Abstract
Non-conventional yeasts (NCYs) (i.e., non-Saccharomyces) are used as alternative starters to promote aroma complexity of fermented foods (e.g., bakery products). A total of 66 yeasts isolated from artisanal food matrices (bread and pizza sourdoughs and milk whey) from different geographical areas of the Campania region (Italy) were screened for physiological and technological characteristics such as leavening ability, resistance to NaCl and pH, exopolysaccharide and phytase activity production, and carbohydrate assimilation. Selected and isolated microorganisms were also used to study the leavening kinetics in experimental doughs as mixed inocula of two different strains. Volatile organic compounds (VOCs) of the inoculated doughs were analyzed with solid-phase microextraction/gas chromatography–mass spectrometry (SPME/GC-MS). Most of the strains belonged to non-Saccharomyces species (Pichia kudriavzevii, Kluyveromyces marxianus) and Saccharomyces (S. cerevisiae). Several strains produced exopolysaccharides (EPSs), that are important for dough rheological properties. Moreover, yeasts isolated from whey showed extracellular phytase activity. The mixed starter culture of the S. cerevisiae and NCY strains showed a synergic effect that enhanced the doughs’ aroma complexity. The use of non-conventional yeasts mixed with S. cerevisiae strains can be advantageous in the bakery industry because they improve the bread aroma profiles and nutritional properties by bioactive molecule production.
1. Introduction
Bread represents the most important staple food, with origins dating back to about 23,000 years ago during the Paleolithic Period [1,2], when the long history of bread-making development started. Centuries ago, leavened bread was exclusively produced through the spontaneous fermentation of cereal flour by the autochthonous microbial communities of yeasts and homo- and hetero-fermentative lactic acid bacteria (sourdough microbiota), whose diversity depends on the flour type, water, and dough-processing environment [3]. Since the late 19th century, despite the advantages of sourdough fermentation, including improvements of bread texture, flavor, leavening capacity, shelf life, and nutritional properties [4], the more widespread bread-making process, especially in mechanized industrial bakeries, has relied on controlled processes through the utilization of pure yeast cultures. The conventional baker’s yeast has exclusively been represented by S. cerevisiae, an Ascomycota, characterized by easy marketability and manipulation and exhibiting excellent leavening performances in a relatively short fermentation time. In recent studies, the awareness that production of carbon dioxide and ethanol from the flour’s simple sugars (leavening capacity) is not the only important function of a baker’s yeast has been increasingly established [5,6]. Generating the aromatic profile and the release of bioactive compounds such as vitamins, antioxidant compounds, peptides, and amino acids are important roles of yeasts’ microflora in the determination of baked products sensorial and nutritional properties. Moreover, the capability of metabolizing the wide array of sugars (mono-, oligo-, and polysaccharides) contained in flour and resistance to several stressful conditions of the baking process (high temperatures, presence of salt, air drying, osmotic and ethanol stress) are required from a bakery yeast. Despite its longstanding utilization, the main drawbacks linked to the utilization of S. cerevisiae, such as a flat aroma complexity, too basic range of sugar utilization, and vulnerability to baking stress conditions, led to overcoming its monopoly in bread-making processes.
The current market interest in leavened baked products with novel and more interesting aroma and sensory profiles obtained through natural ingredients has increasingly prompted the exploration of non-conventional yeast diversity. NCYs of complex aroma profile-generation capacity are widely appreciated and exploited to overcome flavor flattening in several fermented foods and beverages. Novel starters based on these microorganisms can also confer original and distinctive traits to final products that make them attractive [7]. A relevant leavening potential is emerging among the alternative yeasts that are often Crabtree-positive, capable of fermenting several sugars, including oligo- and polysaccharides, and tolerant to stress conditions associated with the baking process.
The increasing number of studies on the relationship between bread flavor and microbial microflora reveals that different yeast species have a more significant impact on bread’s aromatic profile than different types of wheat flour [8]. The role of yeasts is of paramount importance in determining bread taste and aroma complexity, principally of bread crumb, and indirectly of crust to a limited extent; then, there are the types and amounts of VOCs derived from aroma precursors synthesized by yeast during dough leavening [9,10]. People’s acceptance of strains is hugely important for their utilization in the food sector. Still, the improvement of bakery traits of conventional strains by genetic modification is perceived negatively by consumers. One of the possible strategies that can fulfill the market’s demands for natural bakery products with distinctive tastes and aromas is the selection of non-conventional yeasts from natural environments [11].
Currently, the number of non-conventional yeasts with good performance in bread-making processes still needs to be increased, and due to the strict adaptation to their habitat conditions, several efforts are required to find strains able to survive and have desirable performances in stressful baking conditions. Moreover, due to their recently emerged exploitation, adequate knowledge about safety aspects and the lack of virulence effects are among the major challenges related to the novel strains’ application in modern bakeries. Designation of GRAS status will be the important target for each strain in the near future. However, NCYs’ isolation from traditional fermented foods is already an appreciable guarantee for their safety. Fortunately, the increasing availability of genome sequences in specialized databases will significantly improve the safe application of non-conventional yeasts by identifying genes responsible for producing undesirable toxic compounds for human health. Knowledge at the genomic level will also be beneficial for an in-depth understanding of their physiological mechanisms. It would allow for the development of better technological strategies to promote their adaptation and performance in the stressful bakery environment.
Several fermented products or their by-products have been utilized to isolate non-conventional yeasts. Different strains were isolated depending on the raw materials used as sources and their geographical origins. Due to their fermentative capacity and aroma generation, Geotrichium lactis, Saccharomyces ellipsoideus, Debariomyces hansenii, Zygosaccharomyces balii, and several Candida spp. isolated from fermented cassava revealed their potentiality as bakery yeasts. Moreover, other yeasts of the Zygosaccharomyces, Debariomyces, and Geotrichium genera, together with strains of S. cerevisiae and Schizosaccharomyces pombe isolated from Nigerian palm wine, were recognized as interesting baking features [12]. Also, the utilization of wine yeasts in bread making is very attractive due to their aroma profiles, which are more interesting than those produced by baker’s strains [13]. In this regard, Aslankoohi et al. [5] showed that the aroma profile of bread obtained with a strain of Saccharomyces bayanus, isolated from Champagne, was significantly different with respect to the control, offering a pleasant fruity flavor. Six volatile compounds, including phenylacetaldehyde and 2-phenylethanol, found in bread crumbs, were responsible for distinctive aroma.
Other non-Saccharomyces yeasts have been isolated from sourdough and doughs; despite these starters containing no more than one or two strains, a wide yeast diversity has been demonstrated among different preparations [14,15]. In addition to Saccharomyces cerevisiae strains, non-conventional yeasts such as Candida humilis, Torulaspora delbrueckii, Pichia kudriavzevii, Kazachstania gamospora, Wickerhamomyces subpelliculosus, and Candida glabrata were also retrieved [16,17]. Nevertheless, scientific attention has been so far focused mainly on lactic acid bacteria (LAB); due to their numerical prevalence, yeasts play a fundamental role in the mature starter. Sourdough yeasts can survive and maintain an excellent leavening capacity in the stressful environment that they share with LAB, characterized by low pH and oxygen concentration, high sugar content, and they are responsible for the production of bioactive molecules and enzymes. Moreover, the aroma profiles of sourdough products, different than those of straight bread and previously attributed to LAB, are now recognized to be mainly due to yeast metabolism. Among several examples, the unique aroma profile of bread fermented with a S. cerevisiae strain from an Australian sourdough was reported, which was completely different from that of the control made with commercial baker’s yeast [18].
Residues from fermented food manufacturing activities are additional sources of non-conventional yeast diversity. Whey, obtained from milk after the coagulation of high-molecular-weight proteins, like casein, in cheese curd production [19], is characterized by a complex microbiota. LAB are the main components but non-conventional yeasts, such as Kluyveromyces marxianus, Wickerhamiella pararugosa, Torulaspora delbrueckii [20], Candida tropicalis, Trichosporon beigelii, Blastoschizomyces capitatus, and Pichia kudriavzevii [21] are retrieved. Recent studies revealed the attractive potentiality of whey non-conventional strains in producing important aromatic compounds including 2-phenylethanol, an alcohol with a rosy fragrance and preservative properties, which are highly required in the food and cosmetic industries [22,23].
In the present study, yeast strains isolated from artisanal sourdough starters for pizza and bread and from buffalo and cow milk whey obtained during mozzarella cheese production were characterized by their leavening capacity, resistance to bread-making conditions, and aroma production activity. The autochthonous strains with the most promising technological characteristics were tested for bread dough starter formulations based on different compositions.
2. Materials and Methods
2.1. Yeasts Isolation
In this study, 66 strains belonging to ISACC2 (Istituto di Scienze dell’Alimentazione, yeast Culture Collection n° 2—Institute of Food Science-CNR, Avellino, Italy), isolated from artisanal food sources from different geographical areas of the Campania region (Italy) were tested (Table 1). Microbial isolates from pizza and bread sourdough were obtained utilizing 10 g samples mixed with 90 mL of physiological solution (9 g/L NaCl), which were put in Stomacher bags and homogenized by two cycles of 30 s at 230 rpm in a Stomacher 400 Circulator (Seward, UK). One milliliter of the sample was treated from buffalo and cow milk whey. The appropriate dilutions were plated in triplicate on different agar media comprising YPD agar (10 g/L yeast extract, 20 g/L bacteriological peptone, 20 g/L dextrose, and 20 g/L bacteriological agar) supplemented with 0.1 g/L of chloramphenicol (Sigma-Aldrich, Milan, Italy), WL agar (Wallersteins Laboratory nutrient agar) (ThermoFischer, Milan, Italy), and MEA (Malt Extract Agar) (Difco, Basingstoke, UK). PCA (Plate Counts Agar), VRBGA (Violet Red Bile Glucose Agar), and VRBLA (Violet Red Bile Lactose Agar) (ThermoFisher, Milan, Italy), instead, were used to isolate Total Mesophilic Bacteria and Enterobacteriaceae. The plates were incubated for 72–120 h at 28 °C for yeast media, and at 36–44 °C for total and fecal coliforms, respectively. After counting the colonies to enumerate the native microflora of sourdoughs, five colonies were randomly selected and purified by streaking on the correspondent isolation medium. Then, colony features, such as color, surface, and texture, were analyzed on agar, while shape and cell morphology were observed using a Zeiss Axiolab microscope (Carl Zeiss Ltd., Cambridge, UK). Single colonies were streaked on YPD slants and stored at 4 °C. Simultaneously, pure yeast strains were stored at 80 °C in cryovials containing an aliquot of each broth culture, grown for 16 h, and 25% (v/v) glycerol (Carlo Erba, Milan, Italy) as components of ISACC2. The yeast strains stored at 80 °C in cryovials were sub-cultured in YPD broth and incubated at 28 °C for 24 h before the screening.

Table 1.
The yeast strains grouped according to sample sources and geographical area of the Campania region.
2.2. Sodium Chloride Tolerance
The resistance to different NaCl concentrations (2.0, 2.5, 3.0, 4.0, 5.0, and 6.0%) was evaluated following Perricone et al. [24]. Briefly, yeast cultures were sub-cultured in YPD broth at 28 °C for 24 h. The pellet obtained with centrifugation from 1 mL of liquid culture (6000 speed for 5 min), washed twice with sterile physiological solution (9 g/L NaCl), resuspended in 1 mL of the same solution, and utilized to inoculate at 1% several tubes containing YPD (9 mL) and NaCl at the concentrations above listed. Tubes containing liquid YPD without NaCl were also inoculated as a control to eliminate yeast strains that were unable to grow. All the tubes were incubated at 28 °C for 24 h. NaCl tolerance was determined by verifying, through visual inspection, the presence of turbidity or a pellet at the bottom of each tube, containing YPD broth added with NaCl. Each test was performed in duplicate.
2.3. Tolerance to Acidic Conditions
Sub-cultures of yeast strains in YPD broth, obtained after incubation at 28 °C for 24 h, were utilized to inoculate tubes containing YPD adjusted to pH 2.5 and 3.5 with HCl 1 N. YPD tubes were also inoculated as a control. After incubating the tubes at 28 °C for 24 h, the survival capacity was determined by seeding on YPD agar plates followed by incubation at 28 °C for 72 h. The residual yeast population was defined as the survival % calculated with the formula: %S = CFU/mLYPD acid/CFU/mLYPD control × 100, where CFU/mLYPD acid and CFU/mLYPD control were the counts after growth in acidified YPD broth and the YPD control respectively.
2.4. Carbohydrate Assimilation Tests
Test tubes containing 6.7 g/L of YNB (Yeast Nitrogen Base) (Difco, Basingstoke, UK), and 20 g/L of carbohydrate (glucose, maltose, and sucrose), and YNB without any added carbon sources as a negative control, were used. The different tubes were inoculated at 1% with resuspended yeast biomass obtained after two subsequent sub-culturing processes in YPD broth at 28 °C for 24 h. Inoculated test tubes were incubated at 28 °C for 48 h [25,26]. The tests were performed in duplicate.
2.5. Qualitative Screening of Phytate-Degrading Yeasts with Plate Assay
Plate assay was performed following protocols [27,28]. Briefly, the solid medium containing glucose (20 g/L), calcium chloride (2 g/L), ammonium nitrate (5 g/L), potassium chloride (0.5 g/L), magnesium sulfate (0.5 g/L), iron sulfate (0.01 g/L), manganese sulfate (0.01 g/L), and bacteriological agar (15 g/L) was sterilized at 121 °C for 15 min. When the medium temperature reached 50 °C, sodium phytate (sodium myoinositol hexaphosphate) (Sigma-Aldrich, Merck, Darmstadt, Germany) (4 g/L), previously sterilized through 0.2 μm filters (Whatman, Merck, Darmstadt, Germany), was added. Yeast colonies from YPD agar were transferred to the prepared solid medium and incubated at 28 °C for 72 h. Then, the plates were washed with distillated water to determine if the transparent halos surrounding the colonies were due to phytase action or acid production. The plates were flooded with 10 mL of 2% (w/v) cobalt chloride solution and incubated for 10 min at room temperature. Subsequently, this solution was replaced with a mixed solution containing equal volumes of 6.25% (w/v) aqueous ammonium molybdate and 0.42% (w/v) ammonium vanadate solutions. Upon its removal, the colonies surrounded by a clarified halo were checked and recognized as phytase-positive isolates.
2.6. Exopolysaccharide (EPS) Production Test
The yeast’s ability to produce EPS was assayed following Syal and Vohra’s [27] modified protocol. Briefly, yeasts sub-cultured in YPD broth at 28 °C for 24 h were streaked on Ruthenium red milk agar medium containing (100 g/L skim milk powder, 10 g/L sucrose, 20 g/L bacteriological agar, and 0.08% Ruthenium red) and sterilized at 121 °C for 5 min. After incubating the plates at 28 °C for 24 h, white colonies were identified as EPS-producers, different from the red ones. The tests were performed in duplicate.
2.7. Molecular Identification of Selected Yeast Isolates
The yeast strains were identified by analyzing the D1/D2 domain of 26S rDNA sequence. The genetic region was PCR-amplified directly from individual yeast colonies following the protocol described by Arroyo-Lopez et al. [29]. The standard primers utilized are commonly referred to in the literature as NL1 and NL4 [30].
2.8. Leavening Ability Test
Among the 66 yeast isolates from sourdough and milk whey, 25 strains, selected through the preliminary screening for their physiological features, carbohydrate assimilation capacity, resistance to saline and low pH stresses, and ability to produce phytase activity and extracellular EPS, were subjected to a leavening ability test.
For each strain, after growth at 28 °C for 24 h in YPD broth, the vital count was evaluated by seeding on YPD plates, which were incubated at 28 °C for 72 h. After centrifugation (6000 speed for 5 min) of 9 mL from each liquid culture, the collected biomass was washed twice with a physiological solution to remove culture medium residues and metabolites and then resuspended in the same solution. Aliquots were withdrawn to obtain 107 CFU/mL suspensions of single or mixed yeast strains (co-cultures), which were added to each dough, reaching 106 CFU/g final inoculum. In particular, in a glass beaker, tap water (40 mL), NaCl (2% w/w), type 00 soft wheat flour (Divella, Rutigliano, Bari, Italy) (50 g), and cell suspension were mixed for 5 min with a spatula to reach a dough yield (DY) of 180. Aliquots were collected for T0 physico-chemical and microbiological analyses such as pH, total titrable acidity (TTA), and count of vital blastomycetic populations. Then, the mixture was transferred into a glass 250 mL graduated cylinder, and the initial volume (V0) was determined. Doughs without inoculum (NY) and inoculated with a commercial bakery yeast (LSC) (Lievital; Lesaffre, Parma, Italy) were used as negative and positive controls, respectively. After placing the covered cylinders in a thermostated incubator (Heraeus, Hanau, Germany) at 28 °C for 24 h, pH, total titrable acidity (TTA), load of vital blastomycetic population, final volume (Vfin), volume percentage increase (% VI), and the magnitude order of growth of single or mixed inoculated strains were determined. Leavening tests were performed in triplicate.
2.8.1. Determination of the Blastomycetic Population of Doughs
The ability of the inoculated yeasts to adapt and grow in experimental dough conditions was studied by spreading dough suspensions onto solid YPD medium. Briefly, after kneading (T = 0 h) and 24 h of proofing (T = 24 h), 10 g of dough were transferred to 90 mL of physiological solution and homogenized with two cycles of 30 s at 230 rpm utilizing a Stomaker 400 circulator (Seward, UK). Serial dilutions were prepared, plated onto solid YPD medium, and incubated for 72 h at 28 °C. Plate counts were performed in duplicate.
2.8.2. Determination of pH and TTA
The pH values were determined with a pH meter (Hanna Instruments, Padova, Italy). TTA wasmeasured on 10 g of dough samples homogenated in 90 mL of distilled water for 3 min. TTA was expressed as a quantity (mL) of 0.1 M NaOH to reach a pH of 8.5.
2.8.3. Determination of Volume Increase
To assess dough volume increase, on each graduated cylinder, the volume of the dough (in mL) was recorded at time 0 h and after 24 h of fermentation at 28 °C. The volume increase was calculated using the following formula: %VI = (Vfin − V0)/V0 × 100, where Vfin was the volume after 24 h of fermentation and V0 was the initial volume.
2.9. Solid Phase Microextraction Gas Chromatography/Mass Spectrometer Analysis of Volatile Compounds
The most interesting strains, selected on the basis of the performances reported after the several technological tests described in the previous Section 2.2, Section 2.3, Section 2.4, Section 2.5, Section 2.6 and Section 2.8, were evaluated for the ability to affect the aroma profile of the doughs after 24 h of fermentation according to Zotta et al. [13]. The doughs were prepared as described in Section 2.8, and NY (doughs without inoculum) was used to represent the average of the NY volatile organic compounds (VOCs) of all the dough preparations.
VOCs were extracted from doughs using Solid Phase MicroExtraction (SPME) and analyzed with gas chromatography coupled to mass spectrometry (SPME/GC-MS). Briefly, 2 g of the samples were placed into a 20 mL headspace vial, and 5 µL of 3-octanol (internal standard, 100 mg/L standard solution) was added. The vial was placed in a thermostatic block (40 °C) on a stirrer, the fiber was inserted and maintained in the sample headspace for 30 min, then removed and immediately inserted into the GC/MS injector for the desorption of compounds. The extraction was performed automatically with the multipurpose sampler of the GC/MS system. A silica fiber, coated with 75 µm of Carboxen/Polydimethylsiloxane (CAR/PDMS) (Supelco, Bellefonte, PA, USA) was used for analysis. The SPME-GC/MS analysis was performed using an Agilent GC 7890A/MSD 5975 system with a Gerstel MPS2 autosampler (Agilent Technologies, Santa Clara, CA, USA); the operating conditions were as follows: HP-Innowax capillary column (Agilent Technologies, 30 m × 0.25 mm ID, film thickness 0.25 µm), the gas carrier was helium (flow 1.5 mL/min), and SPME injections were splitless (straight glass line, 0.75 mm ID) at 240 °C for 20 min, during which time thermal desorption of the analytes from the fiber occurred. The oven parameters were as follows: initial temperature of 40 °C held for 3 min, followed by an increase to 240 °C at a rate of 5 °C/min, and then held for 10 min. The injector temperature was 240 °C. The mass spectrometer operated in scan mode over a mass range from 33 to 300 amu (2 s/scan) at an ionization potential of 70 eV. VOC identification was achieved by comparing mass spectra with the Nist library (NIST 20) and by matching the retention indices (RIs) calculated according to the equation of Van Den Dool and Kratz [31] and based on a series of alkanes. The data are expressed as relative peak areas with respect to internal standards. Blank experiments were conducted in two different modalities: blank of the fiber and blank of the empty vial. These types of control were carried out after every 4 analyses. Two technical replicates were carried out for each sample.
2.10. Bread-making Ability Test of Yeast Strain Co-Cultures
Different strains, identified at the molecular level and selected on the technological features of their inoculated doughs, such as % volume increase, pH, TTA, and characterizing odors, were utilized as mixed inocula (co-culture) in leavening ability tests. Different combinations of 2 different strains (YPZWL14+YPZMA14; YPZWL14+YSPR1; YPZWL14+YSMAX7; YPZMA14+YSPR1; YPZMA14+YSMAX7) were used in bread-making tests following the protocol reported in Section 2.8. Doughs from different combinations were evaluated for their leavening ability and sensory profile. The test was performed in duplicate.
2.11. Statistical Analysis
The mean and standard deviation were calculated for each experimental parameter. Principal Component Analysis (PCA) was carried out using Tanagra 1.4 software.
3. Results
3.1. Determination of Yeast Populations in Sourdoughs and Whey
The results from YPD plate counts of artisanal bread sourdoughs revealed the presence of non-conventional yeasts (NCYs) of around 7.0 log CFU/g. In particular, the yeast populations showed slight variation on the different solid media: on YPD, the count order was 6.5 log CFU/g, while on MEA and WL, it was 7.1 and 7.6 log CFU/g, respectively. Concerning the total mesophilic bacteria (TBC) and Enterobacteriaceae, the microbial load was higher, as expected, on PCA, with values of 7.8 log CFU/g and 1.0 log CFU/g on VRBGA and VRBLA. The analyses of artisanal pizza sourdoughs gave similar results (NCY on YPD of 6.9 log/CFU, 7.2 and 7.5 log CFU/g on MEA and WL, respectively) with YPD counts still lower than those on the other two media. Also, total mesophilic bacteria and Enterobacteriaceae populations presented the same sizes, as confirmed by PCA count of 7.7 log CFU/g, and 1.0 log CFU/g on VRBGA and VRBLA, respectively. The whey samples subjected to analysis came from artisanal dairies specialized in producing mozzarella cheese from cow and buffalo milk. For samples of buffalo and cow whey, the indigenous yeast population counts on YPD accounted for 6.0 and 6.1 log CFU/mL, respectively; a similar count was detected on WL medium (7.6 and 7.7 log CFU/mL), while on MEA, 7.4 and 6.5 log CFU/mL were enumerated. The mesophilic bacterial microflora (TBC) showed a load of 8.6 and 8.3 log CFU/mL. In comparison, the Enterobacteriaceae counts at 36 and 44 °C were between 4.5 and 2.4 for the buffalo samples and between 4.3 and 2.2 log CFU/mL for cow whey. On WL medium, the colonies presented smooth or rough surfaces, an umbonate shape; most of them had a cream color, and few were white. Based on microscope observation, 40% of the isolated and purified yeast cells were ovoidal, round with multipolar buds, 30% were cylindrical and budded, 15% were round of small dimensions, and 15% showed pseudomicelium.
3.2. Resistance to Acidic Stress
In the resistance tests to acidic conditions, the counts of YPD liquid cultures of the isolated yeasts at pH 2.5 showed that the vital load was between 4 and 4.9 log CFU/mL, and finally, at pH 3.5, the resistance capacity was higher, as witnessed by counts on YPD of 5.0–5.9 log CFU/mL. The counts of YPD liquid cultures after 24 h growth, utilized as reference, were 7.0–7.9 log CFU/mL. The evaluation of % survival (%S) of different yeast strains, showed that the best values were measured for the 16 artisanal pizza sourdough isolates. In YPD pH 2.5, %S values >50% and >67% were revealed for 6 and 10 strains, respectively. The highest survival was detected for growth in YPD pH 3.5 with %S >70% for 11 strains and %S >75% for 5 of them.
3.3. Resistance to NaCl
Non-conventional yeasts isolated from artisanal sourdoughs and the whey of buffalo and cow milk showed 100% resistance at 2.0, 2.5, and 3.5% NaCl concentrations, while at higher NaCl concentrations, the resistance decreased. In fact, at 4% NaCl, growth was evidenced for only 85% of the isolates, and at 5 and 6% NaCl, only 51 and 40% of the different strains cultures were able to survive.
3.4. Carbohydrate Assimilation Test
The native yeasts isolated from different fermented matrixes were able to assimilate glucose, while 64% metabolized maltose. The assimilation capacity was more pronounced for yeasts isolated from sourdoughs than those from whey, 10% of which were able to metabolize the disaccharide maltose (Table 2). Regarding sucrose, 72% of the strains were able to utilize the carbohydrate as a carbon source, and this capacity was higher from the sourdough sources.

Table 2.
Technological features of native yeast strains isolated from sourdoughs and whey. LSC commercial bakery yeast was used as a control.
3.5. Production of Exopolysaccharides (EPSs)
All isolated strains were able to grow on Ruthenium red milk agar and produce white colonies, highlighting their capacity to secrete exopolysaccharides. In particular, among whey isolates, five strains native to buffalo whey (50%) and two strains from cow whey were good producers. Among yeasts from sourdough, a higher number of producers were found, and in particular, a higher percentage was found among yeasts native to pizza sourdough (47%). In comparison, 20% was detected for the isolates from artisanal bread (Table 2).
3.6. Isolated Yeasts with Phytase Extracellular Activity
A sub-group of seven yeast strains, (five from sourdough (YPZWL14, YPZMA14, YPZMA17, YPCWL2, YPCMA15) and two from whey (YSMAX7, YSPR1) showed colonies surrounded by a transparent halo on the solid medium containing sodium phytate as the sole phosphorus source. As reported by several authors, the clear halo around the colonies was produced by the extracellular hydrolytic action of phytase on myoinositol hexaphosphate [27].
3.7. Leavening Test Utilizing Single Strain Inoculum
Small-scale leavening tests were carried out with the isolated yeasts selected based on their physiological characteristics and abilities of technological interest. Twenty-five yeasts (15 from sourdoughs and 10 from whey) and the commercial bakery yeast (LSC) were utilized as single-strain inoculum, and a big difference between the leavening ability of yeasts from two different origins was evidenced (Table 2). In particular, a higher percent volume increase (up to 110%) was detected when inocula were carried out utilizing isolates from sourdoughs, while those from whey gave a maximum %VI of 97%. The bakery commercial yeast (LSC) used for the positive control gave doughs with slightly higher final volumes (140% VI) than the experimental sourdough yeasts. At the same time, it showed a stronger leavening ability compared with the whey yeasts.
3.8. Molecular Identification of Selected Yeast Isolates
The isolated yeasts subjected to identification were selected based on their physiological features, carbohydrate assimilation patterns, resistance to acid and saline stresses, EPS production and phytase activity, leavening capacity, and sensory smell evaluation of the inoculated doughs. After a stringent selection, only 14 isolated yeasts that showed the most interesting characteristics as candidates for starters in the bread-making process were chosen for identification. Among them, seven of the strains were isolated from whey and eight from sourdoughs. Identification at the species level was possible for all of them because of the elevated identity levels (≥99%) of the PCR-amplified sequences of 26S rDNA D1 and D2 domains with those present in the NCBI database. Among the strains isolated from whey, the two predominant species were K. marxianus and P. kudriavzevii while for those from sourdoughs, S. cerevisiae represented the only identified species (Table 3).

Table 3.
Identification of native yeasts isolated with the analysis of the D1/D2 domains of the 26S rDNA gene.
3.9. Determination of Yeast Populations, pH, and TTA in the Experimental Doughs
Only the doughs inoculated with single yeasts (YPZWL14, YPZMA14, YSPR1, and YSMAX7) showed no defects and were tested to determine the yeast population, pH, and TTA.
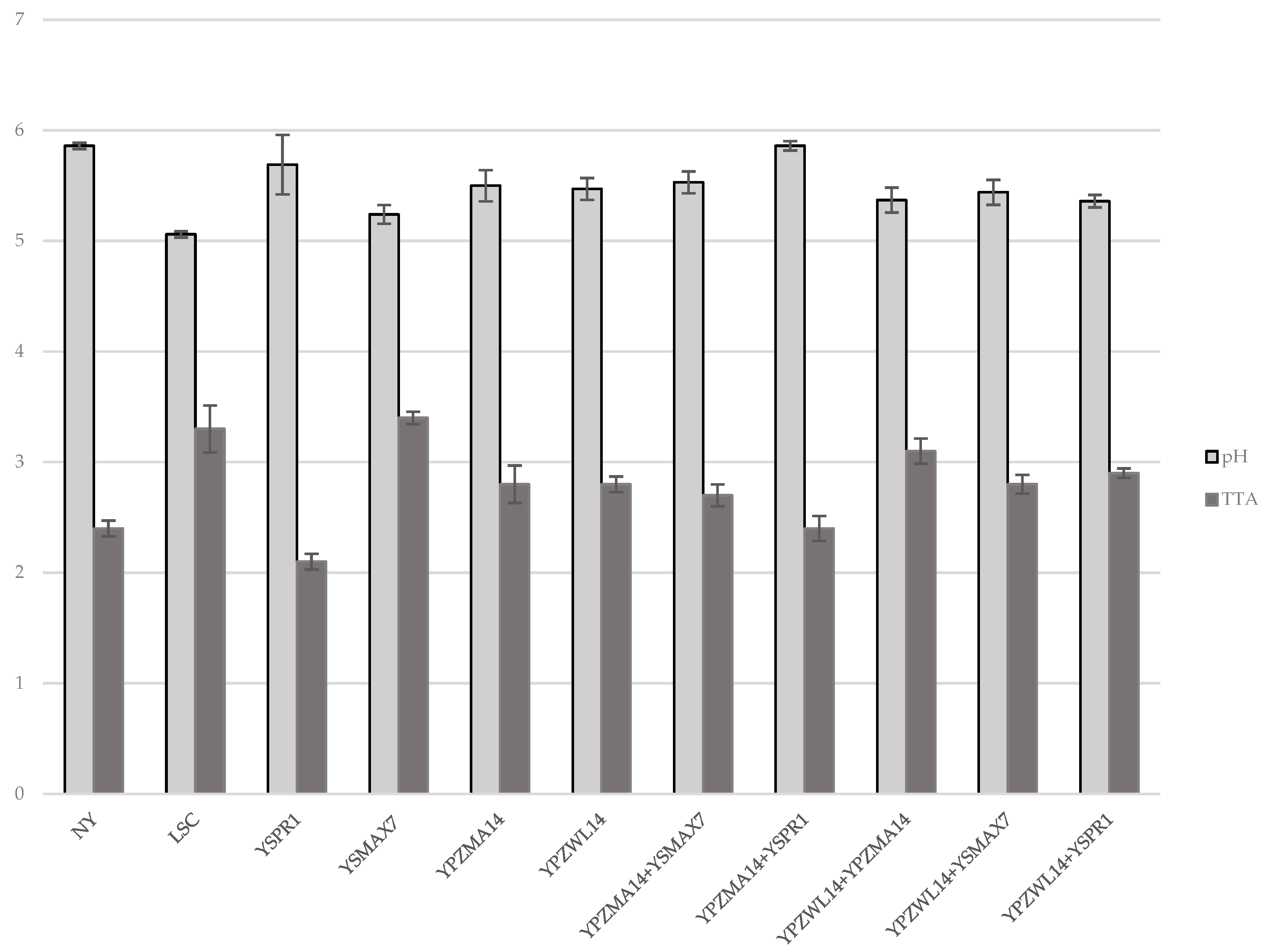
Microbial counts around 8.0 log CFU/mL (7.8 and 8.0 log CFU/mL for yeasts from sourdough and whey, respectively) were detected for liquid YPD culture and utilized for the single strain inoculum. Yeast counts of the experimental doughs were 6.0 log CFU/mL at time 0 h, while for T24h, similar values for all the isolates, between 7.4 and 8.7, were measured. Different pH and TTA values were measured for doughs inoculated with a single inoculum and, in detail, when YPZWL14, YPZMA14, YSPR1, and YSMAX7 were used for a single inoculum, pH varied from the time 0 values around 6.07 to the T24h ones of 5.47, 5.50, 5.69 and 5.24, respectively, for the each of the listed strains. TTA also varied from time 0 values comprised in the interval 1.5–1.7 to T24h TTA of 2.8, 2.8, 2.1, and 3.4 for the single-inoculated doughs with YPZWL14, YPZMA14, YSPR1, and YSMAX7. The T24h pH and TTA of the control doughs (NY) and LSC dough were 5.86, 5.06, and 2.4, 3.3, respectively (Figure 1).
Figure 1.
pH and TTA values at T24h in doughs inoculated with single and double strains.
3.10. Leavening Test Utilizing Double-Strain Inoculum
The leavening tests with mixed inocula were realized with the following co-cultures: YPZWL14 (S. cerevisiae) +YPZMA14 (S. cerevisiae); YPZWL14+YSPR1 (P. kudriavzevii); YPZWL14+YSMAX7 (K. marxianus); YPZMA14+YSPR1; and YPZMA14+YSMAX7. The two combinations involved isolates from different origins (sourdough and whey) or two strains from sourdough. The data highlighted that the mixture of two different strains of S. cerevisiae from sourdough (YPZWL14+YPZMA14) produced a % V I of 142.5%. The best results were obtained from sourdough and whey isolate co-cultures when the doughs were inoculated with YPZWL14 and YSMAX7 or YSPR1, reaching a % V I of 135.7 and 136%, respectively. The combinations of YPZMA14 with YSPR1 or YSMAX7 yielded, instead, a lower % V I (92%; 87%), respectively. Not-yeasted control dough showed a % V I of 12.5% due to the proofing activities of spontaneous microflora of the environment and the ingredients utilized for the preparation. Bakery commercial yeast (LSC) gave a % V I of 140%. Different pH and TTA values were measured for doughs inoculated with different combinations (YPZWL14+YPZMA14; YPZWL14+YSPR1; YPZWL14+YSMAX7; YPZMA14+YSPR1; YPZMA14+YSMAX7). The pH varied from time 0 h values around 6.07 to T24h pH values of 5.37, 5.36, 5.44, 5.86, and 5.53, respectively, for each of the listed co-cultures. TTA also varied from time 0 h values in the interval 1.5–1.7, to T24h TTA of 3.1, 2.9, 2.8, 2.4 and 2.7 for the combination doughs (Figure 1).
Based on previous test results for the isolates from sourdough, YPZMA14 was eliminated from the co-cultures for dough inoculation. The utilization of the YPZWL14 strain, instead, was due to its better leavening activity and aroma production assayed in several experimental proofs.
3.11. Determination of Aromatic Profiles
We analyzed volatile organic compounds (VOCs) in the doughs inoculated with single and co-culture selected yeasts after 24 h of fermentation, comparing LSC bakery commercial yeast and non-inoculated yeast (NY) samples as control. The twenty-eight volatile compounds that were identified with SPME-GC/MS (Table 4) belonged to various chemical classes such as alcohols (12 compounds), esters (six compounds), ketones (four compounds), acids (three compounds), aldehydes (two compounds), and terpenes (one compound). Alcohols were detected in the highest quantities in various samples. The most represented alcohols were ethanol and isoamyl alcohol, which always increased during fermentation with respect to the NY dough’s negative control. Dough preparations inoculated with the two S. cerevisiae strains (YPZWL14 and YPZMA14) showed a higher ethanol production, while for isoamyl alcohol, this was detected also for the YPZWL14+YSMAX7 and YPZWL14+YSPR1 selected mixed-starter cultures. Phenylethyl alcohol was identified in every dough at T24h but was at the highest level in the YPZWL14+YSPR1inoculated doughs. Also, moderate ester production was detected in all doughs inoculated with single strains or co-cultures. In particular, an increase in ethyl acetate was measured with respect to the LSC and NY samples, especially in YSMAX7 dough. Regarding ketones and, in particular, acetoin their production generation was detected for all doughs except those in which YPZWL14+YPZMA14 were utilized. Small amounts of acetic acid were found in all doughs inoculated with single strains or co-cultures except for the YSPR1 and NY doughs. On the other hand, 2,3-butanedione was revealed only after single-strain inoculum YPZWL14. Moreover, limonene was present only at a low level in negative control (NY) and LSC samples.

Table 4.
Profiles of volatile organic compounds that were detected in doughs inoculated with different yeast strains.
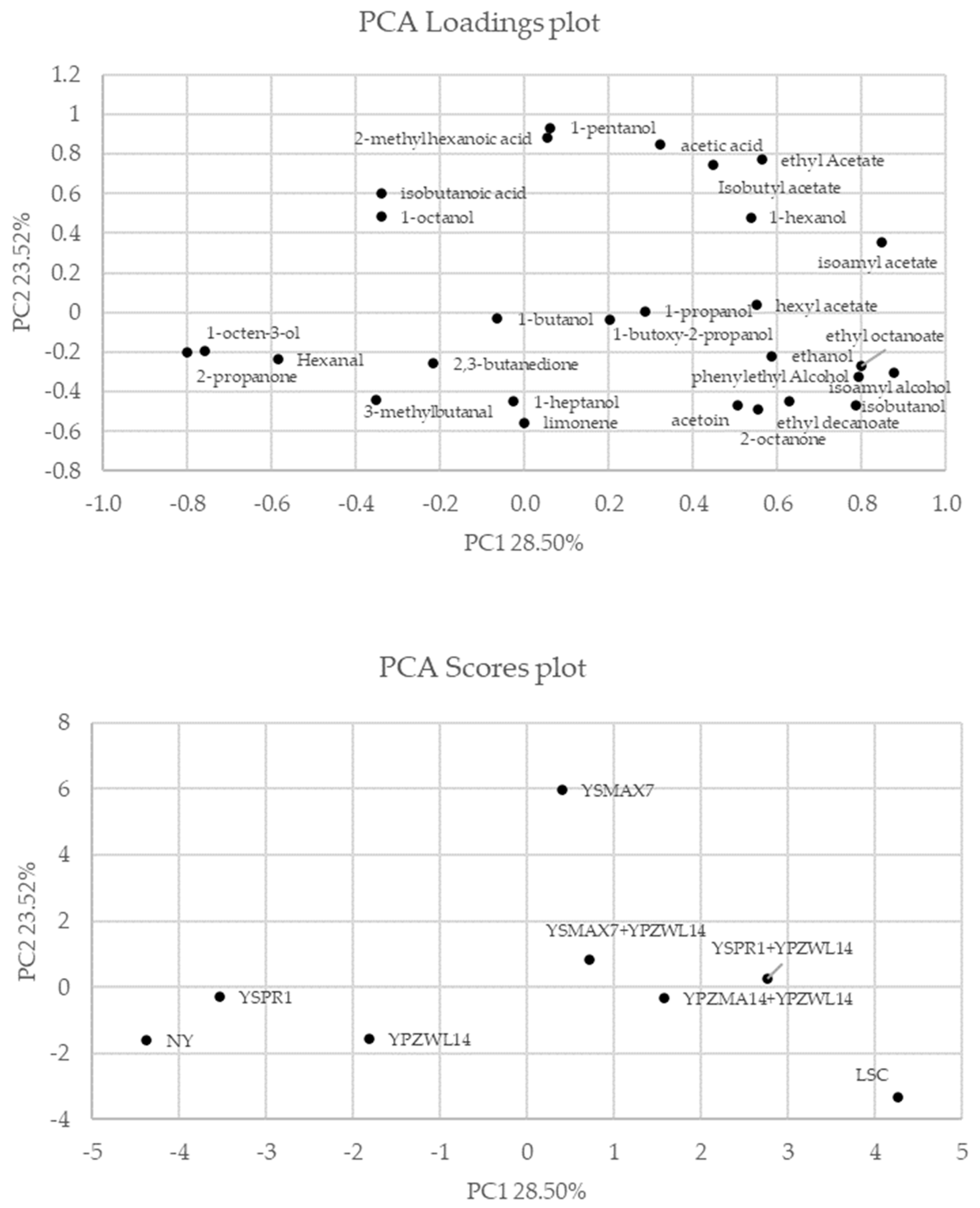
A PCA of the volatile compounds was performed to understand better the differences among the doughs (Figure 2). The two PCAs explained 52.02% of the total variance in the data. The yeast strains K. marxianus (YSMAX7), P. kudriavzevii (YSPR1), and S. cerevisiae (YPZWL14) alone and in the mixed doughs were located in different zones of the plane. Regarding the score plot, a clear separation between doughs was evidenced after 24 h of proofing. In particular, the doughs inoculated with S. cerevisiae in co-culture were grouped in the same cluster as those inoculated with bakery commercial yeast (LSC). In contrast, those inoculated with K. marxianus (YSMAX7) and P. kudriavzevii (YSPR1) alone were located in distinct and separate zones. Moreover, when P. kudriavzevii was utilized as a single inoculum, VOC production was close to the NY value, showing a reduced aromatic complexity with respect to other samples after 24 h of fermentation. The loading plot showed that doughs inoculated with K. marxianus (YSMAX7) were characterized by acetic acid, isobutyl acetate, ethyl acetate, 2-methylhexanoic acid, 1-hexanol, and 1-pentanol, as already reported by other authors [32]. The doughs containing P. kudriavzevii (YSPR1) and NY were characterized by 1-octen-3-ol, hexanal, and 2-propanone, while in those with YPZWL14 (S. cerevisiae) 2,3-butanedione and 3-methylbutanal were detected. Doughs containing YPZWL14 in combination with the whey yeasts, K. marxianus (YSMAX7) and P. kudriavzevii (YSPR1), or the other sourdough strain S. cerevisiae (YPZMA14), and the dough inoculated with LSC were found in the same cluster and were characterized by the same aromatic compounds, in particular, acetoin, ethanol, isoamyl alcohol, phenylethyl alcohol, and isobutanol.

Figure 2.
Principal component analysis (PCA) of volatile organic compounds that mainly differentiated the doughs inoculated with different strains. YPZMA14 and YPZWL14: S. cerevisiae; YSPR1: P. kudriavzevvii; YSMAX7: K. marxianus; LSC: bakery commercial yeast; NY: non-inoculated yeast.
4. Discussion
Bread and pizza artisanal sourdoughs and milk whey from the manufacturing process of mozzarella cheese were screened as they represent natural habitats in which stringent parameters (acidity, high amounts of complex sugars, low availability of fermentable sugars and oxygen) promote natural selection on microbial consortia (yeasts and lactic acid bacteria). The native yeast strains selected in this study were endowed with all the characteristics to be used in baking processes, such as the capacity to ferment the sugars present in wheat flour, represented by maltose, and small amounts of glucose produced by the action of flour endogenous amylases.
As already reported by Johnston et al. [33], in the co-presence of monosaccharides and disaccharides, the first metabolized sugars are represented by the monosaccharide in the order of fructose and glucose, followed by sucrose and maltose. In particular, a correlation between leavening ability and maltose assimilation capacity was reported by several authors [2,34]. This is because of the high increase in this disaccharide after dough mixing, and the yeast cells need to adapt their metabolism to consume maltose after monosaccharide depletion [6]. This explains the lower leavening capacity of whey yeast strains respect to the sourdough strains due to a sensibly reduced maltose assimilation capacity among the isolated strains.
Moreover, the selected yeast strains, especially those isolated from sourdoughs, showed good resistance to NaCl and acid conditions. Such behavior is in agreement with observations of other authors who highlighted the high resistance of sourdough yeasts to saline and acidic conditions due to their adaptation to dough environment conditions determined by the presence of lactic acid bacteria (LAB) [35]. These bacteria, which are responsible for the production of lactate (homo-fermentative strains), also accompanied by acetic acid (hetero-fermentative ones), are responsible for the reduction in pH during fermentation [4]. Notably, the 100% survival of the selected yeasts in the presence of NaCl concentrations up to 3.5% underlines the good adaptability of these strains to baking conditions. In fact, the % NaCl commonly utilized, i.e., in Italian bread, is between 0.7 and 2.3 with mean values of 1.5 and 1.6 for artisanal and industrial products, respectively, and the highest ones (2.5) for industrial bread [36].
The EPS capacity reported for our yeasts was prevalently found among sourdough strains rather than whey ones. This ability is of high technological interest due to the possibility of EPS-producing starters acting as adjuvants and emulsifiers in the kneading phase, favoring the dough and final bread texture. Moreover, EPS are bio-polymers with an interesting role in yeast cell protection from desiccation, toxic compounds, and osmotic stress and can promote biofilm formation and an immunostimulant action [27].
The phytase activity production that represents an important pro-technological character was found in the 20% whey isolated yeasts (P. kudriavzevii, K. marxianus) and in the 33% of yeasts from sourdoughs that belonged to Saccharomyces species. The phytase enzyme has a high nutritional value since it has a fundamental role in the solubilization of phytates, which chelate minerals with important functions in the human diet (iron and zinc), depleting their bioavailability and uptake [35]. In this case, we could hypothesize that the loss of the ability to synthesize the enzyme by the yeast cultures was caused by the characteristics of the substrates used in the laboratory [37].
In the determination of dough leavening abilities, our yeast strains belonging to S. cerevisiae species, utilized as a single inoculum, showed different levels of % V I (60–138). The YPZWL14 strain always showed an interesting proofing performance with respect to YPZMA14, endowed with a more limited capacity to increase dough volume, although both belonged to S. cerevisiae species [2,38]. Furthermore, the evaluation of sensory smell and the results of the technological tests on the isolates from the sourdough led to the decision to eliminate YPZMA14 from the co-cultures for the inoculation of the doughs and choosing YPZWL14 for experimental tests [39].
Regarding the pH and TTA values of the doughs inoculated with the different strains and/or their co-cultures, their ranges of variation after 24 h of fermentation were far more limited than those detected for sourdoughs where LAB were present. A slight pH reduction was measured, and the yeast strains’ succinic acid production has already been recognized by different authors as the main determinant of dough acidification [40].
From a VOC point of view, the aromatic profile of bread represents a crucial qualitative parameter that derives not only from the cooking phase but also from the secondary metabolites (alcohols, esters, aldehydes, and ketones) generated by yeasts during dough fermentation [11]. Yeasts, with their metabolic pathways, contribute not only to dough leavening but also to bread aroma. The utilization of conventional yeasts (S. cerevisiae) yields a limited aromatic compound diversity. In the modern bakery, non-conventional yeasts isolated from different natural sources would also be utilized in association with LAB to improve bread aroma complexity with flavor notes specific to the used strain [13]. K. marxianus, a non-conventional yeast, was tested for many years as an alternative bakery yeast also in combination with LAB. Caballero et al. [41] showed that the use of this yeast did not generate off-flavors, while Plessas et al. [42] demonstrated that K. marxianus alone or in association with S. cerevisiae also enhanced the aroma complexity with pleasant notes despite a predominant acidic one. As already reported by various authors, yeasts produce alcohols and aldehydes mainly via the Ehrlich pathway, starting from wheat flour amino acids. The most important aroma-active aldehydes and alcohols formed by yeast activity are 3-methylbutanal (the compound represented at the highest amounts in our doughs inoculated with the isolated S. cerevisiae), isoamylalcohol, derived from leucine, and phenylethyl alcohol, derived from phenylalanine [32]. These compounds were detected in all doughs inoculated with the S. cerevisiae, K. marxianus, and P. kudriavzevii co-cultures. Another group of volatile compounds in bread aroma is organic acids. Acetic acid is produced from the fatty acid synthase pathway, while branched organic acids (2-methylhexanoic acid) are synthesized via an alternative route to the oxidation of aldehydes from the Ehrlich pathway [43]. Other important volatile components detected in the experimental doughs were esters (isobutyl acetate and ethyl acetate), which are interesting because of their pleasant odors (fruity notes). These compounds originate from trans-esterification reactions of fatty acids-CoA with free alcohols [10]. Also, elevated amounts of alcohols (1-hexanol, and 1-pentanol, among others), produced from the fatty acid lipoxy-degradation pathway, conferred interesting floral nuances, specifically to the doughs inoculated with native K. marxianus yeast.
Several authors evaluated the exploitation of P. kudriavzevii as a single starter or in combination with S. cerevisiae, investigating the generated VOC profiles [44,45,46,47]. As reported by these authors, P. kudriavzevii combined with S. cerevisiae could improve the fruity and floral profile of the fermented products, especially by enhancing the production of 2-phenylethanol and isoamyl acetate. Our data demonstrated that P. kudriavzevii as a single inoculum was not able to improve the dough’s aromatic profile, which, after 24 h of fermentation, remained similar to that of the unyeasted dough. In this regard, both the doughs containing P. kudriavzevii, or the not inoculated ones, were characterized by the presence of 1-octen-3-ol, hexanal, and 2-propanone derived from fatty acid degradation, as suggested by Schrader [48], who also studied the yeast P. pastoris. The last group of aroma compounds identified in the doughs inoculated with the native S. cerevisiae strain alone and in co-cultures was the ketones, in particular, 2,3-butanedione (diacetyl) and 3-hydroxy-2-butanone (acetoin), which, in contrast to aldehydes, alcohols, acids, and esters, are formed outside the yeast cell [5,43]. α-acetolactate, which is an intermediate of valine and leucine biosynthesis, is released out of the yeast cells where, through a nonenzymatic reaction, it produces diacetyl and acetoin [10].
Moreover, an in-depth evaluation of strain exploitation for the formulation of innovative starters will also require the assessment of safety aspects [49] since new starters for bread-making will be not only innovative to taste and nutritional points of view but also reliable with respect to a healthy guarantee.
5. Conclusions
This study highlights that the exploitation of mixed inocula of selected non-conventional autochthonous yeasts from different food-fermented artisanal matrices in the baking sector could meet, in a positive way, the modern consumer requirements for tasty, fragrant, and healthy leavening products. Our data showed that selected yeasts are able to be used in the bread-making process to enhance the dough aroma complexity and help in the release of exopolysaccharides and phytase activity. Thus, the yeasts could be powerful tools in the production of functional compounds that have important effects on human health.
Author Contributions
Conceptualization, A.S., F.B. and E.I.; methodology, A.S., F.B., E.I. and S.D.C.; software, F.B.; validation, A.S., F.B. and E.I.; formal analysis, A.S., F.B., E.I. and S.D.C.; writing—original draft preparation, A.S., F.B. and E.I.; writing—review and editing, A.S., F.B. and E.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the CNR project NUTRAGE FOE-2021 “Nutrizione, Alimentazione & Invecchiamento Attivo” DBA.AD005.225.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that are presented in this study and are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piperno, D.R.; Weiss, E.; Holst, I. Processing of Wild Cereal Grains in the Upper Palaeolithic Revealed by Starch Grain Analysis. Nature 2004, 430, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Therdthai, N.; Hui, Y.H. Bakery Products Science and Technology, 2nd ed.; Zhou, W., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 3–761. [Google Scholar]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A Review of Sourdough Starters: Ecology, Practices, and Sensory Quality with Applications for Baking and Recommendations for Future Research. PeerJ 2021, 9, e11389. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the Sourdough May Affect the Functional Features of Leavened Baked Goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef] [PubMed]
- Struyf, N.; Van der Maelen, E.; Hemdane, S.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Bread Dough and Baker’s Yeast: An Uplifting Synergy. Compr. Rev. Food Sci. Food Saf. 2017, 16, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-Throughput Screening of a Large Collection of Non-Conventional Yeasts Reveals Their Potential for Aroma Formation in Food Fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef]
- Makhoul, S.; Romano, A.; Capozzi, V.; Spano, G.; Aprea, E.; Cappellin, L.; Benozzi, E.; Scampicchio, M.; Märk, T.D.; Gasperi, F.; et al. Volatile Compound Production during the Bread-Making Process: Effect of Flour, Yeast and Their Interaction. Food Bioprocess. Technol. 2015, 8, 1925–1937. [Google Scholar] [CrossRef]
- Cho, I.H.; Peterson, D.G. Chemistry of Bread Aroma: A Review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of Commercial Baker’s Yeasts on Bread Aroma Profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Zhou, N.; Semumu, T.; Gamero, A. Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation 2021, 7, 102. [Google Scholar] [CrossRef]
- Boboye, B.; Dayo-Owoyemi, I. Evaluation of Dough Sensory Properties Impacted by Yeasts Isolated from Cassava. J. Appl. Sci. 2009, 9, 771–776. [Google Scholar] [CrossRef]
- Zotta, T.; Di Renzo, T.; Sorrentino, A.; Reale, A.; Boscaino, F. Selection of Non-Saccharomyces Wine Yeasts for the Production of Leavened Doughs. Microorganisms 2022, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Syrokou, M.K.; Tziompra, S.; Psychogiou, E.-E.; Mpisti, S.-D.; Paramithiotis, S.; Bosnea, L.; Mataragas, M.; Skandamis, P.N.; Drosinos, E.H. Technological and Safety Attributes of Lactic Acid Bacteria and Yeasts Isolated from Spontaneously Fermented Greek Wheat Sourdoughs. Microorganisms 2021, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, A.; Howell, K.; Wittwer, A.; Howell, K. Rising Stars in the Bakery: Novel Yeasts for Modern Bread. Microbiol. Aust. 2022, 43, 75–78. [Google Scholar] [CrossRef]
- Huys, G.; Daniel, H.-M.; De Vuyst, L. Taxonomy and Biodiversity of Sourdough Yeasts and Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer: New York, NY, USA, 2013; pp. 105–154. [Google Scholar] [CrossRef]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of Autochthonous Tuscan Sourdough Yeasts as Potential Starters. Int. J. Food Microbiol. 2019, 302, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Song, K.; Cui, M. Performance of Non-Saccharomyces Yeasts Isolated from Jiaozi in Dough Fermentation and Steamed Bread Making. LWT 2019, 111, 46–54. [Google Scholar] [CrossRef]
- Miller, G.D.; Jarvis, J.K.; McBean, L.D. Handbook of Dairy Foods and Nutrition; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Martini, S.; Bonazzi, M.; Malorgio, I.; Pizzamiglio, V.; Tagliazucchi, D.; Solieri, L. Characterization of Yeasts Isolated from Parmigiano Reggiano Cheese Natural Whey Starter: From Spoilage Agents to Potential Cell Factories for Whey Valorization. Microorganisms 2021, 9, 2288. [Google Scholar] [CrossRef]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. [Google Scholar] [CrossRef]
- Fraud, S.; Rees, E.L.; Mahenthiralingam, E.; Russell, A.D.; Maillard, J.-Y. Aromatic Alcohols and Their Effect on Gram-Negative Bacteria, Cocci and Mycobacteria. J. Antimicrob. Chemoth 2003, 51, 1435–1436. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Sternicka, M.K.; Kowalska, P.D.; Mierzejewska, J. Screening of Yeasts for the Production of 2-Phenylethanol (Rose Aroma) in Organic Waste-Based Media. Lett. Appl. Microbiol. 2018, 66, 153–160. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Technological Characterization and Probiotic Traits of Yeasts Isolated from Altamura Sourdough to Select Promising Microorganisms as Functional Starter Cultures for Cereal-Based Products. Food Microbiol. 2014, 38, 26–35. [Google Scholar] [CrossRef]
- Vrancken, G.; De Vuyst, L.; Van der Meulen, R.; Huys, G.; Vandamme, P.; Daniel, H.-M. Yeast Species Composition Differs between Artisan Bakery and Spontaneous Laboratory Sourdoughs. FEMS Yeast Res. 2010, 10, 471–481. [Google Scholar] [CrossRef]
- Kreger van Rij, J.W.K. The Yeasts: A Taxonomic Study; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Syal, P.; Vohra, A. Probiotic Potential of Yeasts Isolated from Traditional Indian Fermented Foods. Int. J. Microbiol. Res. 2013, 5, 390. [Google Scholar] [CrossRef]
- Erma Suryani, A.; Anggraeni, A.; Istiqomah, L.; Damayanti, E.; Karymi, M. Isolation and Identification of Phytate-Degrading Yeast from Traditional Fermented Food. Biodiversitas J. 2021, 22, 866–873. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Durán-Quintana, M.C.; Ruiz-Barba, J.L.; Querol, A.; Garrido-Fernández, A. Use of Molecular Methods for the Identification of Yeast Associated with Table Olives. Food Microbiol. 2006, 23, 791–796. [Google Scholar] [CrossRef]
- O’Donnel, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic, and Pleomorphic Speciation in Fungal Systematics; Reynold, D.R., Taylor, J.W., Eds.; CAB International Wallingford: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Perpetuini, G.; Tittarelli, F.; Perla, C.; Tofalo, R. Influence of Different Aggregation States on Volatile Organic Compounds Released by Dairy Kluyveromyces marxianus Strains. Foods 2022, 11, 2910. [Google Scholar] [CrossRef]
- Johnston, M. Feasting, Fasting and Fermenting: Glucose Sensing in Yeast and Other Cells. Trends Genet. 1999, 15, 29–33. [Google Scholar] [CrossRef]
- Berthels, N.; Cordero-Otero, R.; Bauer, F.; Thevelein, J.; Pretorius, I.S. Discrepancy in glucose and fructose utilization during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef]
- Palla, M.; Blandino, M.; Grassi, A.; Giordano, D.; Sgherri, C.; Quartacci, M.F.; Reyneri, A.; Agnolucci, M.; Giovannetti, M. Characterization and Selection of Functional Yeast Strains during Sourdough Fermentation of Different Cereal Wholegrain Flours. Sci. Rep. 2020, 10, 12856. [Google Scholar] [CrossRef]
- Carcea, M.; Narducci, V.; Turfani, V.; Aguzzi, A. A Survey of Sodium Chloride Content in Italian Artisanal and Industrial Bread. Foods 2018, 7, 181. [Google Scholar] [CrossRef]
- Olstorpe, M.; Schnürer, J.; Passoth, V. Screening of Yeast Strains for Phytase Activity. FEMS Yeast Res. 2009, 9, 478–488. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Pastor, K.; Orhun, G.E.; Mc Elhatton, A.; Rocha, J.M.F. Traditional European Breads; Springer Nature: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Sun, X.; Wu, S.; Li, W.; Koksel, F.; Du, Y.; Sun, L.; Fang, Y.; Hu, Q.; Pei, F. The Effects of Cooperative Fermentation by Yeast and Lactic Acid Bacteria on the Dough Rheology, Retention and Stabilization of Gas Cells in a Whole Wheat Flour Dough System—A Review. Food Hydrocoll. 2023, 135, 108212. [Google Scholar] [CrossRef]
- Jayaram, V.B.; Cuyvers, S.; Lagrain, B.; Verstrepen, K.J.; Delcour, J.A.; Courtin, C.M. Mapping of Saccharomyces cerevisiae metabolites in fermenting wheat straight-dough reveals succinic acid as pH-determining factor. Food Chem. 2013, 136, 301–308. [Google Scholar] [CrossRef]
- Caballero, R.; Olguín, P.; Cruz-Guerrero, A.; Gallardo, F.; García-Garibay, M.; Gómez- Ruiz, L. Evaluation of Kluyveromyces marxianus as baker’s yeast. Food Res. Int. 1995, 28, 37–41. [Google Scholar] [CrossRef]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and L. helveticus for Sourdough Bread Making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Nieto-Sarabia, V.L.; Ballinas-Cesatti, C.B.; Melgar-Lalanne, G.; Cristiani-Urbina, E.; Morales-Barrera, L. Isolation, Identification, and Kinetic and Thermodynamic Characterization of a Pichia kudriavzevii Yeast Strain Capable of Fermentation. Food Bioprod. Process 2022, 131, 109–124. [Google Scholar] [CrossRef]
- Shankar, S.R.; Sneha, H.P.; Prakash, I.; Khan, M.; Punil Kumar, H.N.; Hari, O.M.; Basavaraj, K.; Murthy, P.S. Microbial Ecology and Functional Coffee Fermentation Dynamics with Pichia Kudriavzevii. Food Microbiol. 2022, 105, 104012. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, B.-Q.; Duan, C.-Q.; Yan, G.-L. Effects of Different Pre-Fermentation Cold Maceration Time on Aroma Compounds of Saccharomyces cerevisiae Co-Fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Shrivastava, A.; Sharma, R.K. Unraveling Non-conventional Yeast Pichia: An Emerging Lignocellulosic Ethanologenic and Exoelectrogenic Yeast. BioEnergy Res. 2023, 16, 1318–1334. [Google Scholar] [CrossRef]
- Schrader, J. Microbial Flavour Production. In Flavours and Fragrances; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 507–574. [Google Scholar]
- Suzzi, G.; Gardini, F. Biogenic Amines in Dry Fermented Sausages: A Review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).