Functional Cheeses: Updates on Probiotic Preservation Methods
Abstract
:1. Introduction
2. Functional Foods
3. Probiotics
Probiotic Strains
4. Probiotic Cheese
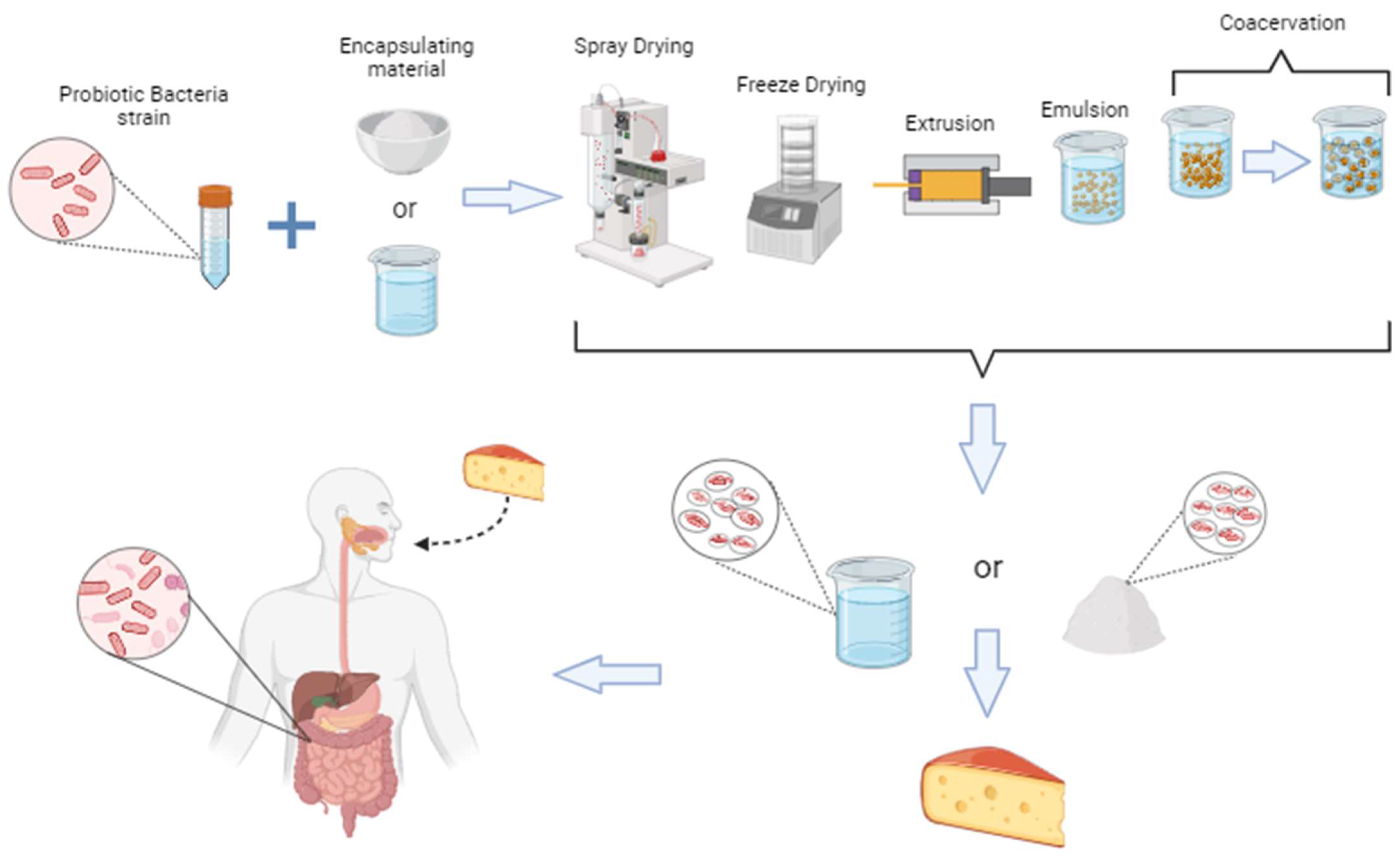
5. Technology for Preserving Probiotics in Cheese
5.1. Extrusion
5.2. Spray-Drying
5.3. Lyophilization/Freeze Drying
5.4. Emulsion
5.5. Coacervation
6. Other Ways of Incorporating Probiotics into Cheese
Edible Coatings
7. Current Applications of Probiotics in Cheese as Health Promoters
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murtaza, M.A.; Anees-Ur-Rehman, M.; Hafiz, I.; Ameer, K.; Celik, O.F. Effects of probiotic adjuncts on physicochemical properties, organic acids content, and proteolysis in cheese prepared from buffalo milk. J. Food Process. Preserv. 2022, 46, e16385. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Parolin, C.; Serrazanetti, D.I.; Vitali, B.; Lanciotti, R. Use of Lactobacillus crispatus to produce a probiotic cheese as potential gender food for preventing gynaecological infections. PLoS ONE 2019, 14, e0208906. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.R.A.; Ahmed, M.S.I. Technological aspects, health benefits, and sensory properties of probiotic cheese. SN Appl. Sci. 2019, 1, 1113. [Google Scholar] [CrossRef]
- Homayouni, A.; Ansari, F.; Azizi, A.; Pourjafar, H.; Madadi, M. Cheese as a potential food carrier to deliver probiotic microorganisms into the human gut: A review. Curr. Nutr. Food Sci. 2020, 16, 15–28. [Google Scholar] [CrossRef]
- Cordeiro, B.F.; Alves, J.L.; Belo, G.A.; Oliveira, E.R.; Braga, M.P.; da Silva, S.H.; Lemos, L.; Guimarães, J.T.; Silva, R.; Rocha, R.S.; et al. Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model. Front. Microbiol. 2021, 12, 623920. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, F.M.; Silva, H.L.A.; Poso, S.M.V.; Barroso, M.V.; Lanzetti, M.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; Freitas, M.Q.; Silva, M.C.; et al. Probiotic Prato cheese attenuates cigarette smoke-induced injuries in mice. Food Res. Int. 2019, 123, 697–703. [Google Scholar] [CrossRef]
- Lopes, L.A.A.; Pimentel, T.C.; Carvalho, R.d.S.F.; Madruga, M.S.; Galvão, M.d.S.; Bezerra, T.K.A.; Barão, C.E.; Magnani, M.; Stamford, T.C.M. Spreadable goat Ricotta cheese added with Lactobacillus acidophilus La-05: Can microencapsulation improve the probiotic survival and the quality parameters? Food Chem. 2021, 346, 128769. [Google Scholar] [CrossRef]
- Rubel, I.A.; Iraporda, C.; Manrique, G.D.; Genovese, D.B. Jerusalem Artichoke (Helianthus tuberosus L.) inulin as a suitable bioactive ingredient to incorporate into spreadable ricotta cheese for the delivery of probiotic. Bioact. Carbohydr. 2022, 28, 100325. [Google Scholar] [CrossRef]
- Anihouvi, E.S.; Kesenkaş, H. Wagashi cheese: Probiotic bacteria incorporation and significance on microbiological, physicochemical, functional and sensory properties during storage. LWT 2022, 155, 112933. [Google Scholar] [CrossRef]
- Kamel, D.G.; Hammam, A.R.A.; Nagm El-diin, M.A.H.; Awasti, N.; Abdel-Rahman, A.M. Nutritional, antioxidant, and antimicrobial assessment of carrot powder and its application as a functional ingredient in probiotic soft cheese. J. Dairy Sci. 2023, 106, 1672–1686. [Google Scholar] [CrossRef]
- Kim, J.-H.; Woo, D.; Nam, Y.; Baek, J.; Lee, J.-Y.; Kim, W. Probiotic cheese improves alcohol metabolism and alleviates alcohol-induced liver injury via the SIRT1/AMPK signaling pathway. J. Funct. Foods 2023, 108, 105736. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Elaaser, M.; El-Sayed, H.S. Ameliorate the processed cheese production by functional microcapsules loaded with mustard seed extract and Bifidobacterium bifidum. Biocatal. Agric. Biotechnol. 2021, 38, 102221. [Google Scholar] [CrossRef]
- de Andrade, D.P.; Bastos, S.C.; Ramos, C.L.; Simões, L.A.; de Andrade Teixeira Fernandes, N.; Botrel, D.A.; Magnani, M.; Schwan, R.F.; Dias, D.R. Microencapsulation of presumptive probiotic bacteria Lactiplantibacillus plantarum CCMA 0359: Technology and potential application in cream cheese. Int. Dairy J. 2023, 143, 105669. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Banerjee, P.; Ray, D.P. F3unctional food: A brief overview. Int. J. Bioresour. Sci. 2019, 6, 57–60. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Granato, D.; Zabetakis, I.; Koidis, A. Sustainability, nutrition, and scientific advances of functional foods under the new EU and global legislation initiatives. J. Funct. Foods 2023, 109, 105793. [Google Scholar] [CrossRef]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Statista. Size of the Worldwide Functional Food and Beverage Market 2020–2028. Available online: https://www.statista.com/statistics/1264080/functional-food-andbeverages-global-market-size/ (accessed on 19 September 2023).
- Fortune Business Insights. Functional Foods. Available online: https://www.fortunebusinessinsights.com/functional-foods-market-102269 (accessed on 19 September 2023).
- Ayyash, M.; Liu, S.-Q. Special Issue “Probiotics, Prebiotics and Functional Foods: Health Benefits and Biosafety&rdquo. Microorganisms 2023, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, K.M.G.M.M.; Lee, N.-K.; Paik, H.-D. Fermented dairy products as delivery vehicles of novel probiotic strains isolated from traditional fermented Asian foods. J. Food Sci. Technol. 2021, 58, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- IPA—Europe International Probiotics Associantion. 2021. Available online: https://www.ipaeurope.org/legal-framework/market-data/ (accessed on 23 August 2023).
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, N.; Ouoba, L.I.I.; Naghizadeh Raeisi, S.; Sutherland, J.; Ghoddusi, H.B. Probiotic Lactobacilli in fermented dairy products: Selective detection, enumeration and identification scheme. Microorganisms 2021, 9, 1600. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Aghamirzaei, M.; Mostashari, P.; Sarbazi, M.; Tizchang, S.; Madahi, H. 3—The impact of biotechnology on dairy industry. In Microbial Biotechnology in Food and Health; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 53–79. [Google Scholar] [CrossRef]
- Motalebi Moghanjougi, Z.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M.; Almasi, H.; Amiri, S. Bio-preservation of white brined cheese (Feta) by using probiotic bacteria immobilized in bacterial cellulose: Optimization by response surface method and characterization. LWT 2020, 117, 108603. [Google Scholar] [CrossRef]
- Cruz, A.G.D.; Zacarchenco, P.B.; Oliveira, C.A.F.D.; Corassin, C.H. Processamento de Produtos Lácteos: Queijos, Leites Fermentados, Bebidas Lácteas, Sorvete, Manteiga, Creme de Leite, Doce de Leite, Soro em Pó e Lácteos Funcionais, 1st ed.; Elsevier: Rio de Janeiro, Brazil, 2017; pp. 1–343. [Google Scholar]
- Nwachukwu, U.; George-Okafor, U.; Ozoani, U.; Ojiagu, N. Assessment of probiotic potentials of Lactobacillus plantarum CS and Micrococcus luteus CS from fermented milled corn-soybean waste-meal. Sci. Afr. 2019, 6, e00183. [Google Scholar] [CrossRef]
- Liu, B.; Fu, N.; Woo, M.W.; Chen, X.D. Heat stability of Lactobacillus rhamnosus GG and its cellular membrane during droplet drying and heat treatment. Food Res. Int. 2018, 112, 56–65. [Google Scholar] [CrossRef]
- Kullar, R.; Goldstein, E.J.C.; Johnson, S.; McFarland, L.V. Lactobacillus bacteremia and probiotics: A review. Microorganisms 2023, 11, 896. [Google Scholar] [CrossRef]
- Cruz, A.G.d.; Zacarchenco, P.B.; Oliveira, C.A.F.d.; Corassin, C.H. Microbiologia, Higiene e Controle de Qualidade no Processamento de Leites e Derivados, 1st ed.; Elsevier: Rio de Janeiro, Brazil, 2019; pp. 1–384. [Google Scholar]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Stewart, A.; Urban, J.F.; Huang, Y.; Chen, S.; Wu, L.-G.; Chesler, A. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity 2021, 54, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Igbafe, J.; Kilonzo-Nthenge, A.; Nahashon, S.N.; Mafiz, A.I.; Nzomo, M. Probiotics and antimicrobial effect of Lactiplantibacillus plantarum, Saccharomyces cerevisiae, and Bifidobacterium longum against common foodborne pathogens in poultry. Agriculture 2020, 10, 368. [Google Scholar] [CrossRef]
- Walker, C.; Thomas, M.G. Cultural Evolution and Diet. Oxf. Handb. Cult. Evol. 2023. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimarães, J.T.; Silva, R.; Filho, E.G.A.; Brito, E.S.; Pimentel, T.C.; Rodrigues, S.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.L.; et al. Effect of probiotic Minas Frescal cheese on the volatile compound and metabolic profiles assessed by nuclear magnetic resonance spectroscopy and chemometric tools. J. Dairy Sci. 2021, 104, 5133–5140. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.U.F.O.; Bevilaqua, G.C.; da Silva Nascimento, Í.R.; da Cruz Ximenes, G.N.; Andrade, S.A.C.; dos Santos Cortez Barbosa, N.M. Antagonist action of Lactobacillus acidophilus against pathogenic strains in goat milk yogurt. J. Food Sci. Technol. 2023, 60, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shi, X.; Wang, B. A review on the general cheese processing technology, flavor biochemical pathways and the influence of yeasts in cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics. Statistical Books. European Union. Eurostat. Production of Cheese. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics#Milk_products (accessed on 1 September 2023).
- Statista. Annual Consumption of Cheese Worldwide in 2019, by Selected Country (in 1000 Metric Tons). Available online: https://www.statista.com/statistics/868231/global-annual-consumption-of-cheese-by-country/ (accessed on 12 September 2023).
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Sameer, B.; Ganguly, S.; Khetra, Y.; Sabikhi, L. Development and characterization of probiotic buffalo milk ricotta cheese. LWT 2020, 121, 108944. [Google Scholar] [CrossRef]
- Valente, G.L.C.; Acurcio, L.B.; Freitas, L.P.V.; Nicoli, J.R.; Silva, A.M.; Souza, M.R.; Penna, C.F.A.M. Short communication: In vitro and in vivo probiotic potential of Lactobacillus plantarum B7 and Lactobacillus rhamnosus D1 isolated from Minas artisanal cheese. J. Dairy Sci. 2019, 102, 5957–5961. [Google Scholar] [CrossRef]
- Soares, M.B.; Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Pereira, E.P.R.; Balthazar, C.F.; Cruz, A.G.; Ranadheera, C.S.; Sant'Ana, A.S. Behavior of different Bacillus strains with claimed probiotic properties throughout processed cheese (“requeijão cremoso”) manufacturing and storage. Int. J. Food Microbiol. 2019, 307, 108288. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Yaqub, S.; Haq, I.u. Production of probiotic Mozzarella cheese by incorporating locally isolated Lactobacillus acidophilus. Ann. Microbiol. 2020, 70, 56. [Google Scholar] [CrossRef]
- Amiri, S.; Kohneshahri, S.R.A.; Nabizadeh, F. The effect of unit operation and adjunct probiotic culture on physicochemical, biochemical, and textural properties of Dutch Edam cheese. LWT 2022, 155, 112859. [Google Scholar] [CrossRef]
- El-Aidie, S.A.M.; Mabrouk, A.M.; Abd-Elgawad, A.R.; El-Garhi, H.-E.M. Physicochemical, textural and organoleptic properties of functional processed cheese manufactured from ultrafiltered milk. Biocatal. Agric. Biotechnol. 2023, 51, 102798. [Google Scholar] [CrossRef]
- El Sayed, H.S.; Mabrouk, A.M. Encapsulation of probiotics using mixed sodium alginate and rice flour to enhance their survivability in simulated gastric conditions and in UF-Kariesh cheese. Biocatal. Agric. Biotechnol. 2023, 50, 102738. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Uribe-Velázquez, T.; Rosales-De la Cruz, M.F.; Raygoza-Murguía, L.V.; Garcia-Amezquita, L.E.; García-Cayuela, T. Utilization of blueberry-based ingredients for formulating a synbiotic Petit Suisse cheese: Physicochemical, microbiological, sensory, and functional characterization during cold storage. LWT 2023, 183, 114955. [Google Scholar] [CrossRef]
- de Oliveira, C.M.S.; Grisi, C.V.B.; Silva, G.d.S.; Lopes Neto, J.H.P.; de Medeiros, L.L.; dos Santos, K.M.O.; Cardarelli, H.R. Use of Lactiplantibacillus plantarum CNPC 003 for the manufacture of functional skimmed fresh cheese. Int. Dairy J. 2023, 141, 105628. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Nagarajan, M.; Kumar, P.K.; Singh, S.S.; Manvi, D.; Gowda, N.A.N. A comprehensive review on microencapsulation of probiotics: Technology, carriers and current trends. Appl. Food Res. 2023, 3, 100248. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, M.; Xiao, H.; Young Quek, S.; Ogawa, Y.; Ma, G.; Zhang, C. Advances in spray-dried probiotic microcapsules for targeted delivery: A review. Crit. Rev. Food Sci. Nutr. 2023, 17, 1–17. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional dairy products as a source of bioactive peptides and probiotics: Current trends and future prospectives. J. Food Sci. Technol. 2022, 59, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.P.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. Peptide profile and angiotensin-converting enzyme inhibitory activity of Prato cheese with salt reduction and Lactobacillus helveticus as an adjunct culture. Food Res. Int. 2020, 133, 109190. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Y.; Wang, Y.; Xie, L.; Liang, S.; Li, D.; Wang, Y.; Wang, J.; Zhan, X. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics. Carbohydr. Polym. 2022, 289, 119438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol. Spectr. 2022, 10, e01651-22. [Google Scholar] [CrossRef] [PubMed]
- Mirković, M.; Mirković, N.; Miočinović, J.; Radulović, A.; Paunović, D.; Ilić, M.; Radulović, Z. Probiotic yogurt and cheese from ultrafiltered milk: Sensory quality and viability of free-living and spray dried Lactiplantibacillus plantarum 564 and Lactiplantibacillus plantarum 299v. J. Food Process. Preserv. 2021, 45, e15713. [Google Scholar] [CrossRef]
- Sharma, R.; Rashidinejad, A.; Jafari, S.M. Application of spray dried encapsulated probiotics in functional food formulations. Food Bioproc. Tech. 2022, 15, 2135–2154. [Google Scholar] [CrossRef]
- Mudgil, P.; Aldhaheri, F.; Hamdi, M.; Punia, S.; Maqsood, S. Fortification of Chami (traditional soft cheese) with probiotic-loaded protein and starch microparticles: Characterization, bioactive properties, and storage stability. LWT 2022, 158, 113036. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; El-Sayed, H.S.; Abo-Elwafa, G.A. Co-encapsulation of probiotic bacteria, fish oil and pomegranate peel extract for enhanced white soft cheese. Food Biosci. 2022, 50, 102083. [Google Scholar] [CrossRef]
- Estilarte, M.L.; Tymczyszyn, E.E.; Serradell, M.d.l.Á.; Carasi, P. Freeze-drying of Enterococcus durans: Effect on their probiotics and biopreservative properties. LWT 2021, 137, 110496. [Google Scholar] [CrossRef]
- Lopes Neto, J.H.P.; Santos, M.C.G.d.; Leite, K.S.; Silva, L.A.d.; Campos, M.I.F.; Silveira, E.S.d.; Amaral, J.B.S.; Madruga, M.S.; Braga, A.L.M.; Cardarelli, H.R. Development and characterization of Lactobacillus acidophilus (LA-3) microparticles with reducing substances and its addition to Reino cheese. LWT 2021, 143, 111083. [Google Scholar] [CrossRef]
- Silva, R.; Pimentel, T.C.; de Matos Junior, F.E.; Esmerino, E.A.; Freitas, M.Q.; Fávaro-Trindade, C.S.; Silva, M.C.; Cruz, A.G. Microencapsulation with spray-chilling as an innovative strategy for probiotic low sodium requeijão cremoso processed cheese processing. Food Biosci. 2022, 46, 101517. [Google Scholar] [CrossRef]
- Sharifi, S.; Rezazad-Bari, M.; Alizadeh, M.; Almasi, H.; Amiri, S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: Enhanced viability of probiotic in Iranian white cheese. Food Hydrocoll. 2021, 113, 106496. [Google Scholar] [CrossRef]
- Summo, C.; De Angelis, D. The importance of edible films and coatings for sustainable food development. Foods 2022, 11, 3221. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.G.; Atasoy, A.F. New bioactive edible packing systems/symbiotic edible films/coatings as carries of probiotics and prebiotics. Food Bioproc Tech. 2023, 16, 1413–1428. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Puertas, C.; Marcet, I.; Rendueles, M.; Díaz, M. Bioactive synbiotic coatings with lactobionic acid and Lactobacillus plantarum CECT 9567 in the production and characterization of a new functional dairy product. J. Funct. Foods 2020, 75, 104263. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. Optimization and characterization of prebiotic concentration of edible films containing Bifidobacterium animalis subsp. lactis BB-12® and its application to block type processed cheese. Int. Dairy J. 2022, 134, 105443. [Google Scholar] [CrossRef]
- Olivo, P.M.; Scapim, M.R.D.S.; Maia, L.F.; Miazaki, J.; Rodrigues, B.M.; Madrona, G.S.; Bankuti, F.I.; Pozza, M.S.D.S. Probiotic coating for ripened cheeses with Lactobacillus acidophilus and Lactobacillus Helveticus inclusion. J. Agric. Stud. 2020, 8, 152. [Google Scholar] [CrossRef]
- Guadalupe, F.; Ruiz-López, I.; Ochoa, V.C.E.; Hernandez Carranza, P. Effect of edible films’ application on the quality characteristics of manchego-type cheese during storage. Food Bioproc. Tech. 2023, 16, 2910–2920. [Google Scholar] [CrossRef]
- Rolim, F.R.L.; Freitas Neto, O.C.; Oliveira, M.E.G.; Oliveira, C.J.B.; Queiroga, R.C.R.E. Cheeses as food matrixes for probiotics: In vitro and in vivo tests. Trends Food Sci. Tech. 2020, 100, 138–154. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Maggiolino, A.; Faccia, M.; Franco, D.; De Palo, P.; Lorenzo, J.M. Buffalo milk as a source of probiotic functional products. Microorganisms 2021, 9, 2303. [Google Scholar] [CrossRef] [PubMed]
- Olajugbagbe, T.E.; Elugbadebo, O.E.; Omafuvbe, B.O. Probiotic potentials of Pediococuss acidilactici isolated from wara; A Nigerian unripened soft cheese. Heliyon 2020, 6, e04889. [Google Scholar] [CrossRef] [PubMed]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-enriched milk and dairy products increase gut microbiota diversity: A comparative study. Nutr. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Ganatsios, V.; Mantzourani, I.; Bosnea, L. White brined cheese production by incorporation of a traditional milk-cereal prebiotic matrix with a candidate probiotic bacterial strain. Appl. Sci. 2021, 11, 6182. [Google Scholar] [CrossRef]
- Machado, M.; Sousa, S.C.; Rodríguez-Alcalá, L.M.; Pintado, M.; Gomes, A.M. Functional lipid enriched probiotic cheese: Gastrointestinal stability and potential health benefits. Int. Dairy J. 2023, 144, 105700. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic acid bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ali Esmail, G.; Fahad Alzeer, A.; Valan Arasu, M.; Vijayaraghavan, P.; Choon Choi, K.; Abdullah Al-Dhabi, N. Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 293–299. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of COVID-19: The Zhejiang experience. J. Zhejiang Univ. Med. Sci. 2020, 49, 147–157. [Google Scholar]
- Mushtaq, M.; Gani, A.; Masoodi, F.A. Himalayan cheese (Kalari/Kradi) fermented with different probiotic strains: In vitro investigation of nutraceutical properties. LWT 2019, 104, 53–60. [Google Scholar] [CrossRef]
- Grom, L.; Rocha, R.; Balthazar, C.; Guimarães, J.; Coutinho, N.; Barros, C.; Pimentel, T.; Venâncio, E.; Junior, I.C.; Silva, P. Postprandial glycemia in healthy subjects: Which probiotic dairy food is more adequate? J. Dairy Sci. 2020, 103, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Yang, W.; Zhu, Q.; Zhang, G.; Zhang, X.; Liu, L.; Li, X.; Hussain, M.; Ni, C.; Jiang, X. Proteolysis and ACE-inhibitory peptide profile of Cheddar cheese: Effect of digestion treatment and different probiotics. LWT 2021, 145, 111295. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Ashfaq, K.; Ashfaq, T.; Ghaffari, M.A.; Ali, N.; Kazmi, F.; Sohail, N. The antithrombotic potential of bioactive peptides induced by buffalo milk probiotic cheddar cheese: Potential of bioactive peptides induced by buffalo milk probiotic cheddar cheese. Pak. Med. J. 2022, 5, 324–328. [Google Scholar] [CrossRef]
- Cuffia, F.; George, G.; Godoy, L.; Vinderola, G.; Reinheimer, J.; Burns, P. In vivo study of the immunomodulatory capacity and the impact of probiotic strains on physicochemical and sensory characteristics: Case of pasta filata soft cheeses. Food Res. Int. 2019, 125, 108606. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Rabah, H.; Carmo, F.L.R.; Ariute, J.C.; Aburjaile, F.F.; Brenig, B.; Guédon, E.; Le Loir, Y.; Jan, G.; Azevedo, V. Functional Swiss-type experimental cheeses diet promotes beneficial effects in mice gut microbiome during homeostasis and inflammation. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional foods, nutraceuticals and probiotics: A focus on human health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health benefits of consuming foods with bacterial probiotics, postbiotics, and their metabolites: A review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef]
- Chelladhurai, K.; Ayyash, M.; Turner, M.S.; Kamal-Eldin, A. Lactobacillus helveticus: Health effects, current applications, and future trends in dairy fermentation. Trends Food Sci. Tech. 2023, 136, 159–168. [Google Scholar] [CrossRef]
| Genus | Species | pH | Temperature Range (°C) | Applications | Reference |
|---|---|---|---|---|---|
| Lactococcus | Lactococcus lactis ssp. lactis | 4.6 | Tmi: 8; Ti:30; Tma: 40 | Mesophilic probiotic starter used for different types of dairy products such as cheese (Gouda and Edam). | [30,32] |
| Lactococcus lactis ssp. lactis biovar diacetylactis | 4.6 | Tmi:8; Ti:22–28; Tma:40 | [30,32] | ||
| Lactococcus lactis ssp. cremoris | 5.6 | Tmi:4; Ti:20–28; Tma:37 | [30,32] | ||
| Streptococcus | Streptococcus thermophilus | 4.5 | Tmi:22; Ti:40; Tma:52 | Thermophilic probiotic starter used mainly for yogurts | [30,32] |
| Lactobacillus | Lactobacillus acidophilus | 4.2 | Tmi:27; Ti:37; Tma:48 | Mesophilic probiotic starter used for different types of dairy products, especially in cheeses during ripening | [30,32] |
| Lactobacillus delbrueckii ssp. bulgaricus | 3.8 | Tmi:22; Ti:45; Tma:52 | [30,32] | ||
| Lactobacillus delbrueckii ssp. lactis | 3.8 | Tmi:18; Ti:40; Tma:50 | [30,32] | ||
| Lactobacillus helveticus | 3.8 | Tmi:22; Ti:42; Tma:54 | - | [30,32] | |
| Lacticaseibacillus casei | - | Ti:30 | - | [30,32] | |
| Lactiplantibacillus plantarum | - | Tmi:12; Ti:37; Tma:40 | - | [33] | |
| Lacticaseibacillus rhamnosus | - | Ti:37 | - | [34] | |
| Leuconostoc | Leuconostoc mesenteroides ssp. cremoris | 4.5 | Ti:35 | Mesophilic starter culture used for cheeses like sour cream | [30] |
| Type of Cheese | Probiotic Microorganism Used | Type of Probiotic Culture | Quantity | References |
|---|---|---|---|---|
| Minas artisanal cheese | Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus | Isolated culture | 108 cfu/g | [48] |
| Prato cheese | Lacticaseibacillus casei-01 | DVS Culture | 107–108 CFU/g | [6] |
| Processed cheese (“requeijão cremoso”) | Bacillus coagulans MTCC 5856, Bacillus coagulans GBI-30 6086, Bacillus subtilis PXN 21, Bacillus subtilis PB6 and Bacillus flexus HK1 | Isolated culture | 106–107 spores/g | [49] |
| Feta | Lactobacillus acidophilus and Bifidobacterium animalis | DVS Culture | 1010 CFU/mL | [31] |
| Mozzarella cheese | Lactobacillus acidophilus | Isolated culture | 1010 CFU/g | [50] |
| Ricotta Cheese prepared from buffalo milk | Lactobacillus acidophilus La-05 | DVS Culture | 108 CFU/mL | [47] |
| Cheddar prepared from buffalo milk | Lactobacillus acidophilus and Bifidobacterium bifidum | DVS Culture | 108–1010 CFU/g | [1] |
| Minas Frescal Cheese | Lactococcus lactis NCDO 2118 | DVS Culture | 107–108 CFU/g | [5] |
| Spreadable ricotta cheese | Lacticaseibacillus paracasei BGP1 | DVS Culture | 1010 CFU/mL | [8] |
| Dutch Edam cheese | Lacticaseibacillus casei LAFTI-L26 | DVS Culture | 108 CFU/mL | [51] |
| Wagashi cheese | Lacticaseibacillus rhamnosus and Lactiplantibacillus plantarum | DVS Culture | 108–109 CFU/mL | [9] |
| Processed cheese | Lactiplantibacillus plantarum NRC AM10 and Limosilactobacillus reuteri NBIMCC 1587 | Isolated culture | 3% (1:1) | [52] |
| Cream cheese | Lactiplantibacillus plantarum CCMA 0359 | Isolated culture | 1010 CFU/g | [13] |
| Kariesh cheese | Bifidobacterium lactis BB-12, Lacticaseibacillus rhamnosus NRRL B-442 and Lactobacillus gasseri NRRL B-14168 | Isolated culture | 3% | [53] |
| Petit Suisse | Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12 | DVS Culture | 1010 CFU/g | [54] |
| Fresh cheese prepared from skimmed milk | Lactiplantibacillus plantarum CNPC 003 | Isolated culture | 109 CFU/L | [55] |
| Technique Encapsulation | Disadvantages | Benefits | Reference |
|---|---|---|---|
| Extrusion |
|
| [46,57] |
| Emulsion |
|
| [46,57] |
| Coacervation |
|
| [57] |
| Spray drying |
|
| [46,57,59,64,65] |
| Freeze-drying |
|
| [46,57] |
| Type of Cheese | Probiotic Strain | Preservation Technology | Encapsulating Material | Process Conditions | Application Stage | Storage Time (Days) | Main Results | Log CFU/g/log CFU/mL | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cream cheese | Lactiplantibacillus plantarum | Spray-drying | Whey powder | T = 150 °C | After homogenization of the mass | 90 | High viability at the simulated gastrointestinal tract; It did not alter the organoleptic properties of the cheese. | I = 8.69; F = 8.31 | [13] |
| Chami (traditional soft cheese) | Pediococcus pentosaceus | Freeze-drying | Camel milk proteins and wheat starch | T = −80 °C | After homogenization of the mass | 9 | Camel milk proteins revealed higher cell viability (98.6%) than probiotic cells encapsulated with wheat starch (70.7%). | I = 9.23/9.57; F = 9.10; 6.77 | [66] |
| Kariesh cheese | Bifidobacterium lactis BB-12, Lacticaseibacillus rhamnosus NRRL B-442 and Lactobacillus gasseri NRRL B-14168 | Extrusion | Sodium alginate and rice flour | Rice flour (1.3 and 5%); 0.2 M of CaCl2 solution | After homogenization of the mass | 21 | The survival rate of probiotics when exposed to in-vitro simulated gastrointestinal solutions was recorded at 72.9, containing 5% of rice flour. | I = 8.59, 8.69, 8.87; F = 5.39, 6.33, 6.89 | [53] |
| White soft cheese | Bifidobacterium lactis BB12 | Extrusion | Sodium alginate, fish oil, and pomegranate peel extract (PPE) | Fish oil (5 and 10%); PPE (36.55 μg/mL); 0.2 M of CaCl2 solution | Before coagulation | 30 | The probiotic + fish oil + PPE emulsion protected the probiotic bacteria during storage for 30 days. | I = 8.08; F = 5.5 | [67] |
| Feta cheese | Lactobacillus acidophilus and Bifidobacterium animalis | Emulsification method | Sodium alginate and pectin | Solutions of sodium alginate or pectin (2% w/v) | After pressing and cutting the mass | 45 | Sodium alginate proved to be more efficient in relation to the viability of probiotics compared to pectin. | I = 9.3; F = 8.6 | [31] |
| Cream cheese | Enterococcus durans | Freeze-drying | Maltodextrin and cryoprotectant (sucrose and lactulose) | T = −50 °C | After homogenization of the mass | 92 | E. durans strains show high resistance to the freeze-drying process in the presence of all cryoprotectants used, and the best results were obtained when lactulose, a prebiotic sugar was used. | I = 8.38; F = 8.04 | [68] |
| Goat Ricotta | Lactobacillus acidophilus (La-05) | Ionic gelation | Alginate and chitosan | Solution of CaCl2 at 0.5 mol/L | After salting the mass | 7 | Microencapsulation of probiotic cultures resulted in increased probiotic survival. | I = 7.18; F = 6.88 | [7] |
| Reino cheese | Lactobacillus acidophilus (LA-3) | Ionic gelation | Biopolymeric solution (ascorbic acid (0.04% w/v), L-cysteine hydrochloride (0.04% w/v), and sodium alginate (4% (w/v)) | Solution of CaCl2 at 4% (w/v) | After heating the milk | 25 | High viability in the cheese containing the microcapsules | I = 9.34; F = 8.49 | [69] |
| Processed cheese (Requeijão cremoso) | Lactobacillus acidophilus | Spray chilling | Air pressures (2.5 kgf/cm2) | Added at the beginning of the mass heating process | 90 | The formulation containing microcapsules showed greater sensory acceptance of texture and probiotic counts greater than 6 log CFU/g during storage and simulation of gastrointestinal conditions. | I = 7.52; F = 7.15 | [70] | |
| Iranian white cheese | Lactiplantibacillus plantarum ATCC 8014 | Liophilization and spray drying | Whey protein isolate (WPI) and Gum Arabic (GA) | T = −80 and 100 °C | After heating the milk | 61 | High survivability of L. plantarum ATCC 8014 in freeze-dried microcapsules than in spray-dried microcapsules during storage time (60 days). | I = 9.11; F = 6.44 | [71] |
| Type of Cheese | Probiotics Strains | Objective | Beneficial Potential | Reference |
|---|---|---|---|---|
| Prato | Lacticaseibacillus casei-01 | Efficacy of repeated consumption of probiotic Prato cheese against the inflammatory and oxidative condition induced by cigarette smoke in a mouse model | Repeated intake of probiotic cheese reduced oxidative stress in the lungs, intestine, and liver, and alleviated inflammation in the lungs. | [6] |
| Kalari | Lactobacillus plantarum (NCDC 012), Lacticaseibacillus casei (NCDC 297), Levilactobacillus brevis (NCDC 021) | Evaluate the in vitro anti-proliferative, immunomodulatory, and antidiabetic potential of Kalari cheese incorporated with probiotics | The addition of probiotics enhanced the antiproliferative (against human breast and colon cancer cells, neuroblastoma), antidiabetic, antimicrobial, and immunomodulatory activity of the Kalari cheese | [88] |
| Minas Frescal (Brazil) | Lactococcus lactis NCDO 2118 | To investigate the probiotic therapeutic effects of a Minas Frescal cheese containing L. lactis NCDO 2118 on ulcerative colitis induced by dextran sodium sulfate in mice. | Mice that consumed the probiotic cheese exhibited reduced severity of colitis, with attenuated weight loss, lower disease activity index, limited shortening of the colon length, and reduced histopathological score. | [5] |
| Minas Frescal and Prato (Brazil) | Lacticaseibacillus casei-01 | To evaluate the effect of different probiotic dairy matrices on antihyperglycemic activity in vitro and in vivo. | Prato cheese showed greater anti-hyperglycemic activity in vitro (greater inhibitory activity of α-amylase and α-glucosidase) and in vivo [less increase in postprandial glycemia and maintenance of other glycemic indices in healthy individuals. | [89] |
| Chami | Pediococcus pentosaceus | Incorporation of microencapsulated probiotic strain in Chami, and evaluation of antidiabetic activity in vitro | Chami fortified with encapsulated probiotic bacteria exhibited greater retention of bioactive properties, in terms of inhibition of α-glucosidase and Dipeptidyl peptidase IV (DPP-IV) during storage. | [66] |
| Cheddar | Lactobacillus helveticus 1.0612, Lacticaseibacillus rhamnosus 1.0911, Lacticaseibacillus casei 1.0319 | To evaluate the influence of digestion and the addition of different probiotics in cheddar cheese regarding the degree of proteolysis and the inhibitory activity of the angiotensin-converting enzyme (ACE) | Cheddar cheese with different probiotics contributed to the release of ACE-I peptides and in vitro digestion further increased their activity in cheese samples. | [90] |
| Fresh cheese | Lactiplantibacillus plantarum 299v, Bifidobacterium animalis Bo | To evaluate the potential of probiotic cheese fortified with bioactive fatty acids using in vitro models with an emphasis on modulating obesity-related metabolism and the immunomodulatory response. | The combination of the strains with the fatty acids in the cheese provided an increase in bacterial survival during passage through the gastrointestinal tract, indicating a possible synergistic effect between both. The digested fractions also stimulated the production of adipokines, reduced lipid accumulation in hepatocytes, increased adipolysis, and had an anti-inflammatory effect. | [83] |
| Fresh cheese | Lactococcus lactis LB1022, Lactiplantibacillus plantarum LB1418 | To evaluate the effect of probiotic cheese on inducing alcohol metabolism | Intake of probiotic cheese improved alcohol metabolism, regulated fatty acid oxidation, and prevented the formation of fat and inflammation in the liver | [11] |
| Cheddar | Lactobacillus acidophilus and distinct mesophilic starter cultures | To evaluate the antithrombotic efficacy of buffalo milk probiotic cheese | The water-soluble extract of probiotic cheddar cheese showed greater antithrombotic activity compared to the control cheese, and the activity increased with the ripening period. | [91] |
| Fior di Latte-type | Lactobacillus rhamnosus GG and Lactobacillus acidophilus LA5 | To study the impact of adding Lactobacillus probiotics in cheese (Fior di Latte-type) on evaluating their immunomodulatory capacity using an in vivo murine model. | Probiotic cheeses (with individual or combined strains) were able to modulate the immune system of mice, reducing the secretion of pro-inflammatory cytokines in the intestine, and increasing the secretion of secretory IgA (S-IgA) | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, H.C.S.; de Jesus, M.S.; Sandes, R.D.D.; Leite Neta, M.T.S.; Narain, N. Functional Cheeses: Updates on Probiotic Preservation Methods. Fermentation 2024, 10, 8. https://doi.org/10.3390/fermentation10010008
Araujo HCS, de Jesus MS, Sandes RDD, Leite Neta MTS, Narain N. Functional Cheeses: Updates on Probiotic Preservation Methods. Fermentation. 2024; 10(1):8. https://doi.org/10.3390/fermentation10010008
Chicago/Turabian StyleAraujo, Hannah Caroline Santos, Mônica Silva de Jesus, Rafael Donizete Dutra Sandes, Maria Terezinha Santos Leite Neta, and Narendra Narain. 2024. "Functional Cheeses: Updates on Probiotic Preservation Methods" Fermentation 10, no. 1: 8. https://doi.org/10.3390/fermentation10010008
APA StyleAraujo, H. C. S., de Jesus, M. S., Sandes, R. D. D., Leite Neta, M. T. S., & Narain, N. (2024). Functional Cheeses: Updates on Probiotic Preservation Methods. Fermentation, 10(1), 8. https://doi.org/10.3390/fermentation10010008





