Transcriptomic Analysis of Cell Stress Response in Wickerhamomyces anomalus H4 Under Octanoic Acid Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification
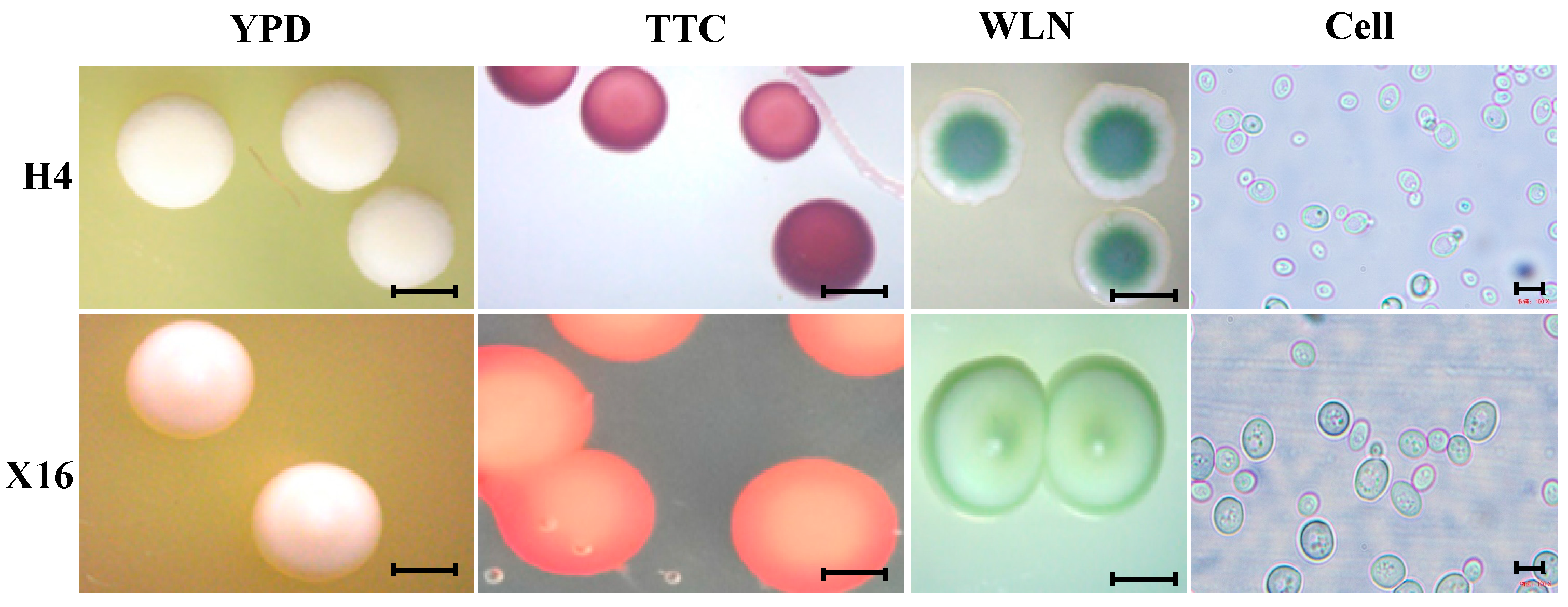
2.1.1. Isolation
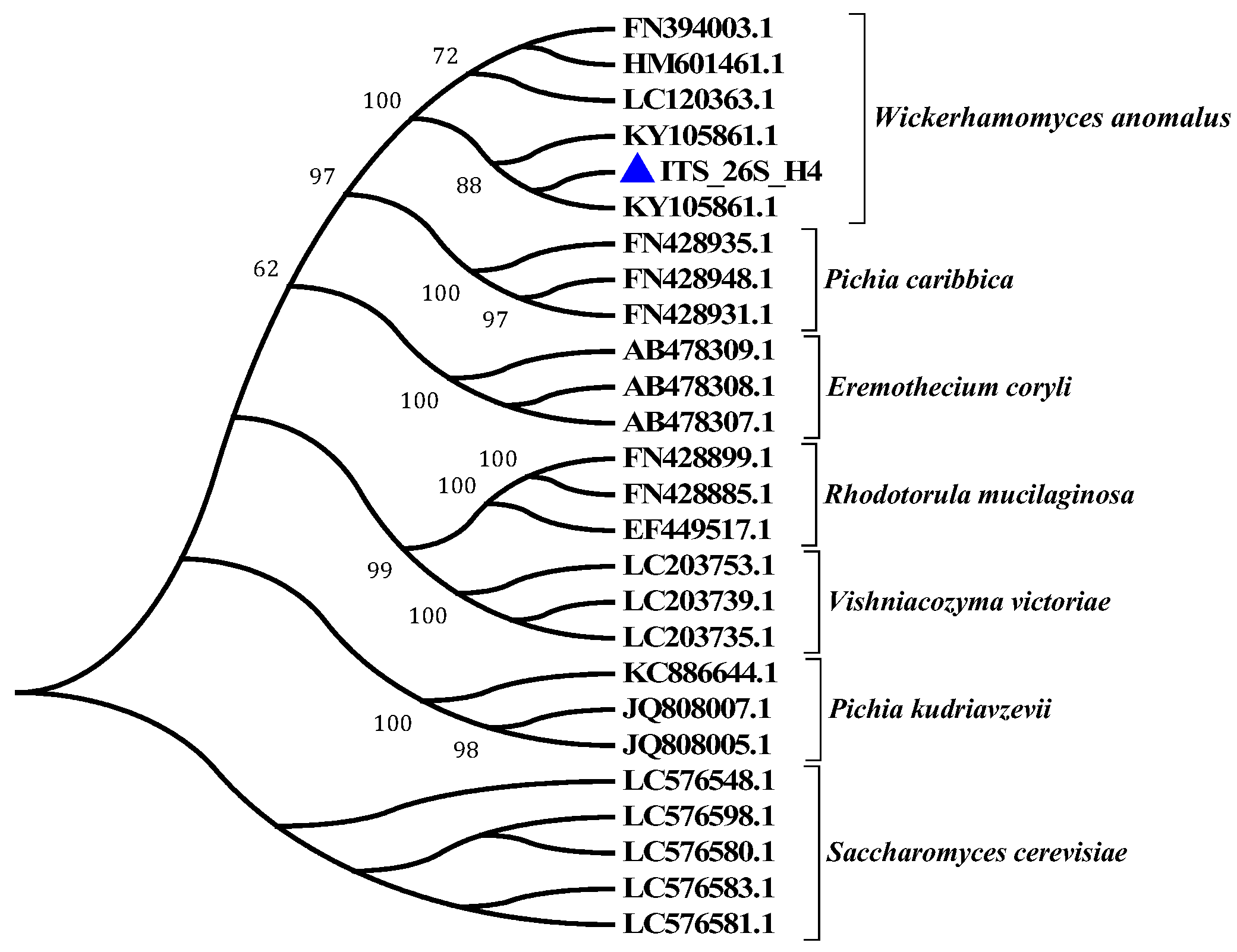
2.1.2. Identification
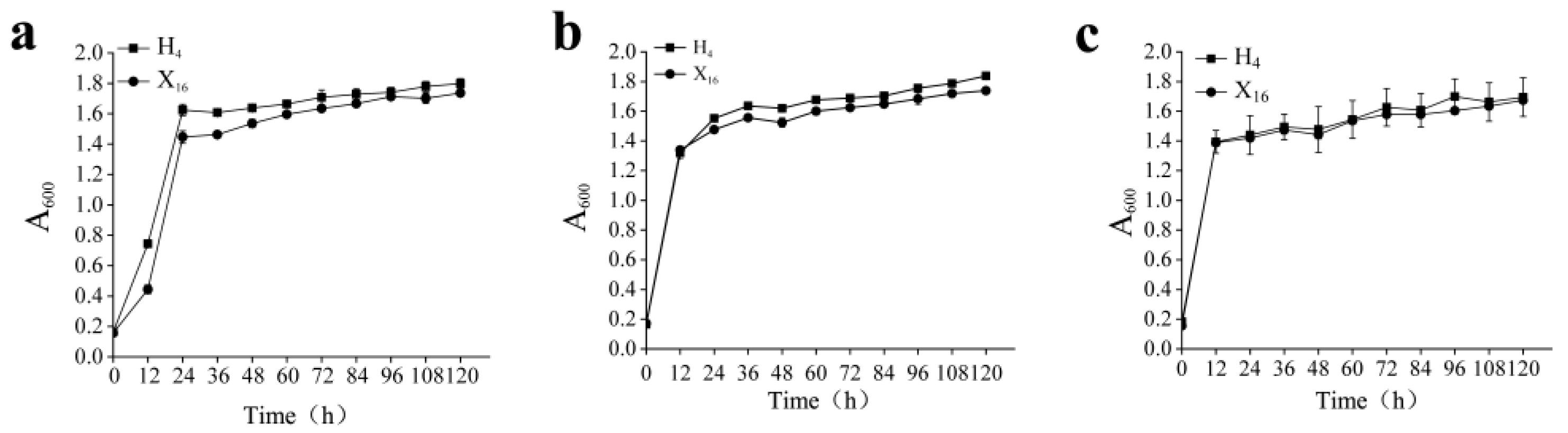
2.2. Growth Curve Analysis
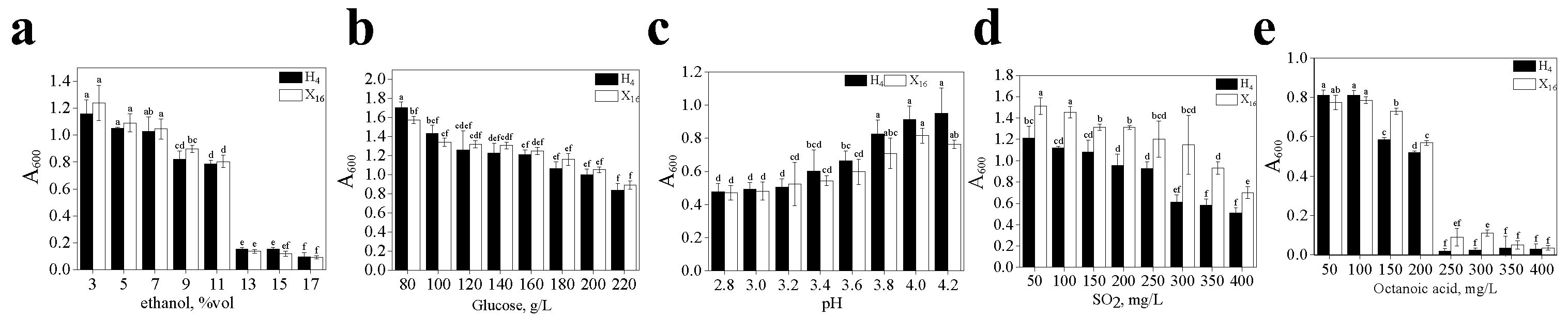
2.3. Brewing Potential and Survival Analysis
2.4. Transcriptomics Analysis
2.5. Statistics
3. Results and Discussion
3.1. Screening and Identification of Yeast Strains
3.2. Growth Curve Analysis
3.3. Traits of H4 for Winemaking
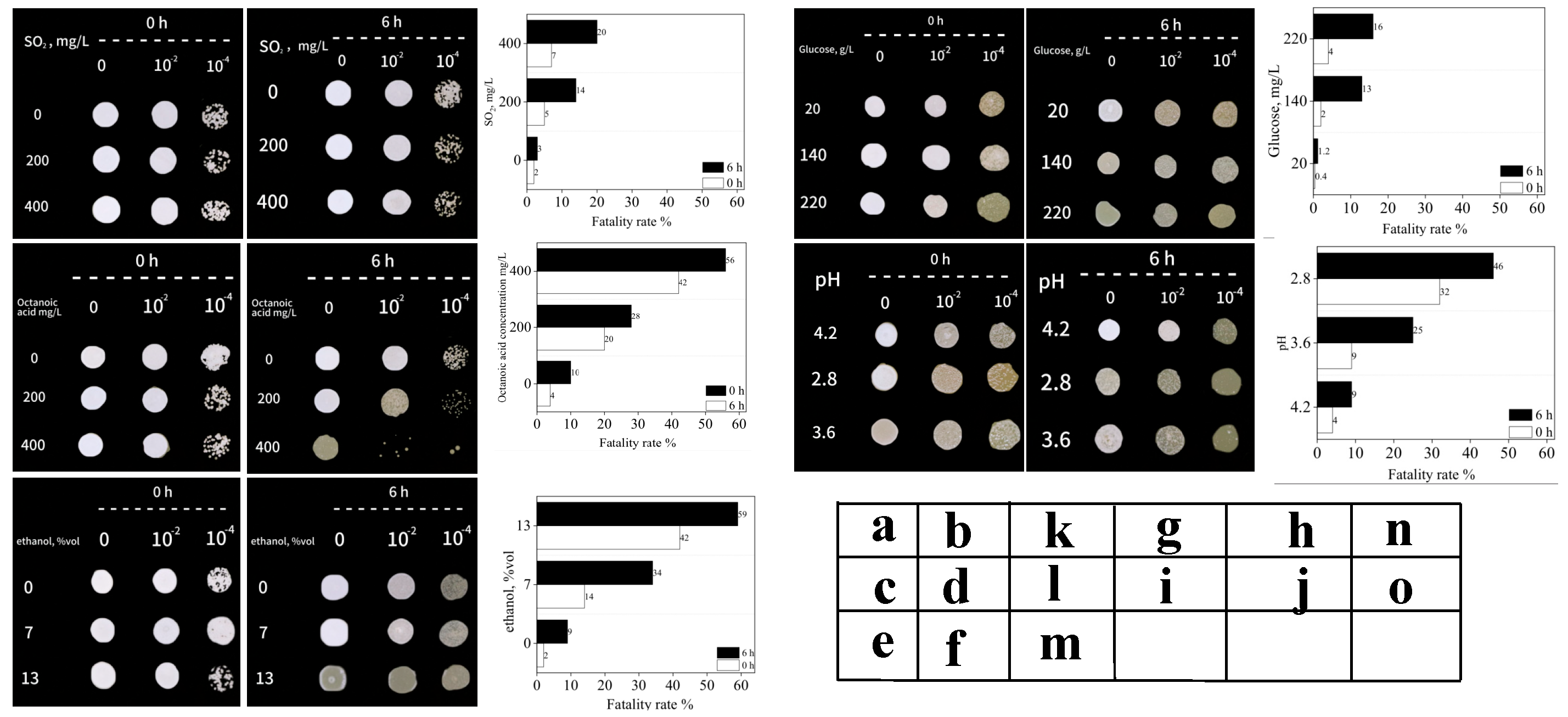
3.3.1. Tolerance Analysis of Strain H4
3.3.2. Survival Analysis of Strain H4
3.4. Transcriptomics Analysis
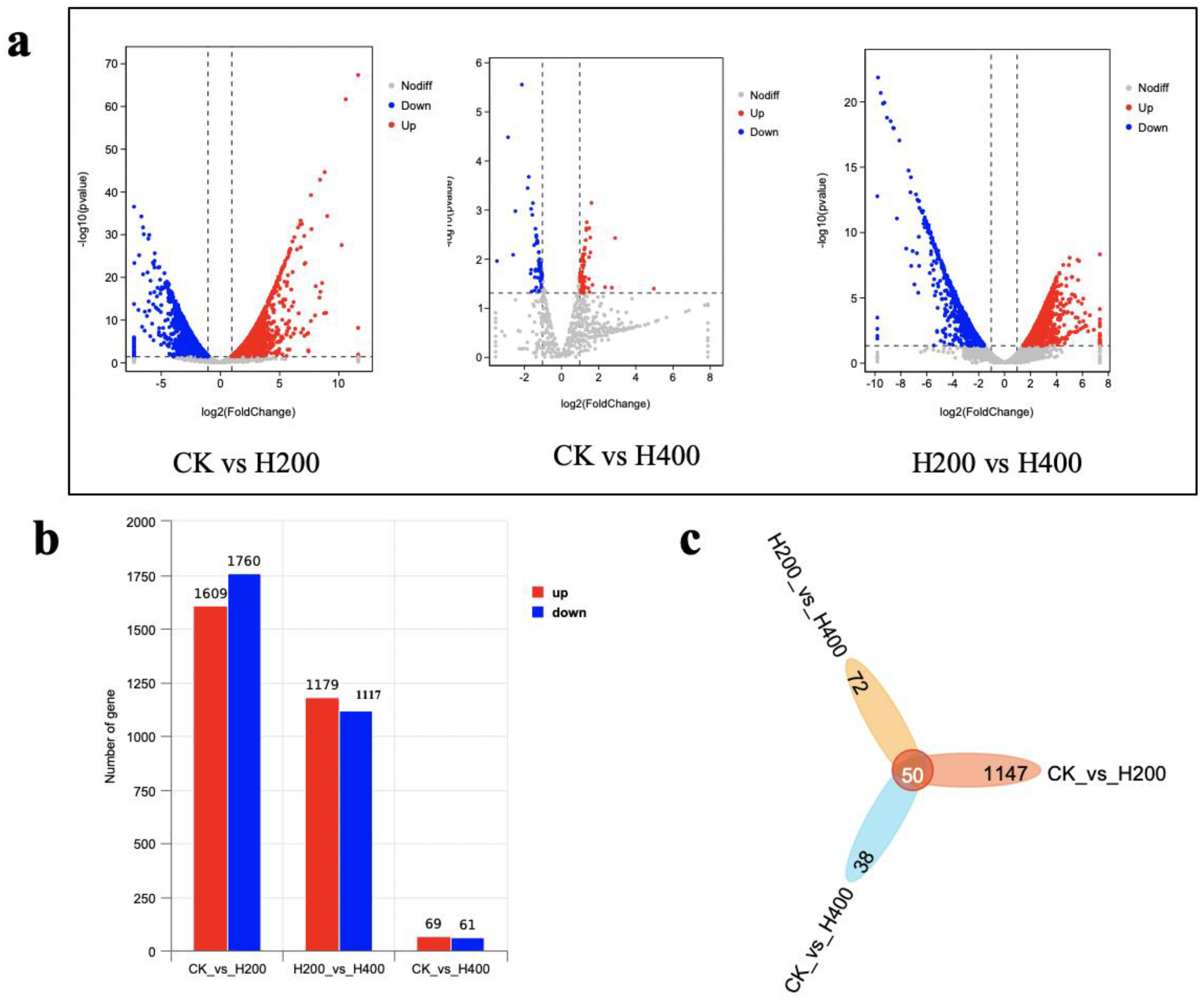
3.4.1. Identification of Differentially Expressed Genes (DEGs) by Transcriptomics
3.4.2. Defining Differentially Expressed Genes
3.4.3. Functional Enrichment Analysis of DEGs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtzman, C.P. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to Wickerhamomyces anomalus. Anton Van Leeuwenhoek 2011, 99, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Baliou, S.; Kofteridis, D.P. Fungemia by Wickerhamomyces anomalus—A Narrative Review. Pathogens 2024, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Yao, Z.; Wang, Z.; Huang, J.; Khan, M.T.; Chen, B.; Zhang, M. Fungal deterioration of the bagasse storage from the harvested sugarcane. Biotechnol. Biofuels 2021, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shi, Y.; Legrand Ngolong Ngea, G.; Zhang, X.; Yang, Q.; Zhang, Q.; Xu, X.; Zhang, H. Changes of the microbial community in kiwifruit during storage after postharvest application of Wickerhamomyces anomalus. Food Chem. 2023, 404 Pt A, 134593. [Google Scholar] [CrossRef]
- Cai, W.; Wan, Y.; Chen, Y.; Fan, H.; Li, M.; Wu, S.; Lin, P.; Zeng, T.; Luo, H.; Huang, D.; et al. Transcriptomics to evaluate the influence mechanisms of ethanol on the ester production of Wickerhamomyces anomalus with the induction of lactic acid. Food Microbiol. 2024, 122, 104556. [Google Scholar] [CrossRef]
- Li, Y.; Long, H.; Jiang, G.; Yu, Z.; Huang, M.; Zou, S.; Guan, T.; Zhao, Y.; Liu, X. Protective effects of thiamine on Wickerhamomyces anomalus against ethanol stress. Front. Microbiol. 2022, 13, 1057284. [Google Scholar] [CrossRef]
- Wang, W.; Fan, G.; Li, X.; Fu, Z.; Liang, X.; Sun, B. Application of Wickerhamomyces anomalus in simulated solid-state fermentation for Baijiu production: Changes of microbial community structure and flavor metabolism. Front. Microbiol. 2020, 11, 598758. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Ai, J.; Huang, W.; Zhan, J.; You, Y. Formation of vinylphenolic pyranoanthocyanins by selected indigenous yeasts displaying high hydroxycinnamate decarboxylase activity during mulberry wine fermentation and aging. Food Microbiol. 2023, 113, 104272. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, G.; Li, Y. Wickerhamyces anomalus as a potential biocontrol agent against Botrytis cinerea. J. Agric. Food Chem. 2017, 65, 7123–7131. [Google Scholar]
- Sadeghi, F.; Torkashvand, H.; Khanabadi, F.; Didehdar, M.; Latifi, R.; Fakhar, M.; Elmi, T. A review on Wickerhamomyces anomalus yeast: A promising eco-friendly approach to biological control of malaria. Ann. Parasitol. 2022, 68, 657–665. [Google Scholar]
- Zhang, J.; He, Y.; Yin, L.; Hu, R.; Yang, J.; Zhou, J.; Cheng, T.; Liu, H.; Zhao, X. Isolation of aroma-producing Wickerhamomyces anomalus yeast and analysis of its typical flavoring metabolites. Foods 2023, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, X.; Xiang, X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. AMB Express 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Valzano, M.; Cecarini, V.; Bozic, J.; Rossi, P.; Mensah, P.; Amantini, C.; Favia, G.; Ricci, I. Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit. Vectors 2019, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-H.; Huang, G.-D.; Huang, X.-Y.; Pu, J.-H.; Wu, J.-S.; Yue, L.-R.; Hardie, W.J.; Liu, X.-Z.; Huang, M.-Z. A comparative study of yeasts for Rosa roxburghii wine fermentation. Fermentation 2022, 8, 311. [Google Scholar] [CrossRef]
- Du, X.; Peng, Y.; Albero, J.; Li, D.; Hu, C.; García, H. Synthetic fuels from biomass: Photo-catalytic hydrodecarboxylation of octanoic acid by Ni nanoparticles deposited on TiO2. ChemSusChem 2022, 15, e202102107. [Google Scholar] [CrossRef]
- Cabral, M.G.; Viegas, C.A.; Sá-Correia, I. Mechanisms underlying the acquisition of resistance to octanoic-acid-induced-death following exposure of Saccharomyces cerevisiae to mild stress imposed by octanoic acid or ethanol. Arch. Microbiol. 2001, 175, 301–307. [Google Scholar] [CrossRef]
- Borrull, A.; López-Martínez, G.; Poblet, M.; Cordero-Otero, R.; Rozès, N. New insights into the toxicity mechanism of octanoic and decanoic acids on Saccharomyces cerevisiae. Yeast 2015, 32, 451–460. [Google Scholar] [CrossRef]
- Van der Aa Kühle, A.; Jespersen, L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S rDNA, the ITS1-5.8S rDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst. Appl. Microbiol. 2003, 26, 564–571. [Google Scholar] [CrossRef]
- Bai, F.Y. Reclassification of the Sporobolomyces roseus and Sporidiobolus pararoseus complexes, with the description of Sporobolomyces phaffii sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2309–2314. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Li, Y.; Long, H.; Jiang, G.; Gong, X.; Yu, Z.; Huang, M.; Guan, T.; Guan, Y.; Liu, X. Analysis of the ethanol stress response mechanism in Wickerhamomyces anomalus based on transcriptomics and metabolomics approaches. BMC Microbiol. 2022, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Thammaket, J.; Srimongkol, P.; Ekkaphan, P.; Thitiprasert, S.; Niyomsin, S.; Chaisuwan, T.; Chirachanchai, S.; Thongchul, N. Isolation, screening, and characterization of the newly isolated osmotolerant yeast Wickerhamomyces anomalus BKK11-4 for the coproduction of glycerol and arabitol. Braz. J. Microbiol. 2024, 55, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; de Vries, M.; Verkleij, G.J.; Crous, P.W.; Boekhout, T.; et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef]
- Turner, W.; Greetham, D.; Du, C. The characterisation of Wickerhamomyces anomalus M15, a highly tolerant yeast for bioethanol production using seaweed derived medium. Front. Bioeng. Biotechnol. 2022, 10, 1028185. [Google Scholar] [CrossRef] [PubMed]
- Sehnem, N.T.; Machado, Â.S.; Matte, C.R.; Morais, M.A., Jr.; Ayub, M.A.Z. Second-generation ethanol production by Wickerhamomyces anomalus strain adapted to furfural, 5-hydroxymethylfurfural (HMF), and high osmotic pressure. An. Acad. Bras. Cienc. 2020, 92 (Suppl. 2), e20181030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Yue, Z.; Tan, P.; Sun, M.; Ji, L.; Bai, Y.; Ma, X. Wickerhamomyces anomalus relieves weaning diarrhea via improving gut microbiota and redox homeostasis using a piglet model. Food Funct. 2022, 13, 11223–11235. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, L.; Shi, G.Y. Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2017, 33, 94. [Google Scholar] [CrossRef]
- Cai, M.; Zhou, J.; Hao, T.; Du, K. Tolerance of phyllospheric Wickerhamomyces anomalus to BDE-3 and heavy metals. Environ. Sci. Pollut. Res. 2022, 29, 56555–56561. [Google Scholar] [CrossRef]
- Coda, R.; Cassone, A.; Rizzello, C.G.; Nionelli, L.; Cardinali, G.; Gobbetti, M. Yeast community dynamics and volatile organic compound production during spontaneous apple juice fermentation. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef]

| Group | Raw Reads | Raw Bases | Clean Reads | Useful Reads % |
|---|---|---|---|---|
| 0 mg/L | 3.98 × 107 ± 3.81 × 106 | 6.01 × 109 ± 5.75 × 108 | 3.75 × 107 ± 3.61 × 106 | 94.15 ± 0.07 |
| 200 mg/L | 4.04 × 107 ± 2.78 × 106 | 6.11 × 109 ± 4.20 × 108 | 3.81 × 107 ± 2.57 × 106 | 94.08 ± 0.23 |
| 400 mg/L | 4.27 × 107 ± 3.34 × 106 | 6.45 × 109 ± 5.05 × 108 | 4.02 × 107 ± 3.13 × 106 | 94.05 ± 0.14 |
| Group | Useful bases % | Clean bases | Q20 % | Q30 % |
| 0 mg/L | 94.15 ± 0.07 | 5.66 × 109 ± 5.44 × 108 | 98.21 ± 0.06 | 94.24 ± 0.16 |
| 200 mg/L | 94.08 ± 0.23 | 5.75 × 109 ± 3.88 × 108 | 98.33 ± 0.30 | 94.52 ± 0.82 |
| 400 mg/L | 94.053 ± 0.14 | 6.07 × 109 ± 4.73 × 108 | 98.13 ± 0.22 | 94.05 ± 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.-H.; Li, L.; Chen, Q.-Y.; Huang, B.-X.; Shi, M.-Z.; Dong, W.-J.; Zu, Y.; Huang, M.-Z.; Liu, X.-Z. Transcriptomic Analysis of Cell Stress Response in Wickerhamomyces anomalus H4 Under Octanoic Acid Stress. Fermentation 2024, 10, 563. https://doi.org/10.3390/fermentation10110563
Yu Z-H, Li L, Chen Q-Y, Huang B-X, Shi M-Z, Dong W-J, Zu Y, Huang M-Z, Liu X-Z. Transcriptomic Analysis of Cell Stress Response in Wickerhamomyces anomalus H4 Under Octanoic Acid Stress. Fermentation. 2024; 10(11):563. https://doi.org/10.3390/fermentation10110563
Chicago/Turabian StyleYu, Zhi-Hai, Li Li, Qiu-Yu Chen, Bing-Xuan Huang, Ming-Zhi Shi, Wan-Jin Dong, Yuan Zu, Ming-Zheng Huang, and Xiao-Zhu Liu. 2024. "Transcriptomic Analysis of Cell Stress Response in Wickerhamomyces anomalus H4 Under Octanoic Acid Stress" Fermentation 10, no. 11: 563. https://doi.org/10.3390/fermentation10110563
APA StyleYu, Z.-H., Li, L., Chen, Q.-Y., Huang, B.-X., Shi, M.-Z., Dong, W.-J., Zu, Y., Huang, M.-Z., & Liu, X.-Z. (2024). Transcriptomic Analysis of Cell Stress Response in Wickerhamomyces anomalus H4 Under Octanoic Acid Stress. Fermentation, 10(11), 563. https://doi.org/10.3390/fermentation10110563





