Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. WPS Silage Chemical Composition Analysis
2.3. WPS Silage Bacterial Community Sequencing Analysis
2.4. WPS Silage Metabolite Analysis
2.5. Statistical Analysis and Graphing
3. Results
3.1. Chemical Compositions of WPS and WPS Silage
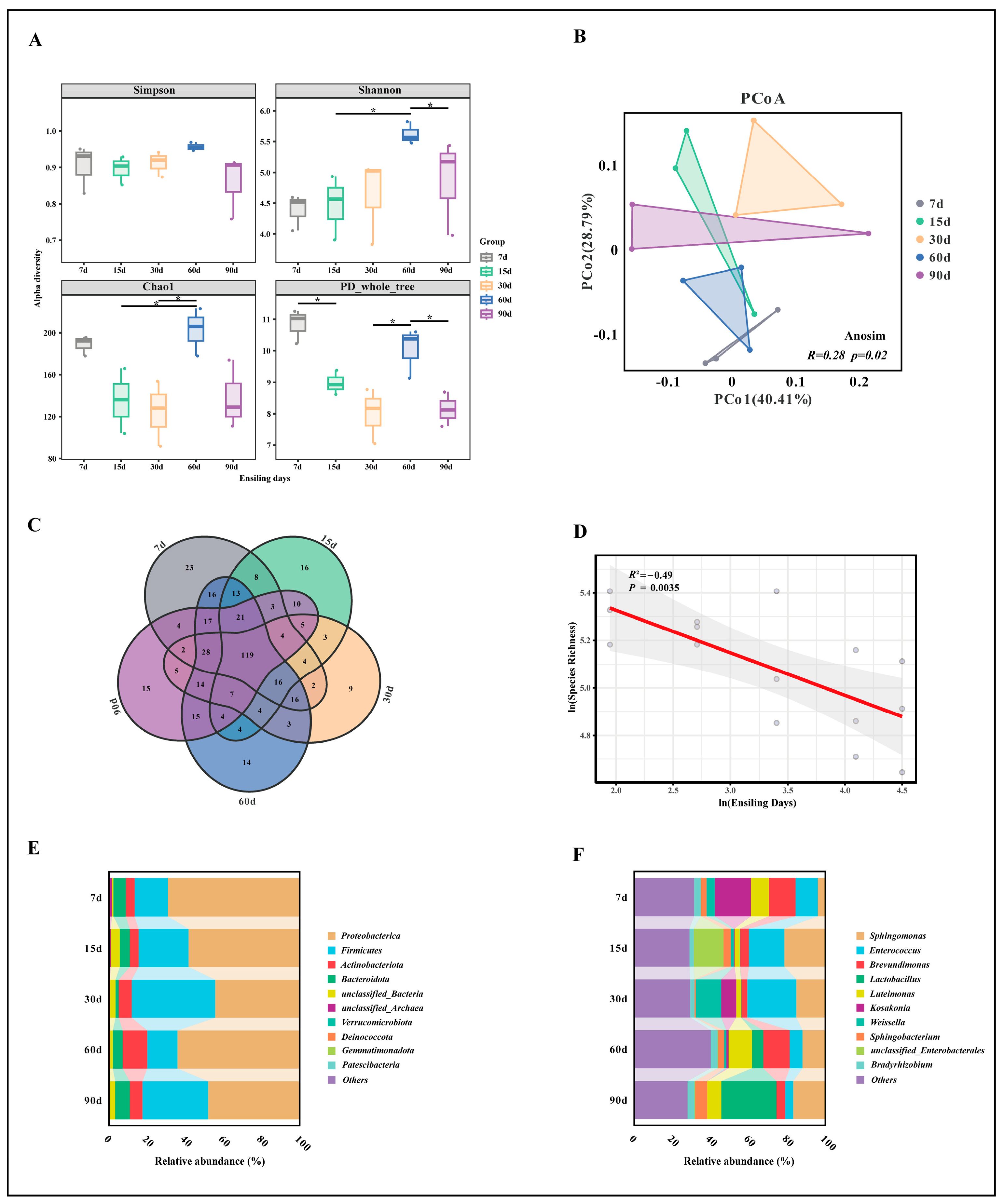
3.2. Microbial Community of the WPS Silage
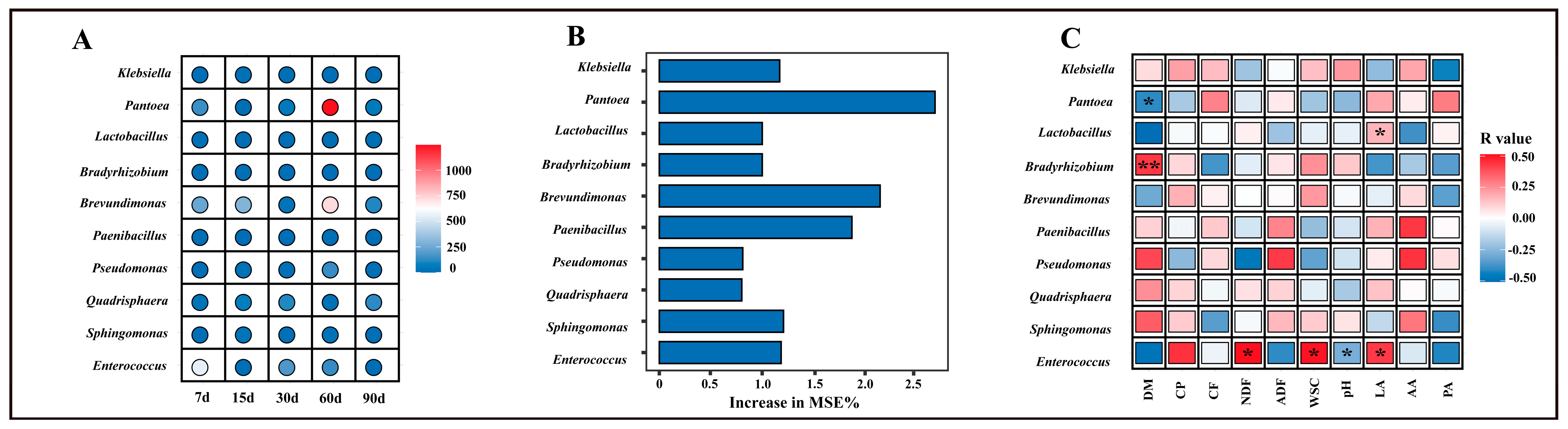
3.3. Biomarker Selection and Correlation with Fermentation and Nutritional Indicators
3.4. Metabolite Analysis of WPS Silage
3.5. Correlations Between Chemical Composition and Microbial Community
4. Discussion
4.1. Fermentation Quality and Nutritional Indicators of the WPS Silage
4.2. Microbial Community and Key Bacteria of the WPS Silage
4.3. Metabolites Analysis and Correlation of Metabolites with Microbiome and Fermentation Indicators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, L.; Bao, W.; Yao, C.; Zhao, F.; Jin, H.; Huang, W.; Li, B.; Kwok, L.; Liu, W. Changes in chemical composition, structural and functional microbiome during alfalfa (Medicago sativa) ensilage with Lactobacillus plantarum PS-8. Anim. Nutr. 2022, 9, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, T.; Ding, X.; Zhang, Q.; Yang, L.; Nie, Z.; Zhao, T.; Gai, J.; Yang, S. Confirmation of GmPPR576 as a fertility restorer gene of cytoplasmic male sterility in soybean. J. Exp. Bot. 2021, 72, 7729–7742. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of gallic acid on fermentation parameters, protein fraction, and bacterial community of whole plant soybean silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef]
- An, D.; Lai, X.; Han, T.; Nsigayehe, J.M.V.; Li, G.; Shen, Y. Crossing latitude introduction delayed flowering and facilitated dry matter accumulation of soybean as a forage crop. J. Integr. Agr. 2024, in press. [CrossRef]
- Liu, Z.; Cao, Y.; Wang, Z.; Ren, H.; Amidon, T.; Lai, Y. The Utilization of Soybean Straw. II. Dissolution & Regeneration of Soybean Straw in LiCl/DMSO. Bioresources 2015, 10, 2305–2317. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Bolsen, K.K.; Lin, C.J. History of silage. Silage Sci. Technol. 2003, 42, 1–30. [Google Scholar]
- de Morais, J.P.G.; Cantoia Júnior, R.; Garcia, T.M.; Capucho, E.; Campana, M.; Gandra, J.R.; Ghizzi, L.G.; Del Valle, T.A. Chitosan and microbial inoculants in whole-plant soybean silage. J. Agric. Sci. 2021, 159, 227–235. [Google Scholar] [CrossRef]
- Driehuis, F.; Elferink, S.O. The impact of the quality of silage on animal health and food safety: A review. Vet. Quart. 2000, 22, 212–216. [Google Scholar] [CrossRef]
- Yang, J.; Tan, H.; Cai, Y. Characteristics of lactic acid bacteria isolates and their effect on silage fermentation of fruit residues. J. Dairy Sci. 2016, 99, 5325–5334. [Google Scholar] [CrossRef]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S. Condensed tannins affect bacterial and fungal microbiomes and mycotoxin production during ensiling and upon aerobic exposure. Appl. Environ. Microb. 2018, 84, e2217–e2274. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Kharazian, Z.A.; Xu, D.; Su, R.; Guo, X. Effects of inoculation and dry matter content on microbiome dynamics and metabolome profiling of sorghum silage. Appl. Microbiol. Biot. 2024, 108, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, M.; Hao, J.; Yan, X.; Fu, Z.; Zhu, N.; Jia, Y.; Wang, Z.; Ge, G. Effects of temperature and lactic acid Bacteria additives on the quality and microbial community of wilted alfalfa silage. BMC Plant Biol. 2024, 24, 844. [Google Scholar] [CrossRef]
- Guo, X.S.; Ke, W.C.; Ding, W.R.; Ding, L.M.; Xu, D.M.; Wang, W.W.; Zhang, P.; Yang, F.Y. Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 2018, 8, 357. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat: A Review. J. Agr. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e177675. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, N.; Rinne, M.; Ke, W.; Weinberg, Z.G.; Da, M.; Bai, J.; Zhang, Y.; Li, F.; Guo, X. The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 2021, 14, 561–576. [Google Scholar] [CrossRef]
- Jiang, Y.; Xue, E.Y.; Lu, W.C.; Cui, G.W.; Li, Y.M.; Han, T.F.; Wang, S.D. Breeding and feeding quality analysis of a new soybean strain deficient in Kunitz trypsin inhibitor. Acta Prataculturae Sin. 2020, 29, 91. (In Chinese) [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Aber, UK, 1991. [Google Scholar]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Meng, H.; Jiang, Y.; Wang, L.; Wang, S.; Zhang, Z.; Tong, X.; Wang, S. Effects of Different Soybean and Maize Mixed Proportions in a Strip Intercropping System on Silage Fermentation Quality. Fermentation 2022, 8, 696. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Zhang, Q. Effects of polyphenol oxidases on proteolysis and lipolysis during ensiling of Moringa oleifera leaves with or without pyrocatechol. Anim. Feed Sci. Tech. 2021, 275, 114870. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Guyader, J.; Baron, V.S.; Beauchemin, K.A. Corn Forage Yield and Quality for Silage in Short Growing Season Areas of the Canadian Prairies. Agronomy 2018, 8, 164. [Google Scholar] [CrossRef]
- Zeng, T.; Li, X.; Guan, H.; Yang, W.; Liu, W.; Liu, J.; Du, Z.; Li, X.; Xiao, Q.; Wang, X.; et al. Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.I.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Smith, L.H. Theoretical Carbohydrates Requirement for Alfalfa Silage Production. Agron. J. 1962, 54, 291–293. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.; Zhou, H.; Lv, R.; Tang, J.; Cai, Y. Silage fermentation and ruminal degradation of cassava foliage prepared with microbial additive. Amb Express 2019, 9, 180. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Shao, T. Effect of ensiling corn stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the Tibetan Plateau. Grassl. Sci. 2017, 63, 236–244. [Google Scholar] [CrossRef]
- Maneerat, W.; Prasanpanich, S.; Tumwasorn, S.; Laudadio, V.; Tufarelli, V. Evaluating agro-industrial by-products as dietary roughage source on growth performance of fattening steers. Saudi J. Biol. Sci. 2015, 22, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hao, F.; Lv, X.; Chen, B.; Yang, Y.; Zeng, X.; Yang, F.; Wang, H.; Wang, L. Diversity of lactic acid bacteria in Moutai-flavor liquor fermentation process. Food Biotechnol. 2020, 34, 212–227. [Google Scholar] [CrossRef]
- Li, R.; Jiang, D.; Zheng, M.; Tian, P.; Zheng, M.; Xu, C. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 2020, 10, 17782. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Abd-Talib, N.; Aziz, R. Review on Crucial Parameters of Silage Quality. Apcbee Procedia 2012, 3, 99–103. [Google Scholar] [CrossRef]
- Gao, R.; Wang, B.; Jia, T.; Luo, Y.; Yu, Z. Effects of different carbohydrate sources on alfalfa silage quality at different ensiling days. Agriculture 2021, 11, 58. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Zheng, M.; Chen, D.; Chen, X. Altering microbial communities: A possible way of lactic acid bacteria inoculants changing smell of silage. Anim. Feed Sci. Tech. 2021, 279, 114998. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biot. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Wang, C.; He, L.; Zhou, W.; Yang, F.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Guo, J.; Shen, Y.; Yang, J. The correlations and spatial characteristics of microbiome and silage quality by reusing of citrus waste in a family-scale bunker silo. J. Clean. Prod. 2019, 226, 407–418. [Google Scholar] [CrossRef]
- Östling, C.; Lindgren, S. Influences of enterobacteria on the fermentation and aerobic stability of grass silages. Grass Forage Sci. 1995, 50, 41–47. [Google Scholar] [CrossRef]
- Dong, C.; Liu, P.; Wang, X.; Zhang, W.; He, L. Effects of Phenyllactic Acid on Fermentation Parameters, Nitrogen Fractions and Bacterial Community of High-Moisture Stylo Silage. Fermentation 2023, 9, 572. [Google Scholar] [CrossRef]
- Baldwin, I.L.; MacCoy, E. Root Nodule Bacteria and Leguminous Plants; University of Wisconsin: Madison, WI, USA, 1932. [Google Scholar]
- Sun, R.; Yuan, X.; Li, J.; Tao, X.; Dong, Z.; Shao, T. Contributions of epiphytic microbiota on the fermentation characteristics and microbial composition of ensiled six whole crop corn varieties. J. Appl. Microbiol. Appl. Microbiol. 2021, 131, 1683–1694. [Google Scholar] [CrossRef]
- Tian, J.; Huang, L.; Tian, R.; Wu, J.; Tang, R.; Zhang, J. Fermentation quality and bacterial community of delayed filling stylo silage in response to inoculating lactic acid bacteria strains and inoculating time. Chem. Biol. Technol. Agric. 2023, 10, 44. [Google Scholar] [CrossRef]
- Sun, L.; Bai, C.; Xu, H.; Na, N.; Jiang, Y.; Yin, G.; Liu, S.; Xue, Y. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 2021, 12, 655095. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, R.; Zhang, L.; Zi, X.; Zhou, H.; Tang, J. Melatonin is a promising silage additive: Evidence from microbiota and metabolites. Front. Microbiol. 2021, 12, 670764. [Google Scholar] [CrossRef]
- He, Q.; Zhou, W.; Chen, X.; Zhang, Q. Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 2021, 329, 129792. [Google Scholar] [CrossRef]
- Xia, G.; Wu, C.; Zhang, M.; Yang, F.; Chen, C.; Hao, J. The metabolome and bacterial composition of high-moisture Italian ryegrass silage inoculated with lactic acid bacteria during ensiling. Biotechnol. Biofuels Bioprod. 2023, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, Z.; Huang, S.; Lon, J.R.; Xie, S. Metabolic Profiling of Serum for Osteoarthritis Biomarkers. Dis. Markers 2022, 2022, 1800812. [Google Scholar] [CrossRef] [PubMed]
- Burlikowska, K.; Stryjak, I.; Bogusiewicz, J.; Kupcewicz, B.; Jaroch, K.; Bojko, B. Comparison of Metabolomic Profiles of Organs in Mice of Different Strains Based on SPME-LC-HRMS. Metabolites 2020, 10, 255. [Google Scholar] [CrossRef] [PubMed]



| Item | WPS |
|---|---|
| DM (%) | 34.26 ± 0.28 |
| CP (%) | 18.78 ± 0.38 |
| ADF (%) | 35.26 ± 0.55 |
| NDF (%) | 48.27 ± 0.58 |
| CF (%) | 3.77 ± 0.09 |
| WSC (%) | 3.84 ± 0.95 |
| Items | 7 d | 15 d | 30 d | 60 d | 90 d | p-Value |
|---|---|---|---|---|---|---|
| DM (%) | 35.01 ± 0.95 b | 37.29 ± 0.37 a | 37.94 ± 0.38 a | 37.16 ± 0.33 a | 37.34 ± 0.36 a | <0.001 |
| CP (%) | 18.32 ± 0.46 a | 18.35 ± 0.07 a | 17.02 ± 0.54 c | 17.92 ± 0.31 ab | 17.37 ± 0.33 bc | <0.001 |
| CF (%) | 3.72 ± 0.07 a | 3.63 ± 0.08 a | 3.18 ± 0.98 c | 3.45 ± 0.05 b | 3.56 ± 0.03 ab | <0.001 |
| NDF (%) | 48.55 ± 0.12 a | 45.87 ± 0.15 b | 46.11 ± 0.58 b | 43.11 ± 0.33 c | 43.77 ± 1.60 c | <0.001 |
| ADF (%) | 31.07 ± 0.49 b | 32.01 ± 0.60 b | 31.78 ± 0.71 b | 32.22 ± 0.82 b | 34.50 ± 1.39 a | 0.009 |
| WSC (%) | 2.51 ± 0.41 a | 1.88 ± 0.02 b | 1.63 ± 0.01 bc | 1.65 ± 0.16 bc | 1.39 ± 0.07 c | <0.001 |
| pH | 5.98 ± 0.14 a | 5.96 ± 0.13 a | 5.96 ± 0.34 a | 5.42 ± 0.07 b | 5.13 ± 0.16 b | <0.001 |
| LA (%) | 2.19 ± 0.04 b | 2.68 ± 0.18 a | 2.72 ± 0.09 a | 2.99 ± 0.16 a | 2.97 ± 0.11 a | <0.001 |
| AA (%) | 1.70 ± 0.02 a | 1.50 ± 0.11 b | 1.45 ± 0.04 b | 1.73 ± 0.18 a | 1.27 ± 0.08 c | <0.001 |
| PA (%) | 0.10 ± 0.02 c | 0.08 ± 0.03 c | 0.16 ± 0.04 ab | 0.11 ± 0.04 bc | 0.17 ± 0.02 a | 0.004 |
| BA (%) | - | - | - | 0.06 ± 0.03 b | 0.12 ± 0.01 a | <0.001 |
| NH3-H (%/TN) | 3.27 ± 0.13 d | 3.85 ± 0.31 c | 3.58 ± 0.10 cd | 4.47 ± 0.29 b | 5.17 ± 0.18 a | <0.001 |
| AS (h) | 54.00 ± 2.00 e | 62.67 ± 2.52 d | 100.67 ± 5.32 c | 108.67 ± 3.38 b | 112.00 ± 2.87 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Jiang, Y.; Wang, L.; Li, Y.; Wang, S.; Tong, X.; Wang, S. Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage. Fermentation 2024, 10, 535. https://doi.org/10.3390/fermentation10100535
Meng H, Jiang Y, Wang L, Li Y, Wang S, Tong X, Wang S. Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage. Fermentation. 2024; 10(10):535. https://doi.org/10.3390/fermentation10100535
Chicago/Turabian StyleMeng, He, Yan Jiang, Lin Wang, Yuanming Li, Sui Wang, Xiaohong Tong, and Shaodong Wang. 2024. "Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage" Fermentation 10, no. 10: 535. https://doi.org/10.3390/fermentation10100535
APA StyleMeng, H., Jiang, Y., Wang, L., Li, Y., Wang, S., Tong, X., & Wang, S. (2024). Dynamic Analysis of Fermentation Quality, Microbial Community, and Metabolome in the Whole Plant Soybean Silage. Fermentation, 10(10), 535. https://doi.org/10.3390/fermentation10100535







