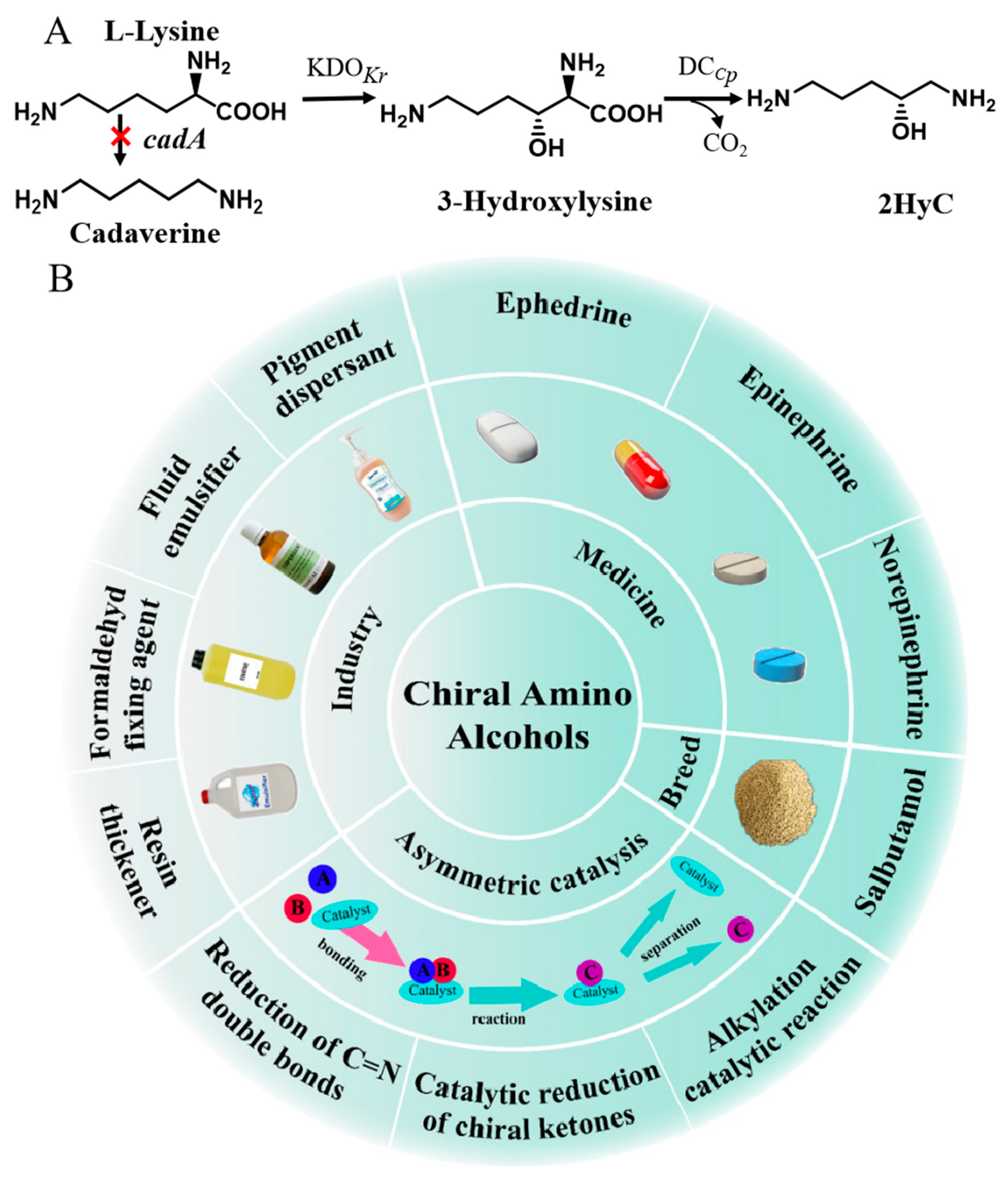
3.1. Construction of a Heterogeneous Pathway for 2HyC Production in Engineered E. coli ML101
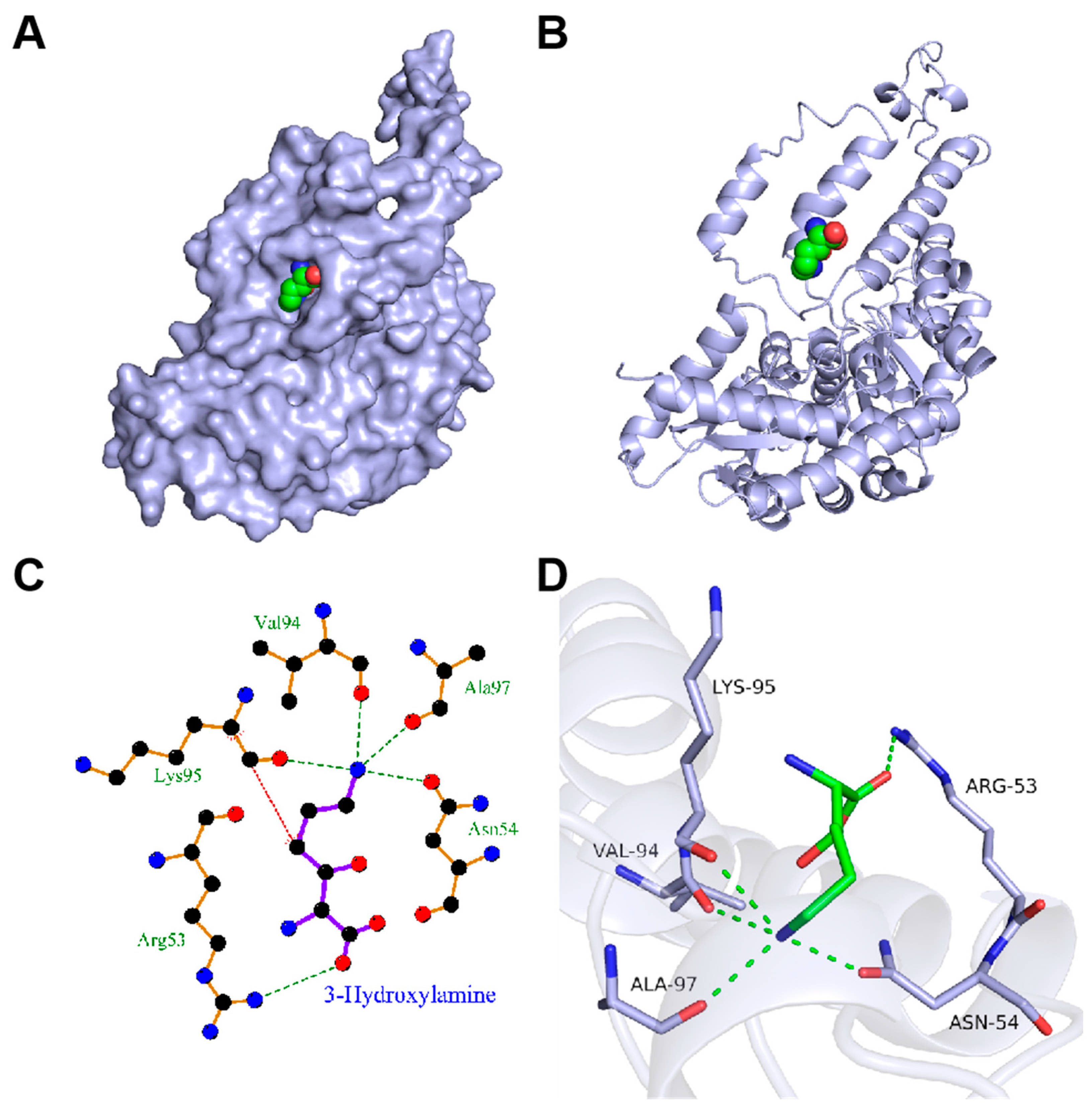
3.2. Molecular Docking Studies
3.3. Iterative Saturation Mutagenesis of DCCp to Improve the Catalytic Activity toward 3-Hydroxylysine
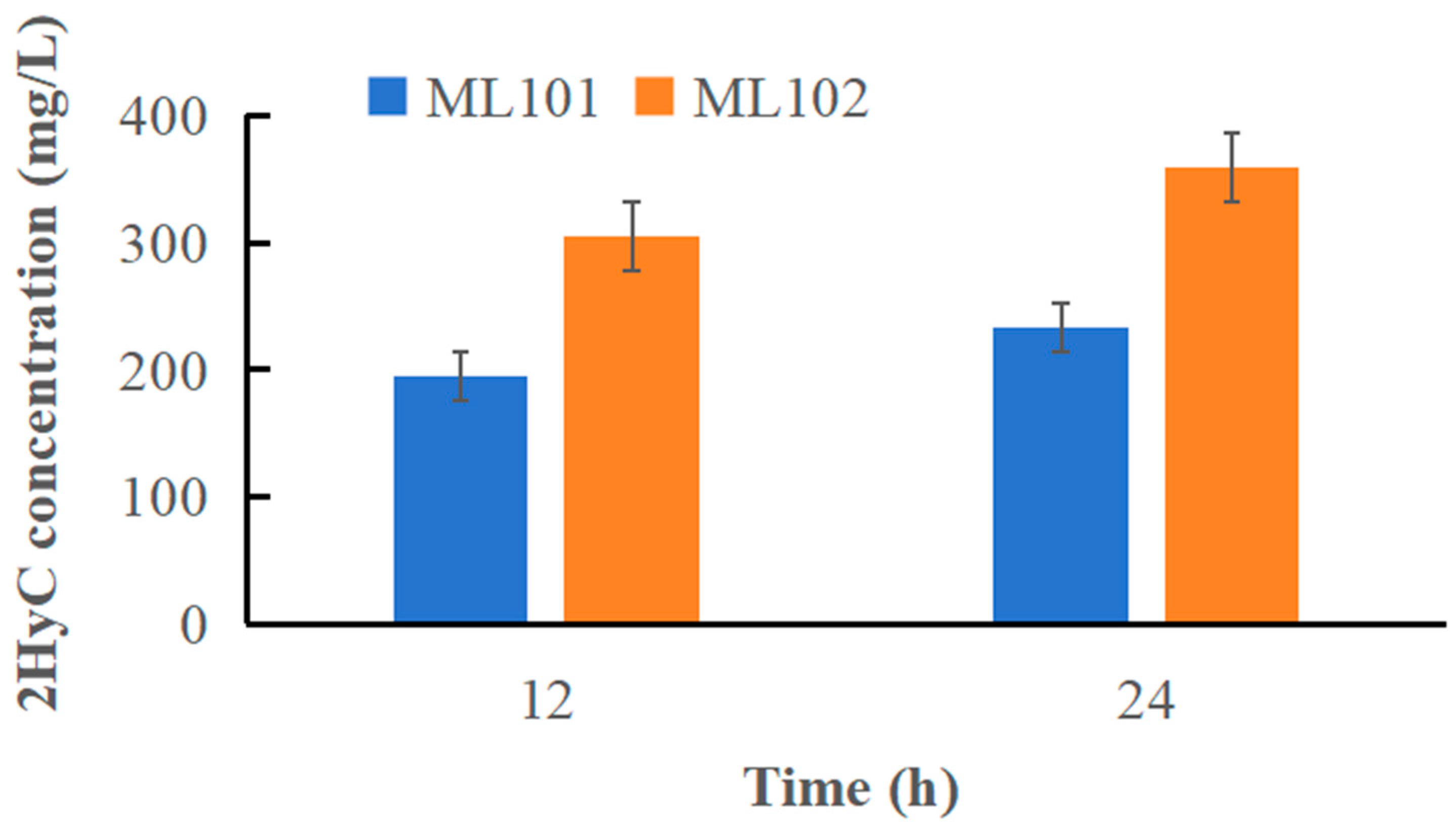
3.4. Metabolic Engineering of E. coli ML102 for 2HyC Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Chen, X.; Jin, X.; Gu, F.; Jiang, W.; Qi, Q.; Liang, Q. Creating Polyploid Escherichia Coli and Its Application in Efficient L-Threonine Production. Adv. Sci. 2023, 10, e2302417. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, F.; Rafatiyan, S.; Abbaspour Motlagh Moghaddam, M.H.; Atkinson, E.; Ledesma-Amaro, R. Applications of synthetic yeast consortia for the production of native and non-native chemicals. Crit. Rev. Biotechnol. 2022, 44, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, Y.; Mi, L.; Chen, W.; Wang, D.; Wang, Q. An economically and environmentally acceptable synthesis of chiral drug intermediate L-pipecolic acid from biomass-derived lysine via artificially engineered microbes. J. Ind. Microbiol. Biotechnol. 2018, 45, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Chen, J.; Qin, Z.; Yu, S.; Zhou, J. Coordinating caffeic acid and salvianic acid A pathways for efficient production of rosmarinic acid in Escherichia coli. Metab. Eng. 2023, 76, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Wang, X.; Gao, S.; Li, D.; Zhou, J. Production of Natural Pigments Using Microorganisms. J. Agric. Food Chem. 2023, 71, 9243–9254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, N.; Yu, S.; Zhou, J. Enhancing caffeic acid production in Escherichia coli by engineering the biosynthesis pathway and transporter. Bioresour. Technol. 2023, 368, 128320. [Google Scholar] [CrossRef]
- Jungmann, L.; Hoffmann, S.L.; Lang, C.; De Agazio, R.; Becker, J.; Kohlstedt, M.; Wittmann, C. High-efficiency production of 5-hydroxyectoine using metabolically engineered Corynebacterium glutamicum. Microb. Cell Factories 2022, 21, 274. [Google Scholar] [CrossRef]
- Yu, S.; Shan, X.; Lyv, Y.; Zhou, J. Bioproduction of quercetin using recombinant thermostable glycosidases from Dictyoglomus thermophilum. Bioresour. Bioprocess. 2022, 9, 48. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Qin, Z.; Yu, S.; Chen, J.; Zhou, J. Combined engineering of l-sorbose dehydrogenase and fermentation optimization to increase 2-keto-l-gulonic acid production in Escherichia coli. Bioresour. Technol. 2023, 372, 128672. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Z.; Su, E.; Wang, L.; Sun, L.; Lei, P.; Xu, H.; Li, S. Recent Strategies for the Biosynthesis of Ergothioneine. J. Agric. Food Chem. 2021, 69, 13682–13690. [Google Scholar] [CrossRef]
- Luckie, B.A.; Kashyap, M.; Pearson, A.N.; Chen, Y.; Liu, Y.; Valencia, L.E.; Romero, A.C.; Hudson, G.A.; Tao, X.B.; Wu, B.; et al. Development of Corynebacterium glutamicum as a monoterpene production platform. Metab. Eng. 2023, 81, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, B.; Chen, W.; Ye, K.; Shen, J.; Liu, P.; Wu, J.; Wang, H.; Chu, X. Highly efficient production of ectoine via an optimized combination of precursor metabolic modules in Escherichia coli BL21. Bioresour. Technol. 2023, 390, 129803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.; Guo, X.; Guo, S.; Kang, Z.; Xiao, D.; Yan, J.; Tao, F.; Zhang, W.; Dong, W.; et al. Increased glutarate production by blocking the glutaryl-CoA dehydrogenation pathway and a catabolic pathway involving L-2-hydroxyglutarate. Nat. Commun. 2018, 9, 2114. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, F.; Blenner, M.; Ledesma-Amaro, R. Editorial: Synthetic Biology of Yeasts for the Production of Non-Native Chemicals. Front. Bioeng. Biotechnol. 2021, 9, 730047. [Google Scholar] [CrossRef]
- Wu, L.; An, J.; Jing, X.; Chen, C.-C.; Dai, L.; Xu, Y.; Liu, W.; Guo, R.-T.; Nie, Y. Molecular Insights into the Regioselectivity of the Fe(II)/2-Ketoglutarate-Dependent Dioxygenase-Catalyzed C–H Hydroxylation of Amino Acids. ACS Catal. 2022, 12, 11586–11596. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Su, W.; Zhang, H.; Xu, W.; Chu, X. Metabolic engineering strategy for synthetizing trans-4-hydroxy-L-proline in microorganisms. Microb. Cell Factories 2021, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.A.; Deive, F.J.; Alvarez, M.S.; Longo, M.A.; Rodriguez, A.; Bernal, C.; Martinez, R. Effect of process parameters and surfactant additives on the obtained activity of recombinant tryptophan hydroxylase (TPH1) for enzymatic synthesis of 5-hydroxytryptophan (5-HTP). Enzym. Microb. Technol. 2022, 154, 109975. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Z.; Wang, B.; Lu, Y.; Yan, L.; Zhao, Z.; Bai, T.; Zhang, J.; Li, H.; Wang, W.; et al. An Artificial Pathway for N-Hydroxy-Pipecolic Acid Production From L-Lysine in Escherichia coli. Front. Microbiol. 2022, 13, 842804. [Google Scholar] [CrossRef]
- Kawahara, T.; Itoh, M.; Kozone, I.; Izumikawa, M.; Sakata, N.; Tsuchida, T.; Shin-Ya, K. MBJ-0110, a novel cyclopeptide isolated from the fungus Penicillium sp. f25267. J. Antibiot. 2016, 69, 66–68. [Google Scholar] [CrossRef]
- Wang, B.X.; Xiao, S.J.; Zhao, X.T.; Zhao, L.M.; Zhang, Y.; Cheng, J.; Zhang, J.M. Recent Advances in the Hydroxylation of Amino Acids and Its Derivatives. Fermentation 2023, 9, 285. [Google Scholar] [CrossRef]
- Baud, D.; Peruch, O.; Saaidi, P.-L.; Fossey, A.; Mariage, A.; Petit, J.-L.; Salanoubat, M.; Vergne-Vaxelaire, C.; de Berardinis, V.; Zaparucha, A. Biocatalytic Approaches towards the Synthesis of Chiral Amino Alcohols from Lysine: Cascade Reactions Combining alpha-Keto Acid Oxygenase Hydroxylation with Pyridoxal Phosphate- Dependent Decarboxylation. Adv. Synth. Catal. 2017, 359, 1563–1569. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, M.; Song, Z.; Li, C.; Wang, Y.; Zhu, Z.; Sun, D.; Lu, F.; Qin, H.-M. Reshaping the Binding Pocket of Lysine Hydroxylase for Enhanced Activity. ACS Catal. 2020, 10, 13946–13956. [Google Scholar] [CrossRef]
- Zhang, X.; King-Smith, E.; Renata, H. Total Synthesis of Tambromycin by Combining Chemocatalytic and Biocatalytic C-H Functionalization. Angew. Chem. 2018, 57, 5037–5041. [Google Scholar] [CrossRef] [PubMed]
- Amatuni, A.; Renata, H. Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential. Org. Biomol. Chem. 2019, 17, 1736–1739. [Google Scholar] [CrossRef]
- Seide, S.; Arnold, L.; Wetzels, S.; Bregu, M.; Gätgens, J.; Pohl, M. From Enzyme to Preparative Cascade Reactions with Immobilized Enzymes: Tuning Fe(II)/α-Ketoglutarate-Dependent Lysine Hydroxylases for Application in Biotransformations. Catalysts 2022, 12, 354. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, A.; Hu, S.; Chen, K.; Ouyang, P. Efficient and scalable synthesis of 1,5-diamino-2-hydroxy-pentane from L-lysine via cascade catalysis using engineered Escherichia coli. Microb. Cell Factories 2022, 21, 142. [Google Scholar] [CrossRef]
- Zhang, J.D.; Zhao, J.W.; Gao, L.L.; Chang, H.H.; Wei, W.L.; Xu, J.H. Enantioselective synthesis of enantiopure beta-amino alcohols via kinetic resolution and asymmetric reductive amination by a robust transaminase from Mycobacterium vanbaalenii. J. Biotechnol. 2019, 290, 24–32. [Google Scholar] [CrossRef]
- Fossey-Jouenne, A.; Vergne-Vaxelaire, C.; Zaparucha, A. Enzymatic Cascade Reactions for the Synthesis of Chiral Amino Alcohols from L-lysine. J. Vis. Exp. JoVE 2018, 132, e56926. [Google Scholar] [CrossRef]
- Steinreiber, J.; Schürmann, M.; van Assema, F.; Wolberg, M.; Fesko, K.; Reisinger, C.; Mink, D.; Griengl, H. Synthesis of Aromatic 1,2-Amino Alcohols Utilizing a Bienzymatic Dynamic Kinetic Asymmetric Transformation. Adv. Synth. Catal. 2007, 349, 1379–1386. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bettinger, C.J.; Langer, R.S.; Borenstein, J.T. Biodegradable microfluidic scaffolds for tissue engineering from amino alcohol-based poly(ester amide) elastomers. Organogenesis 2010, 6, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Hryc, C.F.; Baker, M.L. AlphaFold2 and CryoEM: Revisiting CryoEM modeling in near-atomic resolution density maps. iScience 2022, 25, 104496. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, P.; Li, N.; Li, J.; Chen, J.; Zhou, J. Production of 2-keto-L-gulonic acid by metabolically engineered Escherichia coli. Bioresour. Technol. 2020, 318, 124069. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Y.; Tuyishime, P.; Gao, N.; Li, Q.; Zheng, P.; Sun, J.; Ma, Y. Engineering Artificial Fusion Proteins for Enhanced Methanol Bioconversion. ChemBioChem 2018, 19, 2465–2471. [Google Scholar] [CrossRef]
- Annamalai, N.; Veeramuthu Rajeswari, M.; Vijayalakshmi, S.; Balasubramanian, T. Purification and characterization of chitinase from Alcaligenes faecalis AU02 by utilizing marine wastes and its antioxidant activity. Ann. Microbiol. 2011, 61, 801–807. [Google Scholar] [CrossRef]
- Zhang, K.; Sawaya, M.R.; Eisenberg, D.S.; Liao, J.C. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc. Natl. Acad. Sci. USA 2008, 105, 20653–20658. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, H.; Guo, T.; Zhang, Y.; Ma, L. Integrated multi-spectroscopic and molecular modeling techniques to study the formation mechanism of hidden zearalenone in maize. Food Chem. 2021, 351, 129286. [Google Scholar] [CrossRef]
- Prell, C.; Vonderbank, S.-A.; Meyer, F.; Pérez-García, F.; Wendisch, V.F. Metabolic engineering of Corynebacterium glutamicum for de novo production of 3-hydroxycadaverine. Curr. Res. Biotechnol. 2022, 4, 32–46. [Google Scholar] [CrossRef]
- Liu, K.; Yu, H.; Sun, G.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Semi-rational design of L-amino acid deaminase for production of pyruvate and D-alanine by Escherichia coli whole-cell biocatalyst. Amino Acids 2021, 53, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Yin, X.; Zhang, G.; Liu, S.; Zhou, J.; Li, J.; Du, G. Current progress and prospects of enzyme technologies in future foods. Syst. Microbiol. Biomanuf. 2020, 1, 24–32. [Google Scholar] [CrossRef]

| Strains or Plasmids | Relevant Genotype or Description | Source |
|---|---|---|
| Strains | ||
| BL21(DE3) | Wild type | [35] |
| ML03 | BL21(DE3) ΔcadA | [3] |
| ML10 | ML03 harboring pET22b-KDOKr | This study |
| ML101 | ML03 harboring pET22b-KDOKr-DCCp | This study |
| ML102 | ML03 harboring pET22b-KDOKr-DCCp (R53D/V94I) | This study |
| ML11 | ML03 harboring pET22b-DCCp | This study |
| Plasmids | ||
| pET22b-KDOKr | pET22b carries a lysine hydroxylase gene from Kineococcus radiotolerans (KDOKr), AmpR | This study |
| pET22b-DCCp | pET22b carries a pyridoxal phosphate-dependent decarboxylase gene from Chitinophage pinensis (DCCp), AmpR | This study |
| pET22b-KDOKr-DCCp | pET22b carries a lysine hydroxylase gene from Kineococcus radiotolerans (KDOKr), and a pyridoxal phosphate-dependent decarboxylase gene from Chitinophage pinensis (DCCp), AmpR | This study |
| pET22b-KDOKr-DCCp (R53D/V94I) | pET22b carries a lysine hydroxylase from Kineococcus radiotolerans (KDOKr), and a pyridoxal phosphate-dependent decarboxylase (DCCp) mutant (R53D/V94I) from Chitinophage pinensis (DCCp), AmpR | This study |
| Strains | Time (h) | 2 g/L Lysine | 4 g/L Lysine | ||
|---|---|---|---|---|---|
| 3-Hydroxylysine Production (g/L) | 2HyC Production (mg/L) | 3-Hydroxylysine Production (g/L) | 2HyC Production (mg/L) | ||
| ML10 | 12 | 0.64 ± 0.05 | - | 1.15 ± 0.11 | - |
| ML101 | 12 | 0.47 ± 0.05 | 106.18 ± 5.48 | 0.89 ± 0.08 | 195.37 ± 8.95 |
| Enzymes | Km (mM) | Kcat (s−1) | Kcat/Km (mM−1s−1) |
|---|---|---|---|
| DCCp | 20.72 ± 1.24 | 1.86 ± 0.12 | 0.0898 ± 0.0035 |
| DCCp (R53D) | 18.05 ± 1.02 | 2.08 ± 0.15 | 0.1152 ± 0.0058 |
| DCCp (R53E) | 19.21 ± 0.89 | 2.13 ± 0.18 | 0.1109 ± 0.0043 |
| DCCp (V94A) | 18.48 ± 1.07 | 2.24 ± 0.16 | 0.1212 ± 0.0066 |
| DCCp (V94I) | 17.96 ± 1.35 | 2.19 ± 0.13 | 0.1097 ± 0.0074 |
| DCCp (R53E/V94I) | 17.12 ± 1.09 | 2.25 ± 0.19 | 0.1314 ± 0.0063 |
| DCCp (R53D/V94I) | 16.33 ± 1.18 | 2.39 ± 0.17 | 0.1466 ± 0.0067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Xiao, S.; Luo, Q.; Wang, B.; Zeng, R.; Zhao, L.; Zhang, J. Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design. Fermentation 2024, 10, 56. https://doi.org/10.3390/fermentation10010056
Cheng J, Xiao S, Luo Q, Wang B, Zeng R, Zhao L, Zhang J. Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design. Fermentation. 2024; 10(1):56. https://doi.org/10.3390/fermentation10010056
Chicago/Turabian StyleCheng, Jie, Shujian Xiao, Qing Luo, Bangxu Wang, Rumei Zeng, Liming Zhao, and Jiamin Zhang. 2024. "Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design" Fermentation 10, no. 1: 56. https://doi.org/10.3390/fermentation10010056
APA StyleCheng, J., Xiao, S., Luo, Q., Wang, B., Zeng, R., Zhao, L., & Zhang, J. (2024). Engineering an Artificial Pathway to Improve the Bioconversion of Lysine into Chiral Amino Alcohol 2-Hydroxycadaverine Using a Semi-Rational Design. Fermentation, 10(1), 56. https://doi.org/10.3390/fermentation10010056






