Abstract
Corrosion is the deterioration of metals due to environmental exposure. Commercial inhibitors used to control corrosion often contain heavy metal salts, which are highly toxic to both the environment and human health. A biosurfactant produced by the bacterium Pseudomonas cepacia CCT 6659 was tested as a corrosion inhibitor on carbon steel and galvanized iron surfaces. Matrices based on plant ingredients with different compositions were tested in a laboratory-constructed accelerated corrosion chamber (ACC) simulating a critical maritime atmosphere in conditions of 40 °C, 5% NaCl, and 100% humidity. The most stable matrix was selected for biosurfactant incorporation in different concentrations, expressed as critical micellar concentration (CMC), and was applied to metal surfaces to evaluate its ability to inhibit corrosion. Additionally, to evaluate the potential of the biosurfactant as a low-toxicity corrosion inhibitor additive in paint systems, iron and carbon steel samples were coated with three biosurfactant-containing commercial paints and subjected to critical atmospheric conditions for testing coating effectiveness. The formulation containing vegetable resin as a plasticizer, oleic acid, ethanol, and CaCO3 was chosen to incorporate the biosurfactant. The addition of the biosurfactant at twice its CMC led to a reduction in carbon steel sample mass loss from 123.6 to 82.2 g/m2, while in the galvanized iron plates, the mass loss decreased from 285.9 to 226.7 g/m2 at the same biosurfactant concentration. When supplemented with the biosurfactant, the alkyd resin-based paint (A) ensured less mass loss in samples (46.0 g/m2) compared to the control without biosurfactant (58.0 g/m2). Using the paint formulated with oil-based resin (B), the mass loss decreased from 53.0 to 24.1 g/m2, while with that based on petroleum derivatives (C), it decreased from 82.2 to 27.6 g/m2. These results confirm the feasibility of using biosurfactants in biodegradable coatings, reducing the need for commercial corrosion inhibitors.
1. Introduction
Corrosion is basically defined as the deterioration that can be commonly found in metal structures exposed to the atmosphere or submerged in natural water [1]. This process involves redox reactions that convert the metal or metal component into oxide, hydroxide, or salt when in contact with harmful gases or moisture. A destructive oxidative process damages the structural elements of the attacked material, altering its characteristics and causing wear, chemical variations, or structural modifications [2]. Thus, the metal material loses its essential qualities, such as mechanical resistance, elasticity, ductility, aesthetics, and, unavoidably, durability and performance, no longer meeting the applications for which it is intended [3,4].
Damage caused by corrosion not only requires the replacement of these metal parts but can also cause environmental risks associated with the improper disposal of the resulting waste [5]. One of the most efficient methods adopted by industries to prevent this process is the use of corrosion inhibitors, which are chemical compounds that act when a metal comes into contact with an aggressive environment [6,7].
The growing awareness regarding human health and environmental pollution has led researchers to search for anticorrosive compounds with non-toxic, ecological, safe, and exceptional corrosion inhibition efficiency [8]. Several studies demonstrated the viability of using surfactants as potential corrosion inhibitors, since the surfactant molecules accumulate in multilayers or form micelles, resulting in a protective layer under the metal [9,10]. Biosurfactants are natural compounds with surfactant properties originating from plant sources or as metabolic byproducts of bacteria, yeasts, and filamentous fungi, which represent an ecological alternative to traditional chemical surfactants because they offer sustainable benefits to both the environment and human health [11]. In addition, the surface and interfacial activities of biosurfactants are more effective than those of conventional surfactants (anionic sulfated detergents), due to lower surface tension at lower active product concentration, lower toxicity, and greater biodegradability [12]. Bacteria belonging to the genus Pseudomonas are known to produce rhamnolipid-type biosurfactants, which, because of their remarkable surface tension reduction properties and high emulsification capacity, stand out for their efficiency and environmental compatibility [13].
Thanks to their excellent chemical properties and stability under extreme conditions of temperature, pH, and salinity, biosurfactants are widely recognized as low-molecular-weight surfactants, attracting the interest of researchers and industries. In addition, they act by modifying the surface tension of liquids, favoring better adhesion and spreading on metal surfaces, thus contributing to a slowing of the corrosive process [14].
Accelerated corrosion tests are a widely used method to simulate the effects of long-term corrosion in a short time interval [15]. These tests, which are performed in controlled environmental conditions, can be classified into single-factor simulations or coupled multi-factor simulations. Currently, commercial equipment allows salt spray and humidity tests to be performed on metallic or non-metallic test specimens coated with organic or inorganic layers of products. These tests are performed in accordance with specific technical standards, ensuring the quality and standardization of results. This equipment meets the needs of a variety of customers, including industries in the automotive, petrochemical, naval, energy, and construction sectors, as well as research centers and universities, which seek to ensure the durability and resistance of products and materials. Among these companies, we can mention the automobile manufacturers Volkswagen, Honda, Mercedes-Benz, etc. [16,17,18,19].
In this study, the possibility of using a previously characterized rhamnolipid biosurfactant as a corrosion inhibitor on metal surfaces was evaluated. The biosurfactant was produced from the cultivation of the bacterium Pseudomonas cepacia CCT 6659 using industrial waste as substrate, as described in Soares da Silva et al. [20]. To analyze its effectiveness, a bench-scale accelerated corrosion chamber (ACC) was built, enabling the simulation of critical conditions, such as exposure to salt spray. The tests evaluated the corrosion resistance of metal surfaces protected by coatings made from biosurfactants compared to commercial coatings. This study emphasizes the sustainable potential of biosurfactants as corrosion inhibitors due to their biodegradability, low toxicity, and effectiveness in aggressive environments.
2. Materials and Methods
2.1. Microorganism
The bacterial strain Pseudomonas cepacia CCT 6659, obtained from the culture bank of the André Tosello Foundation for Research and Technology, located in the city of Campinas, Brazil, was used as the biosurfactant producer. The cultures were subcultured every 30 days and kept refrigerated at 5 °C in inclined test tubes containing nutrient agar solid medium.
2.2. Coating Matrices
Rosin resin, or simply rosin, a vegetable resin obtained from several species of the Pinaceae family, was purchased from a local market (Recife, Brazil) and used in its commercially available form to prepare the coating matrix for the test specimens (TSs). The product was selected based on the appropriateness of its physical properties and biodegradability for the purposes of the study.
A synthetic coating (corrosion inhibitor primer), available on the Brazilian market as a gray anticorrosive primer and chemically characterized by the manufacturer as a resin based on polyacids, polyalcohols, drying oils, active and inert pigments, additives, and aliphatic solvents, was applied to TSs for a comparative analysis of efficiency compared with biodegradable matrices based on vegetable resin and subsequently with the selected biosurfactant-containing matrix. The preparation of TSs will be described in Section 2.6.
To evaluate the biosurfactant as an ecological additive in dyeing systems, a test was carried out using synthetic finishing paints or enamels (900 mL) from different Brazilian companies incorporating the P. cepacia biosurfactant. The paints were in accordance with their respective chemical product safety information sheets, as described in Table 1.

Table 1.
Characteristics and compositions of commercial synthetic enamels (paints).
2.3. Production of the Biosurfactant
To produce the biosurfactant, P. cepacia CCT 6659 was cultivated at 28 °C and 250 rpm for 60 h in a mineral medium composed of 0.05% KH2PO4, 0.1% K2HPO4, 0.05% MgSO4.7H2O, 0.01% KCl, and 0.001% FeSO4.7H2O and supplemented with 2.0% residual frying canola oil and 3.0% corn stearate as substrates (pH 7.0). An inoculum of 107 CFU/mL was used [20]. Fermentations were carried out in 2 L Erlenmeyer flasks containing 600 mL of the culture medium. At the end of the cultivation, samples were collected to determine the surface tension, and the resulting fermented medium was used to extract the biosurfactant.
2.4. Extraction of the Biosurfactant
The biosurfactant was extracted from the culture medium as per Farias et al. [21]. Briefly, after removing the cells by centrifugation (4000 rpm, 15 min, 4 °C), the cell-free broth was transferred to a separating funnel, to which the same volume of ethyl acetate (1:1, v/v) was added, and the resulting solution was shaken vigorously for 15 min. After separation of the phases, the organic phase was recovered, and the extraction was repeated using the same volume of solvent. After evaporation of the solvent from the organic phases at 40 °C, the concentrated extract containing the biosurfactant was washed twice with hexane to remove any remaining hydrophobic compounds, such as fatty acids and alcohols resulting from fermentation, and then the solvent was evaporated. The concentration of concentrated biosurfactant was determined gravimetrically, taking into account the volume of the fermented medium sample.
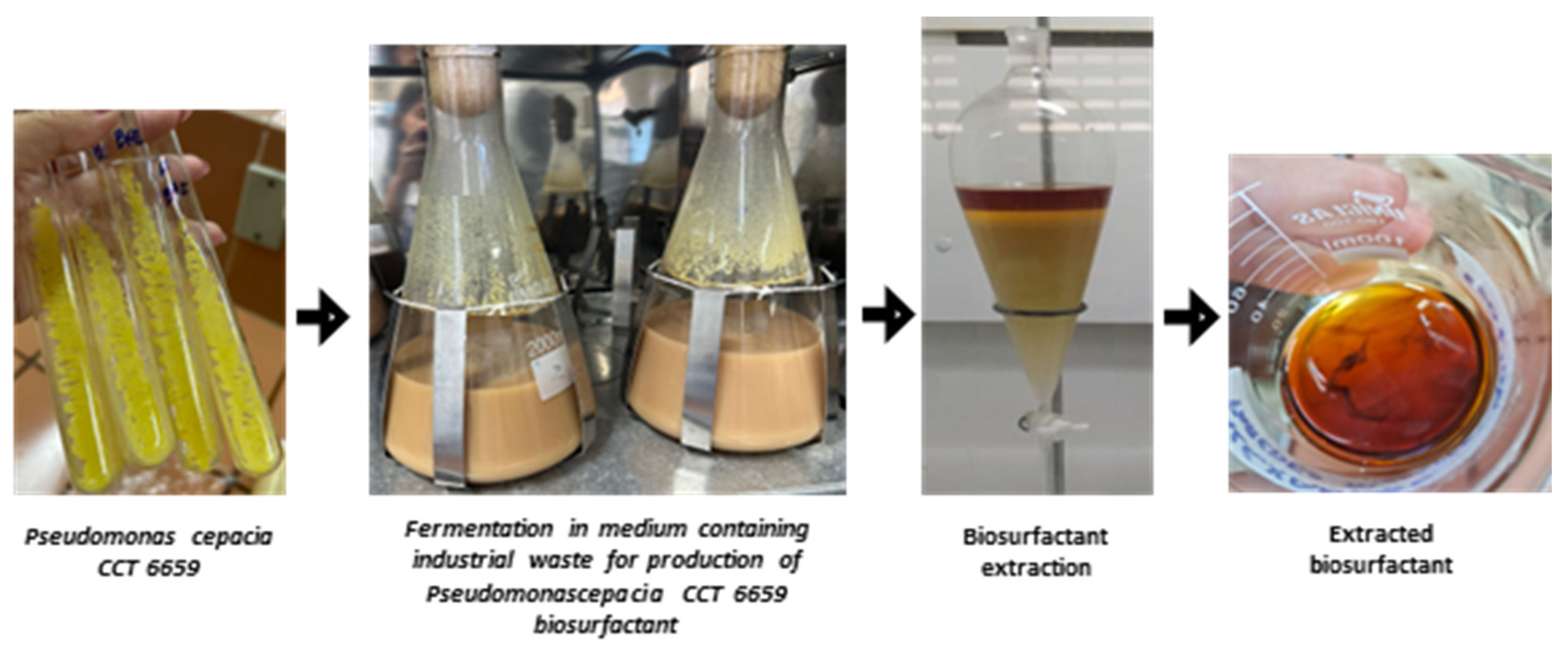
Figure 1 illustrates the fermentation and the extraction of the biosurfactant.
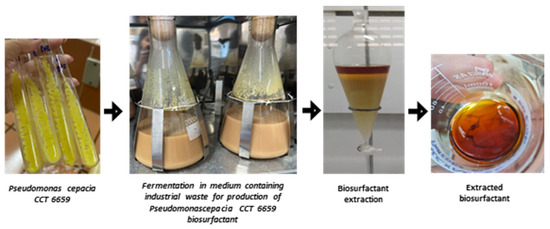
Figure 1.
Stages for obtaining biosurfactant from Pseudomonas cepacia CCT 6659 involving fermentation in a mineral medium enhanced with 2.0% residual frying canola oil and 3.0% corn steep liquor, followed by the recovery of the concentrated biosurfactant extract.
2.5. Construction of the Accelerated Corrosion Chamber (ACC)
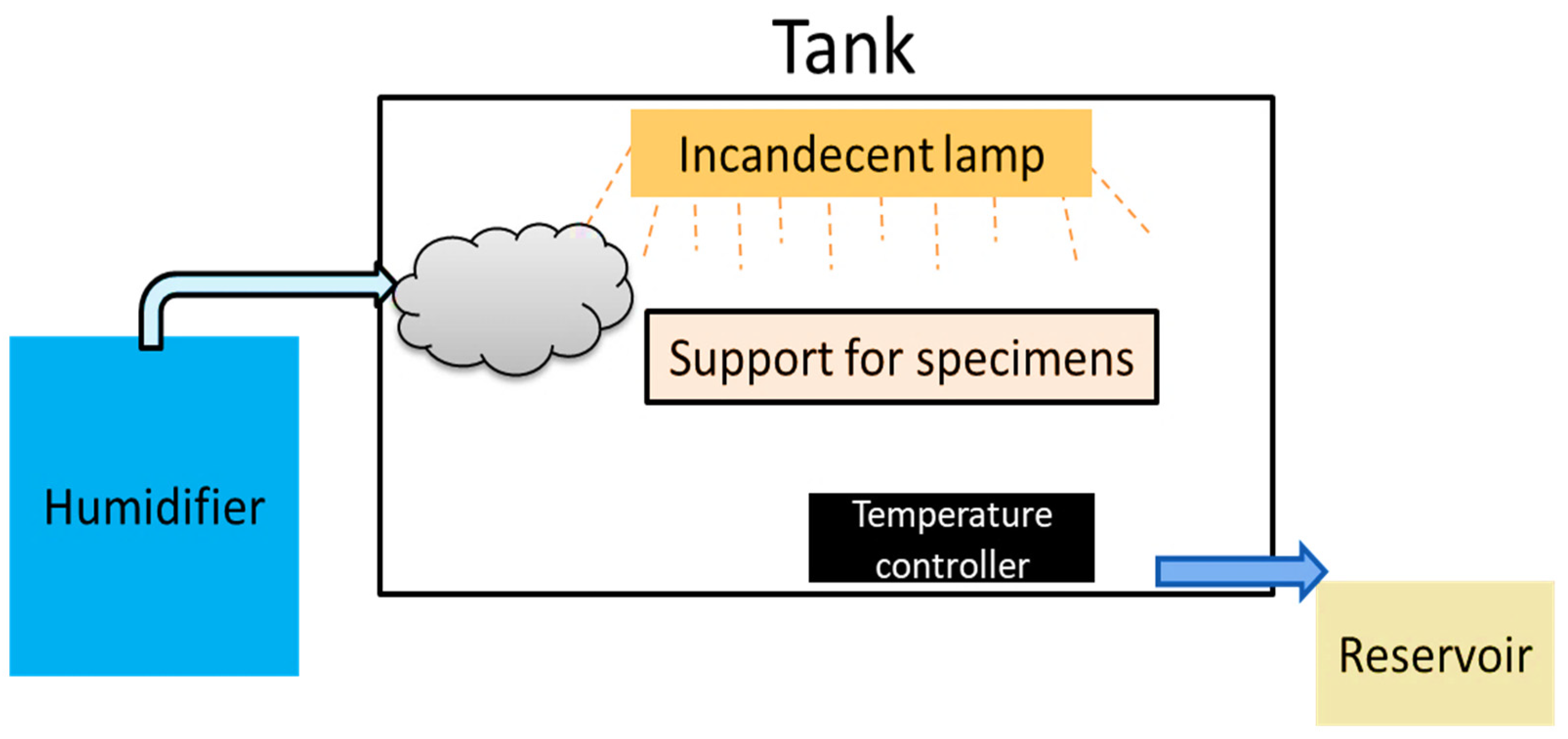
The air-conditioned equipment was built following the regulatory standard ASTM-B-117 of the American Society for Testing and Materials and similar projects from other institutions [22,23,24,25,26]. The accelerated corrosion chamber (ACC) consisted of a humidification system that generated salt mist directed to the interior of the chamber, a temperature controller, and a condensation effluent outlet (Figure 2).
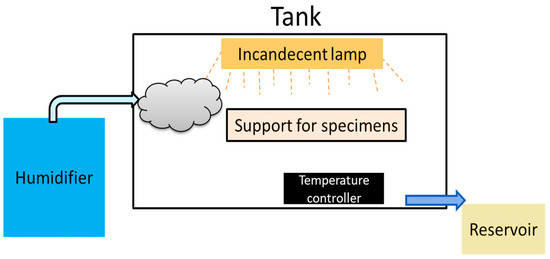
Figure 2.
Flow diagram of physical agents in the accelerated corrosion chamber.
- Assembly of the salt spray chamber: the ACC, constructed using a 1.0 cm-thick acrylic tank, had dimensions of 100.0 cm in length, 40.0 cm in width and 30.0 cm in height. The chamber roof was composed of two triangular and two rectangular 3 mm-thick acrylic sheets with an opening angle of 120° so that the condensate would run down the material, avoiding dripping onto the samples during the test.
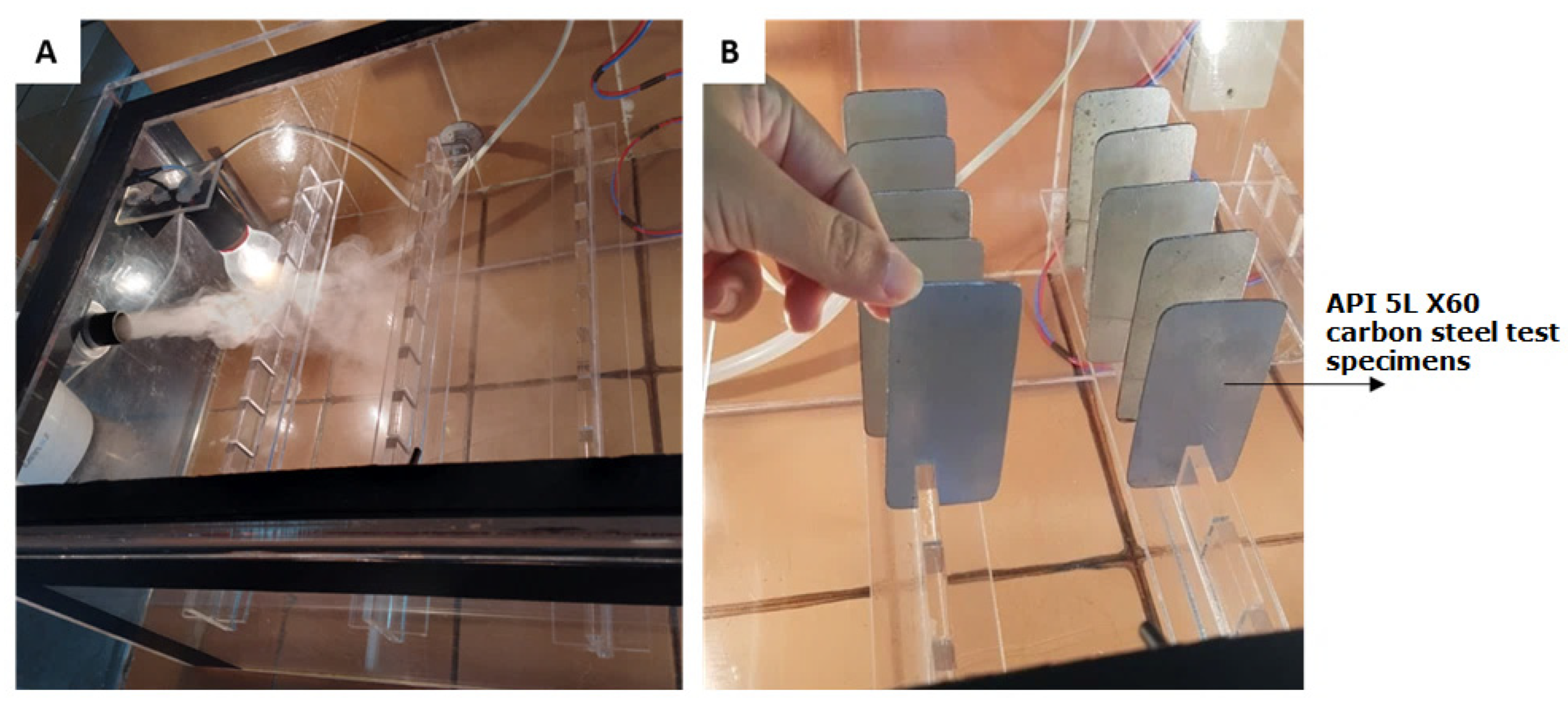
- Sample holder: to suspend the TSs, cut-out acrylic plates measuring 40.0 cm in length and 5.0 cm in width were used, in which slots were made to place the test plates (Figure 3).
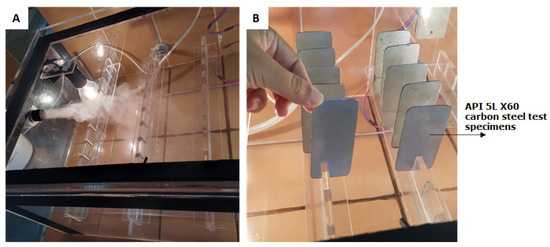 Figure 3. (A) Entry of salt mist into the accelerated corrosion chamber. (B) Carbon steel test specimens placed in the accelerated corrosion chamber.
Figure 3. (A) Entry of salt mist into the accelerated corrosion chamber. (B) Carbon steel test specimens placed in the accelerated corrosion chamber. - Heating system: the electric heating system consisted of incandescent lamps in ceramic sockets and a smaller acrylic box attached to the outside of the chamber, serving as a support for a temperature microprocessor digital electronic controller (model Z31A, COEL, constructed for application with on/off temperature control for heating or refrigeration, fabricated in São Paulo, Brazil).
- Spraying system: for the humidification system, a 3/4″ (19.05 mm) accordion tube coupled with an ultrasonic air humidifier, model NUA-02 (Comfort Air—Mondial, Brazil), with a capacity of 2.2 L and autonomy of 10 h was used. The humidifier was fed with a 5.0% sodium chloride (NaCl) solution.
- Spray solution collection system: the system had a specific outlet at the bottom of the acrylic tank to prevent the accumulation of liquids, allowing the collection of effluents generated by mist condensation inside the tank. The condensate was collected using a silicone hose, which directed the effluents to a reservoir for storage.
These structures and functionalities facilitated the monitoring and evaluation of corrosion under controlled conditions that simulated the aggressive environments typical of coastal atmospheres. The equipment will be shown in the Results and Discussion section.
2.6. Evaluation of Corrosion on Metal Surfaces by Atmospheric Physical Factors
2.6.1. Preparation of Samples
TSs for tests were made from a carbon steel plate and cut into pieces with approximate size of 10.0 cm in length, 5.0 cm in width, and 0.10 cm in thickness. After preparation, the samples were subjected to mass loss tests in ACC, with the objective of evaluating the corrosion resistance under controlled salt spray conditions. First, TSs were sanded with iron sandpaper until reaching granulometries of 120, 180, and 220, cleaned, and weighed to check the initial mass before the test [27].
2.6.2. Test Parameters
To evaluate TS sensitivity to corrosion over time, the ACC was first tested to establish the minimum corrosion conditions necessary to promote oxidation over the entire metal surface. The first tests consisted of exposing the TSs, without any coating, to a mist of 5.0% (w/v) sodium chloride in water (Figure 3). The chamber temperature and relative humidity were maintained at around 40 °C and 100%, respectively, in order to simulate the reality under critical conditions of a maritime atmosphere. The exposure of the TSs continued until the surface showed an evident oxidation process.
2.6.3. Evaluation of the Degree of Corrosion
After exposure in the ACC, TSs were subjected to chemical cleaning with a 26% (v/v) aqueous hydrochloric acid solution to remove the corrosion products, then washed with running water, neutralized with a 10% NaOH solution, and washed again with running water. Then, TSs were dried in an oven at 70 °C, sanded with iron sandpaper to granulometries of 120, 180, and 220, immersed in 10 mL of ethyl alcohol and then in 10 mL of acetone, and cooled to room temperature (28 °C), as described by Dantas [28] and Oliveira [29]. For a careful evaluation of the extent of corrosion and other failures, TSs were weighed on an analytical balance to assess the final mass (W) after the test, expressed in grams (g), and then to calculate the mass loss per area (L), expressed in g/m2, using the formula:
where Wo is the mass (g) of each metal plate before being subjected to corrosion and A is the area (m2).
2.7. Biodegradable Matrices for Incorporation of Pseudomonas cepacia Biosurfactant as a Metal Corrosion Inhibitor
First, matrices were developed using biodegradable inputs to be tested in the ACC to identify the matrix with the best performance.
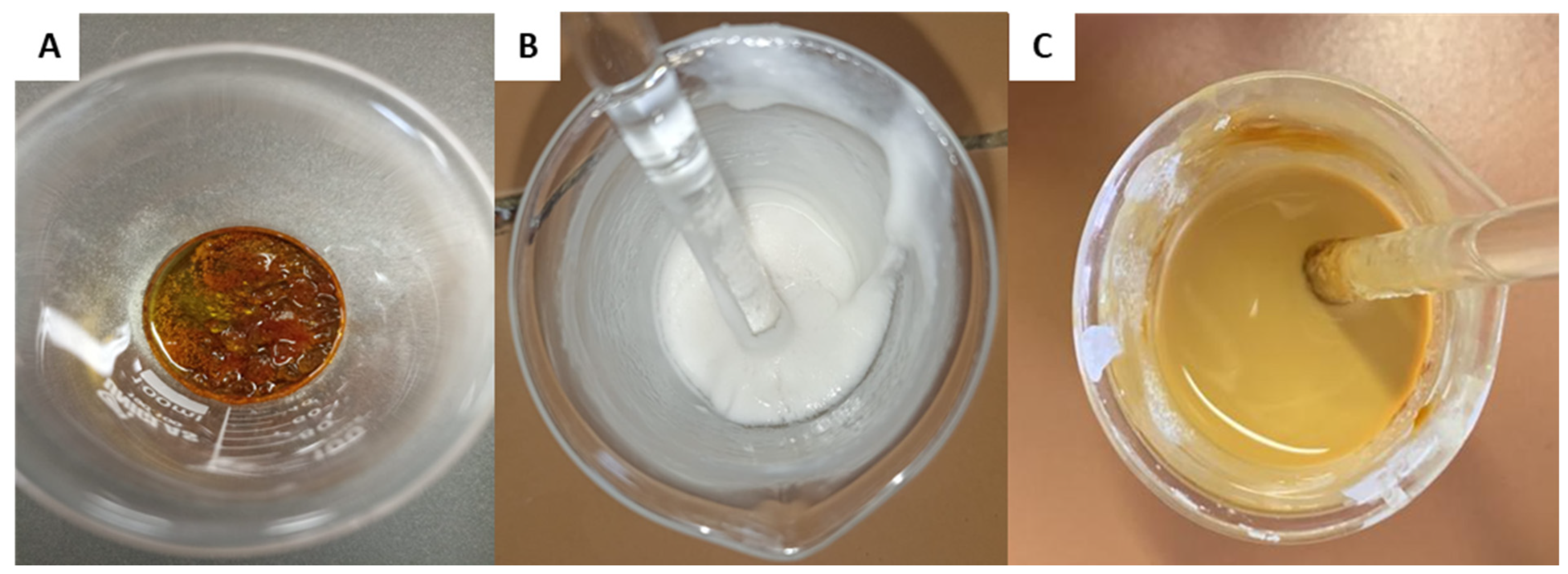
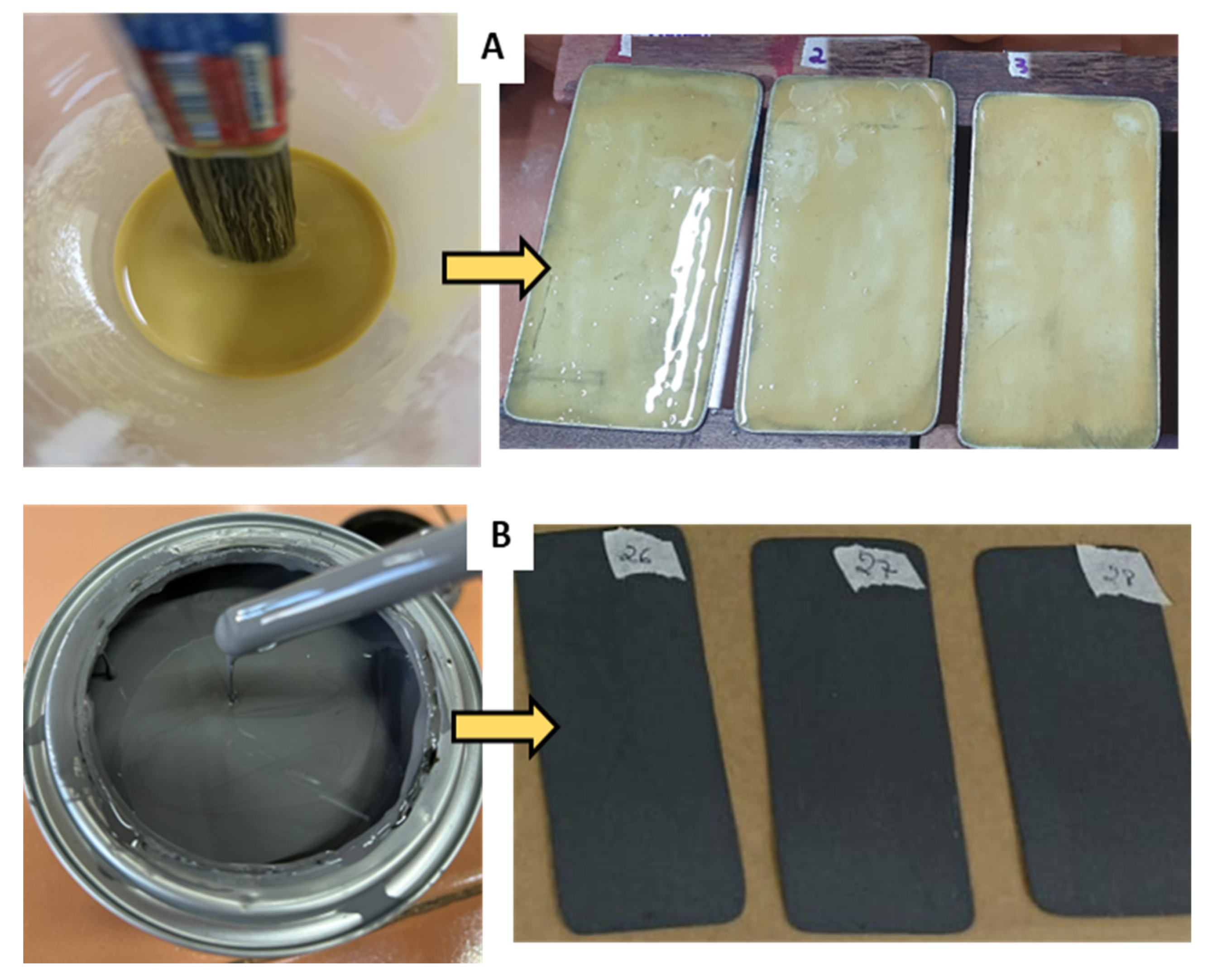
The composition of the formulation used as a base (standard matrix) was adapted from Acevedo et al. [30]. The matrix was prepared by dissolving rosin resin in oleic acid, acting as a plasticizer, after heating. In parallel, calcium carbonate (CaCO3) was dissolved in xylene in a separate system. After complete dissolution, the two mixtures were combined and homogenized, forming a solution with characteristics similar to paint (Figure 4). The homogenization process was aided by heating the components to 150 °C using a hot plate and a bench mixer until the ingredients were completely integrated. Finally, the matrix was used to coat TSs.
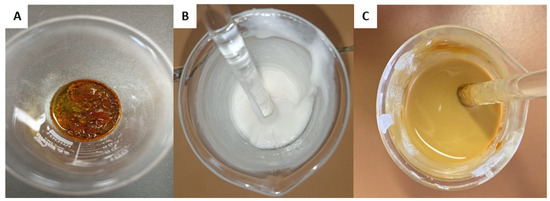
Figure 4.
(A) Preparation of the matrix based on vegetable resin. Heating and solubilization of the resin in oleic acid. (B) Dispersion of CaCO3 in the solvent. (C) Homogenization of the two phases until the formation of a uniform suspension.
Acetone and ethanol were also tested as solvents in addition to xylene, with the aim of obtaining a lower-cost and low-toxicity material. Thus, the four main natural resin-based matrices listed in Table 2 were prepared. Each matrix was also tested after the addition of 1% zinc oxide (ZnO), totaling eight formulations. The commercial anticorrosive coating (corrosion inhibitor primer) described in Section 2.2. was used for comparison.

Table 2.
Compositions of the natural resin-based coating matrices used in this study. All matrices were tested either with or without 1% zinc oxide.
Figure 4 illustrates the development and visual characteristics of the main components (resin, plasticizer, solvent, and CaCO3) during matrix preparation.
A brush was used to deposit homogeneous thin layers of the matrices on the surfaces of the carbon steel TSs without the occurrence of agglomerations. After applying two coats, the matrices were left to dry for 24 h and for the TSs to settle on the ACC supports. Figure 5 shows the TSs coated either with the biodegradable matrix or the commercial coating.
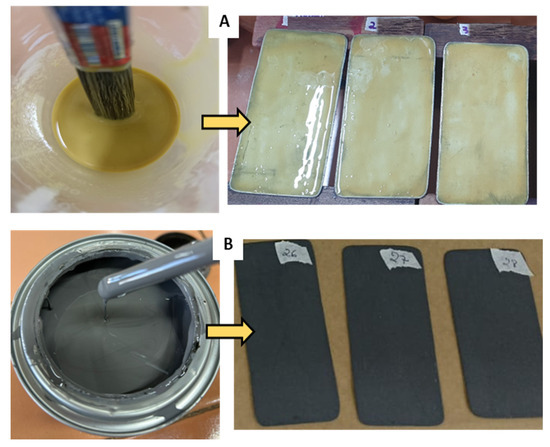
Figure 5.
Test specimens coated with (A) the vegetable resin-based matrix and (B) the synthetic coating (corrosion inhibitor primer).
TSs coated with the matrices and the commercial coating were subjected to simulation of critical atmospheric dynamics in the ACC. For this purpose, TSs were exposed until the surface of one of them showed a very evident oxidation process. After 30 days, the matrix that presented the best performance in terms of integrity of the metal surface and adhesion of the matrix was selected for application of the P. cepacia biosurfactant.
2.8. Incorporation of Pseudomonas cepacia Biosurfactant into the Selected Biodegradable Matrix
After preparing the selected biodegradable matrix as described in the previous section, the biosurfactant from P. cepacia was incorporated into the mixture under stirring to ensure its homogenization. Finally, this matrix containing the biosurfactant was used to coat the TSs, which were then subjected to corrosion tests.
TSs were made from two different metal alloys: one made of carbon steel, as mentioned in the previous section, and the other made of galvanized iron. The galvanized iron was obtained from a rolled flat bar as per the mechanical properties requirements established by the ASTM A36 standard [23], which is widely used in the manufacture of fences, gates, and small and medium-sized industrial and agricultural machinery. The bar, with a width of 1.5″ (25.40 mm), thickness of 1/8″ (3.17 mm), and length of 6 m, was cut into several pieces approximately 3.0 cm wide, 0.5 cm thick, and 7.0 cm long. The application was carried out based on the critical micellar concentration (CMC) of the biosurfactant, which is 600 mg/L [20]. Tests were conducted at concentrations equivalent to 1/2 CMC, CMC, and 2 CMC. After the application of the coatings, TSs were exposed to ACC to evaluate the corrosion resistance and coating performance under controlled exposure conditions [20].
Initially, the metal plates were sanded to ensure a uniform surface, followed by initial weighing. Two coats of the biosurfactant-containing matrix were applied, with a 24 h interval for drying on each side. This process was repeated on the other side of the plates, ensuring complete coverage. After application, TSs were exposed to ACC for 30 days. At the end of the exposure, careful chemical cleaning was performed, followed by drying and final weighing of the plates. With these measurements, it was possible to calculate the mass loss and thus evaluate the efficiency of the bacterial biosurfactant in inhibiting the corrosion of galvanized iron and carbon steel metal alloys.
2.9. Evaluation of Biosurfactant as a Corrosion Inhibitor Additive in Paints (Synthetic Enamel) for Metal Surfaces
Galvanized iron is an ideal material as a substrate for evaluating the performance of popular protective coatings, such as synthetic enamels (used in this test), applied in everyday life by end consumers and in the retail market. Three synthetic enamels (paints) from different brands were applied to the galvanized iron TSs following the method described by Pontes et al. [31] and tested in the ACC. The objective was to select the condition ensuring the best performance and evaluate the effect of the P. cepacia CCT 6659 biosurfactant when incorporated into the paints as an anticorrosive additive. For this evaluation, the paints were prepared by adding the biosurfactant at a concentration of 3%, and the results were compared to those of the respective controls without biosurfactant.
To deposit the paints on the surfaces of the galvanized iron TSs, the same methodology described in the previous section was used.
2.10. Statistical Analyses
Data obtained from tests performed in quintuplicate are expressed as means ± standard deviation. Analysis of variance (ANOVA) was applied to determine significance, considering p-values < 0.05 as statistically significant.
3. Results and Discussion
3.1. Accelerated Corrosion Chamber (ACC)
The accelerated corrosion chamber (ACC) was designed to simulate in a controlled and critical manner the effects of adverse environmental conditions, with temperature, humidity, and atmospheric salinity being significant factors in metal corrosion. Such a controlled environment allowed the metal corrosion process to be accelerated, simulating the impact of years of exposure in a much shorter time. In this way, the ACC offered an effective means of evaluating the performance of anti-corrosive coatings on the surface of metal alloys, testing their resistance under conditions similar to those found in marine environments, where exposure to salt and humidity is especially high.
The chamber proved to be effective in concentrating and dispersing the salt mist, ensuring that the test specimens (TSs) maintained contact with the mist across their entire surface in a homogeneous manner. The humidifying equipment containing the NaCl solution was used at intermediate intensity of the mist controller, with a flow rate of approximately 0.06 mL/s. The transparency of its structure, built in acrylic, allowed visibility during the corrosion process (Figure 6).

Figure 6.
Details of the accelerated corrosion chamber built in the laboratory.
Through efficient air-conditioning, the equipment proved to be effective for test reproducibility. The ACC reproduced a critical maritime atmosphere typical of coastal environments, thanks to the presence of the humidifier, the tank for storing material to be examined, the heating system, and the effluent collection tank.
Qian et al. [32], when performing salt spray tests on electrical installations, emphasized the importance of a standardized bench-scale construction to obtain reliable and reproducible results, which are necessary to predict the durability of equipment and metal installations in adverse environments, and suggested that improvements in testing methodology can lead to better corrosion protection strategies.
Rodrigues [26] developed and patented equipment for tests with salt spray mimicking real weathering conditions to evaluate the corrosion resistance of metallic materials exposed for 48 h to spraying of a concentrated saline solution (5% NaCl). The results obtained confirmed the loss of mass per area in the TSs of the materials and were compared to the range of values specified by the ISO 9227 standard [33], helping to validate the equipment.
3.2. Parameterization Tests in the Accelerated Corrosion Chamber
The use of a calibrated and adjusted test chamber is essential to correctly simulate salt spray and temperature. The test performed in the bench-built ACC, also known as the salt spray test, was essential to evaluate the corrosion resistance of materials exposed to saline environments. To ensure accurate and reliable results, the following specific steps were followed: adequate preparation of clean and degreased samples, determination of the exposure time according to the study objectives, and assessment of the corrosion level and degree of mass loss in samples.
The built equipment made it possible to evaluate the corrosion resistance of carbon steel when exposed to salt spray and compare the results with values specified by ASTM B117 [23] and ISO 9227 standards [33], as well as the results from other studies for equipment validation. The performance was satisfactory, achieving a very evident oxidation process across the entire surface of TSs after 15 days of exposure to a temperature of approximately 40 °C and relative humidity close to 100%, i.e., under conditions mimicking the reality of a critical maritime atmosphere (Figure 7).

Figure 7.
Test specimens after 15 days of exposure to salt spray in the accelerated corrosion chamber.
After cleaning and weighing the TSs, an average reduction of 2.094 g (±10%) in their mass was detected, which was equivalent to an average mass loss per exposed area of approximately 418.8 g/m2 in 15 days. When compared with the value specified by the ISO 9227-06 standard (70 ± 20 g/m2 in 48 h of exposure), the test performed in this study provided an average value approximately 20% lower (55.8 g/m2 in 48 h), i.e., within the acceptable limit for commercial equipment. Therefore, the ACC not only made it possible to study the resistance of metallic materials, protected or not by corrosion inhibitor coatings, to corrosion deriving from the exposure to salt spray but also demonstrated the efficiency of ecological anticorrosive coatings.
In a previous study, Ostroski [34] performed a corrosion test on 1020 carbon steel by the salt spray method in an accelerated test chamber using USCMP-01/2005 model equipment, which revealed aggressive corrosion evidenced by the formation of corrosion products over the entire surface of TSs. These results indicated that steel did not show resistance to the aggressiveness of the saline atmosphere, highlighting the urgency of developing effective protection strategies for materials exposed to corrosive environments such as those found in ports. Santos and Panossian [35] also compared the characteristics and corrosion resistance of zinc coatings produced by electrodeposition in a chloride-based bath with and without additives on steel TSs submitted to accelerated corrosion tests in a salt spray chamber and a wet chamber. It was observed that the coating obtained in a sulfate/chloride bath without additives presented greater corrosion resistance than the coating obtained in a chloride bath with additives. The superiority of the latter was attributed to the texture of the zinc and the absence of residues in the electrodeposit.
3.3. Evaluation of Biodegradable Matrices for Incorporation of Pseudomonas cepacia Biosurfactant as a Metal Corrosion Inhibitor
The study of biodegradable matrices for corrosion inhibitor coatings focused on creating sustainable solutions capable of protecting metals and reducing environmental impact. All matrices produced based on vegetable rosin resin exhibited a homogeneous appearance and viscosity similar to paint.
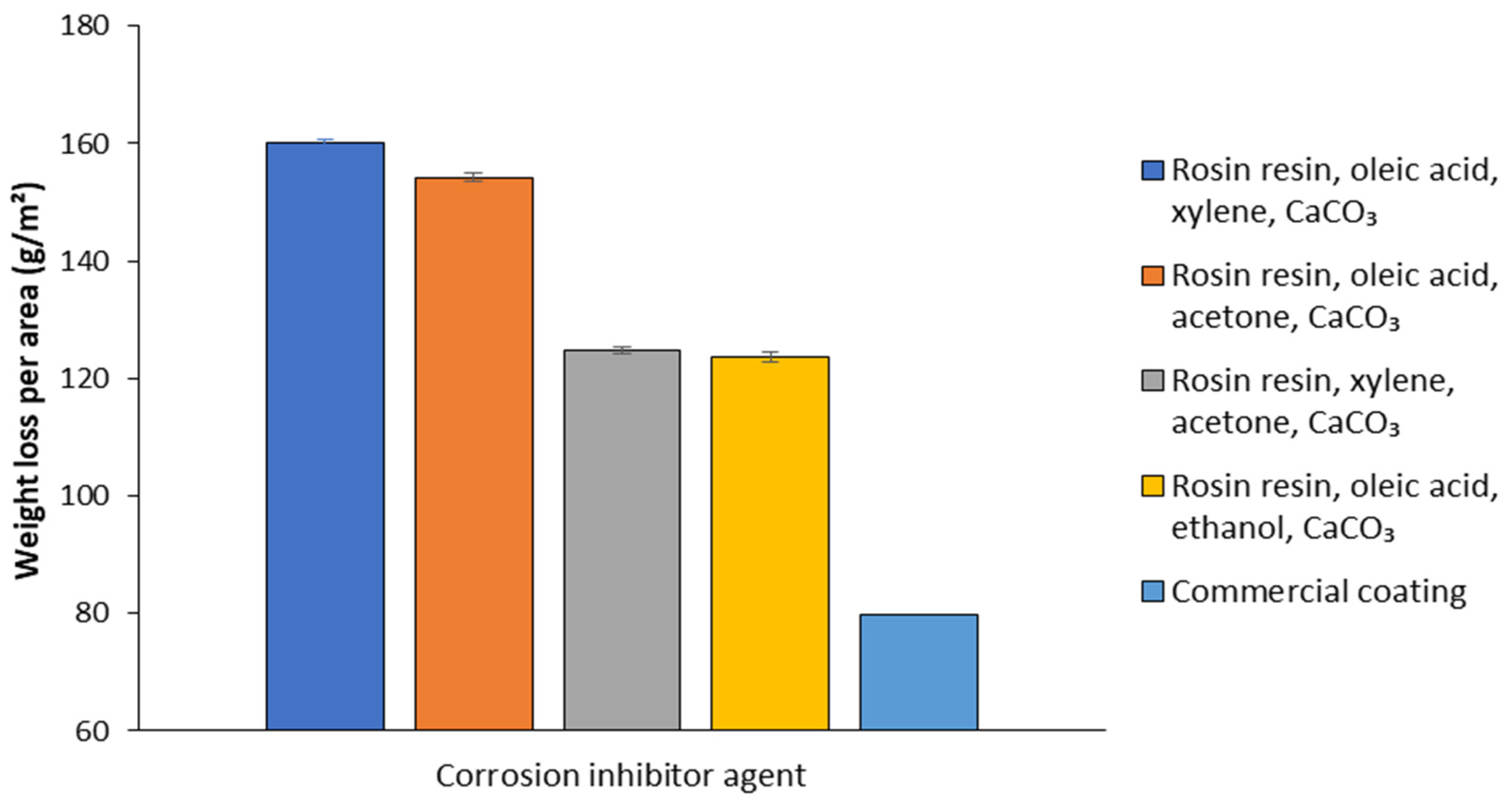
The TSs coated with the matrices and the commercial coating were subjected to critical atmospheric dynamic simulation in the ACC. After 30 days of exposure, matrix D, prepared using ethanol as solvent, showed the greatest inhibition of metal corrosion, ensuring the lowest mass loss per area (123.6 g/m2), and was therefore selected for the successive step of biosurfactant incorporation. On the other hand, the other three matrices containing xylene (A), acetone (B), and xylene + acetone (C) as solvents had lower performance, with mass losses of 160.1, 154.2, and 124.7 g/m2, respectively (Figure 8). Although there was no statistically significant difference in the mass loss results between conditions C and D, matrix D was selected for the next step of biosurfactant incorporation due to the greater viability of ethanol as a solvent, considering safety and sustainability factors compared to the xylene and acetone mixture.
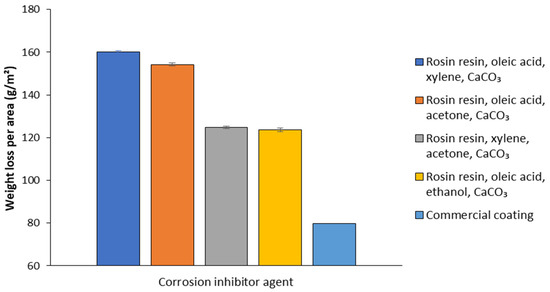
Figure 8.
Mass loss per area after 30 days of critical atmospheric dynamic simulation of carbon steel specimens incorporating matrices based on rosin resin, oleic acid, xylene, and CaCO3; rosin resin, oleic acid, acetone, and CaCO3; rosin resin, xylene, acetone, and CaCO3; rosin resin, oleic acid, ethanol, and CaCO3 and commercial coating (corrosion inhibitor primer).
The selected matrix showed excellent homogeneity, with no evident changes even after replacing the solvent, as suggested by a comparison with the matrix developed by Acevedo et al. [30] using xylene as a solvent. Furthermore, after exposure to controlled conditions in the ACC, the coating maintained its integrity and adhesion, with no signs of wear. These results highlighted the feasibility of modifying the solvents without compromising the effectiveness of the matrix in terms of quality and fixation on the surfaces.
The selected matrix can also be considered the most viable from several standpoints when compared to the others, since ethanol is much more accessible, less expensive, and less toxic than the other solvents tested, which makes it safer to handle and cheaper. Matrices containing zinc oxide (ZnO) as a source of sacrificial metal, with zinc being released instead of the metal to be protected, showed no relevant differences compared to the others. Despite the well-recognized corrosion-inhibiting properties of ZnO, its use in the next testing stage was avoided because it would have interfered with the effectiveness of the alternative inhibitor, the biosurfactant, masking its anticorrosive action.
Although the commercial product (corrosion inhibitor primer) showed the best performance as a corrosion inhibitor, with a mass loss per area (79.7 g/m2) equal to only about one-fifth of that detected for the uncoated TSs (418.8 g/m2), it has toxic ingredients and is not biodegradable in the environment (Figure 8).
The mass loss recorded after 30 days for the selected matrix was equivalent to a corrosion inhibition efficiency of 70.5%. Although this value is significantly lower than that (96.6%) reported by Hemapriya et al. [36] for another biodegradable product consisting of 100 mg/L human hair extract applied on mild steel rods exposed to a 1 mol/L HCl solution, it is noteworthy that the exposure time in that study (only 3 h) was probably insufficient for the electrochemical reactions resulting from corrosion to extend greatly across the metal surface.
In another study by Fernandes et al. [37], a new aniline derivative was investigated as a corrosion inhibitor for mild steel in acidic medium. The research combined theoretical and experimental approaches to evaluate the efficiency of this inhibitor, with an emphasis on the molecular interactions between the compound and the metal surface. The results showed excellent performance of the aniline derivative in inhibiting corrosion, achieving an efficiency of 96.0% at 4.59 mmol/L, acting through adsorption on the steel surface and forming a protective layer.
3.4. Incorporation of Pseudomonas cepacia Biosurfactant into the Plant Resin-Based Matrix
After selecting the plant resin-based matrix, the P. cepacia biosurfactant was incorporated into it to evaluate its performance as a corrosion inhibitor on metal surfaces. For this purpose, the efficacy of the biosurfactant in delaying oxidation was tested, exploring its tensioactive and biodegradable properties in an eco-friendly system with industrial potential.
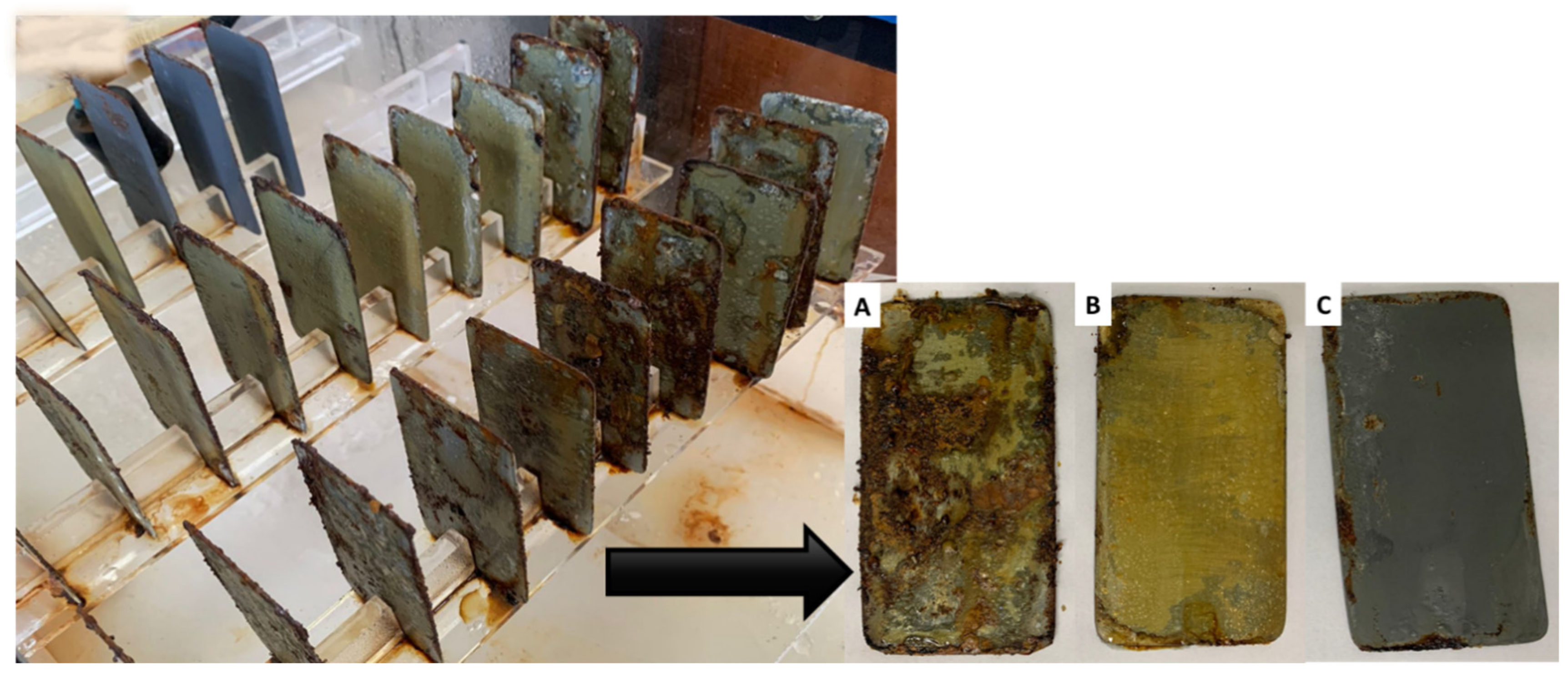
During the preparation of the matrix with the biosurfactant in the form of concentrated extract, a strong interaction between the biosurfactant and the solution was observed, resulting in a creamy and clear formulation. This appearance can be ascribed to the greater dispersion between the phases of the mixture caused by the action of the biosurfactant, which contributed to the homogeneity and stability of the formulation. After 30 days of testing in the ACC, TSs were cleaned and weighed to collect the results. Figure 9 illustrates the effects of the corrosive process on the TSs immediately after the test in the ACC.
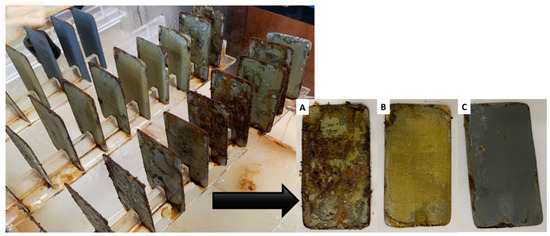
Figure 9.
Test specimens after 30 days of exposure to salt spray in the accelerated corrosion chamber. (A) Control: biodegradable matrix without biosurfactant, (B) biodegradable matrix with incorporated biosurfactant, and (C) commercial coating (corrosion inhibitor primer).
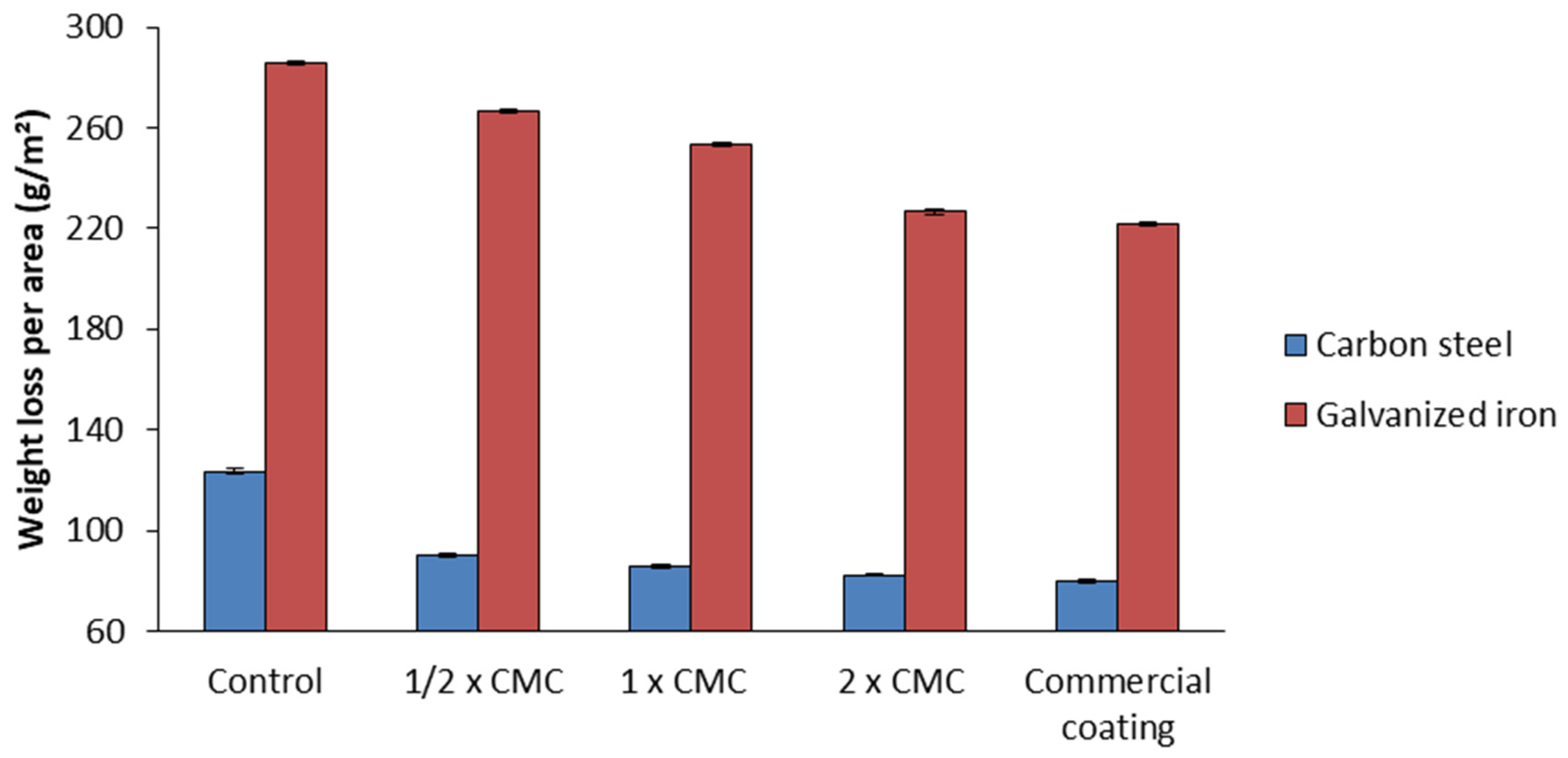
The incorporation of the bacterial biosurfactant up to half the critical micellar concentration (1/2 CMC) into the selected biodegradable matrix resulted in a mass loss per area of carbon steel (90.1 g/m2) approximately 27% lower than that in the control without biosurfactant (123.6 g/m2). By increasing the concentration of the biosurfactant to CMC and 2 CMC, further reductions in mass loss to 85.8 and 82.2 g/m2, respectively, took place. The latter value is close to the one obtained with the commercial coating (79.7 g/m2), but with the considerable advantage of reducing toxicity to the environment.
Under all conditions tested, the mass loss in the galvanized iron plates was greater than in the carbon steel ones, since the former were made of a metal alloy more sensitive to corrosive processes, especially in critical environments. In this case, the biosurfactant reduced the mass loss from 285.9 g/m2 (control) to 266.7 g/m2 at 1/2 CMC, 253.2 g/m2 at CMC, and 226.7 g/m2 at 2 CMC (Figure 10). This last value is comparable to that observed with the commercial coating (221.9 g/m2); however, the biodegradable matrix offers the advantages of low toxicity and high biodegradability, unlike the commercial product, which is toxic and non-biodegradable, representing a risk to the environment.
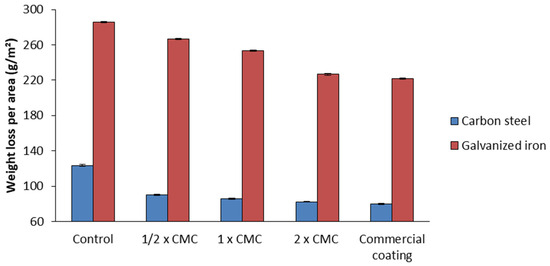
Figure 10.
Mass loss per area after 30 days of exposure to salt spray in the accelerated corrosion chamber in galvanized iron and carbon steel plates in biodegradable matrix with or without biosurfactant at different concentrations, namely, 1/2 CMC, CMC, and 2 CMC, and commercial coating (corrosion inhibitor primer).
According to the literature, the environment to which a material is exposed influences the thermodynamics of corrosive reactions, and factors such as humidity and temperature should be investigated during the evaluation of corrosive processes [25]. The impact of inhibition offered by a green biosurfactant on the dissolution of mild steel alloy (MS-37-2) in aqueous sodium chloride solutions was investigated by Fawzy et al. [38], who related the inhibition efficiency to the evaluated thermodynamic parameters, reinforcing the physical adsorption mechanism of the inhibitors. In a more recent study, the same research group [39], investigating the anticorrosive potential and antibacterial properties of different amino acid-based surfactants, namely, sodium N-dodecyl asparagine, sodium N-dodecyl tryptophan, and sodium N-dodecyl histidine, highlighted the potential of these compounds as sustainable alternatives for the protection of metals against corrosion. After 23 days, 300 mg/L sodium N-dodecyl asparagine as a biosurfactant had led to a mass loss per area (347.5 g/m2) on the copper surface significantly higher than those observed in the present study at the same concentration (equivalent to 1/2 CMC) in both carbon steel (90.1 g/m2) and galvanized iron (266.7 g/m2) plates, evidencing the superior efficacy of the P. cepacia CCT 6659 biosurfactant. These results highlight the efficacy of the proposed biosurfactant in protecting metal structures against corrosion, demonstrating its potential as a corrosion inhibitor in industrial applications.
A study carried out by Faccioli et al. [40] in a static system revealed that the same P. cepacia CCT 6659 biosurfactant used in this work was able to reduce the mass loss in galvanized iron specimens in seawater to 1%, not only acting as a corrosion inhibitor but also mitigating the formation of microbial biofilms. However, under exposure to critical atmospheric conditions in an ACC (temperature of 40 °C in 5% salt spray), the mass loss increased to 2.3%, probably due to a more pronounced oxidative process.
In a study conducted by Ostroski [34], the results of corrosion tests revealed significant differences in the behavior of 1020 carbon steel under different conditions. In the immersion tests, the samples exhibited more homogeneous corrosion, indicating uniformity in the material degradation. In contrast, the salt spray tests showed more aggressive corrosion, with variable intensity, suggesting that the aggressiveness of the saline atmosphere may be more impactful under certain conditions.
3.5. Evaluation of the Biosurfactant as a Corrosion Inhibitor Additive in Paints (Synthetic Enamels) for Metal Surfaces
Corrosion-inhibiting additives in paints such as synthetic enamels are able to protect metal surfaces by forming a barrier against corrosive agents, while biodegradable alternatives can act by inhibiting both corrosion and biofilm formation.
Iron is widely used in residential structures and civil construction due to its abundance and low cost compared to other metals. In previous tests, carbon steel reflected a more industrial scenario, while galvanized iron is suitable for more domestic use. The galvanized iron TSs previously coated with biosurfactant-containing commercial paints were subjected to critical atmospheric dynamic simulation in the ACC for 15 days, a time considered sufficient for the surface of the control samples (paints without biosurfactant) to show a clear oxidation process. In addition, galvanized iron was preferred to carbon steel because it undergoes faster oxidation, offering a faster response for this study.
After 15 days of exposure, some TSs, as soon as being removed from the ACC, showed milder and others more aggressive corrosion, while, as expected, control samples underwent more expressive corrosion characteristics. The biosurfactant-containing paints displayed obviously greater resistance and better adhesion under the severe AAC conditions.
After the tests, TSs were cleaned and weighed, and their conditions related to the oxidation process were exposed more clearly. The integrity (less wear) and corrosion level were the visual parameters considered.
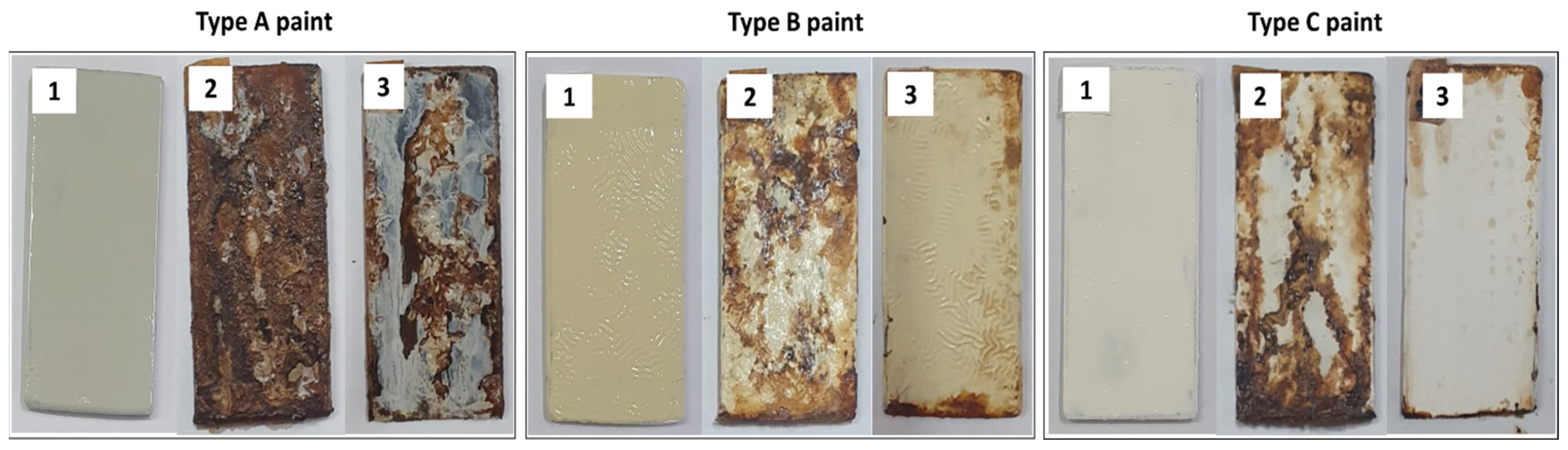
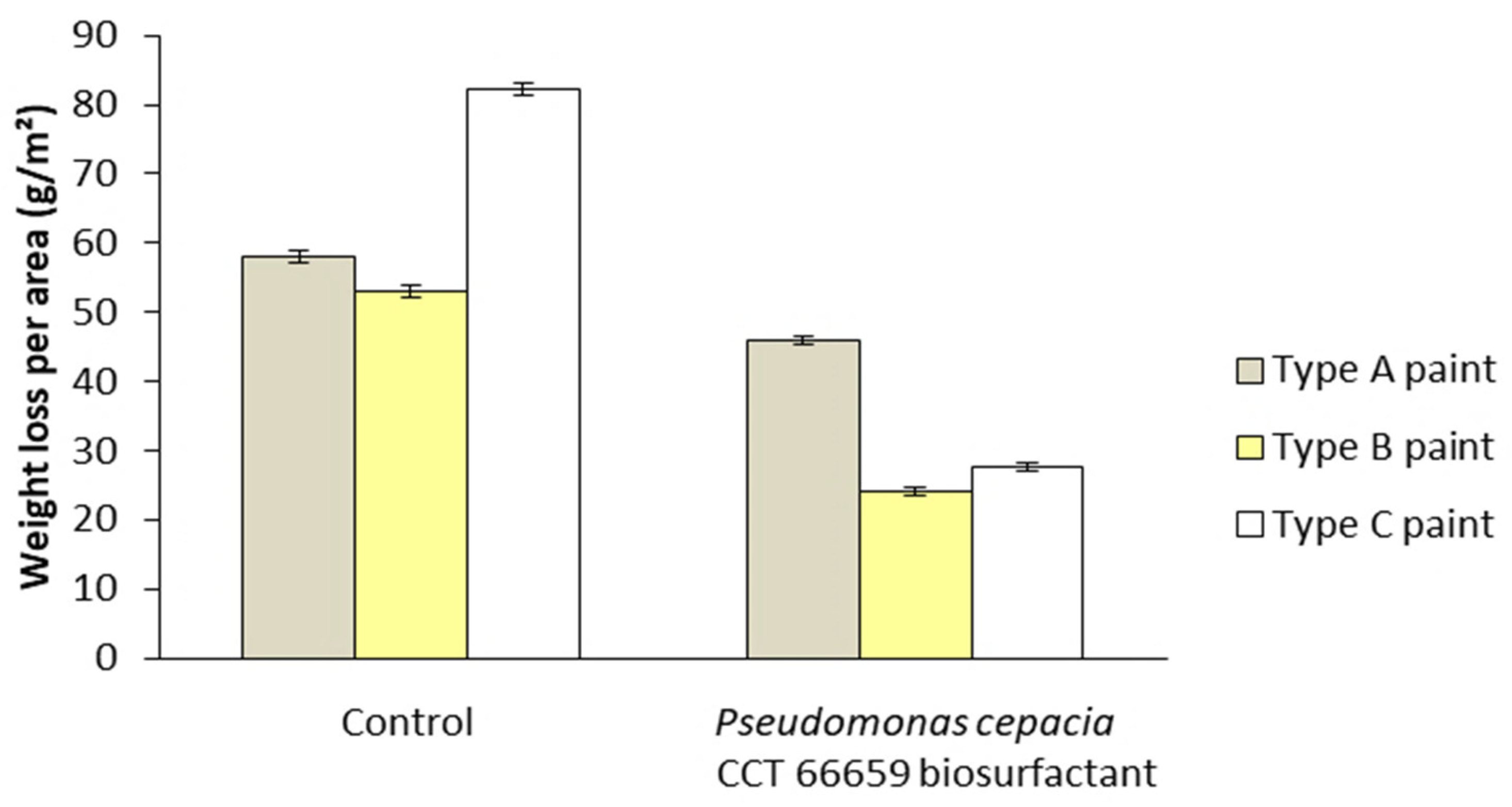
Figure 11 shows the appearance of galvanized iron plates coated with three different commercial paints with or without biosurfactant before and after 15 days of exposure to critical atmospheric conditions simulated in the ACC, while Figure 12 illustrates the respective average mass losses per exposed area. For all the paints tested, the TSs coated with the control paints (without biosurfactant) presented greater mass loss compared with those coated with the biosurfactant-containing ones. Although commercial paints from three different brands were used, the efficiency of the surfactant and its potential as an ecological additive in anticorrosive products were evident. Specifically, the mass losses per area were 46.0, 24.1, and 27.6 g/m2 using type A (ice white), type B (ivory), and type C (snow white) paints containing the bacterial biosurfactant, while significantly higher values (58.0, 53.0, and 82.2 g/m2) were found using their respective controls.

Figure 11.
Corrosion levels observed in the test specimens (TSs) coated with commercial paints before and after 15 days of exposure to critical atmospheric conditions simulated in the accelerated corrosion chamber. (1) TS before exposure; (2) TS coated with paint without biosurfactant; (3) TS coated with paint incorporating the biosurfactant. Paint A—formulated with polyacid-based resin, polyalcohols, drying oils, active pigments, additives, aliphatic solvent, and turpentine. Paint B—formulated with light hydrated petroleum distillates, xylene, toluene, cobalt octoate, manganese octoate, and methyl ethyl ketoxime. Paint C—formulated with oil-based resin, polyacids, polyalcohols, solvents, additives, and pigments.
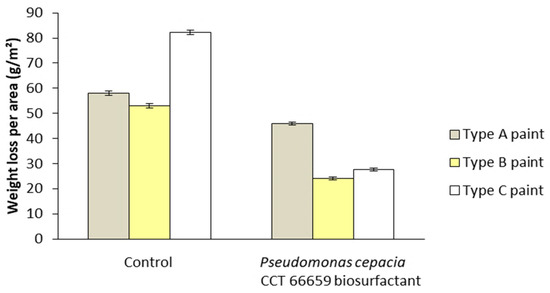
Figure 12.
Mass losses detected in test specimens coated with commercial paints with and without biosurfactant (controls) before and after 15 days of exposure to critical atmospheric conditions simulated in the accelerated corrosion chamber. Paint A—formulated with polyacid-based resin, polyalcohols, drying oils, active pigments, additives, aliphatic solvent, and turpentine. Paint B—formulated with light hydrated petroleum distillates, xylene, toluene, cobalt octoate, manganese octoate, and methyl ethyl ketoxime. Paint C—formulated with oil-based resin, polyacids, polyalcohols, solvents, additives, and pigments.
Faccioli et al. [40], who investigated the use of the same nontoxic biosurfactant produced by P. cepacia CCT6659 as a metal corrosion inhibitor, found a minimal mass loss (15.7%) compared to the control, demonstrating its potential for industrial applications. Semeniuk et al. [40] reported a corrosion rate of only 1.85 10−6 g cm−2 h−1 when using a rhamnolipid biosurfactant from a Pseudomonas sp. as a corrosion inhibitor on steel plates exposed to an aqueous solution of 0.1% NaCl at 20 °C. Even though in the present study the corrosion rate in galvanized iron plates coated with commercial type B paint containing the P. cepacia CCT6659 biosurfactant was higher (6.70 10−6 g cm−2 h−1), it should be taken into account that the conditions tested in the present study were more critical (5% NaCl solution at 40 °C) because they were intended to simulate a maritime atmosphere. Even so, a degree of protection (54.48%) very close to that reported by the above authors [41] (55%) was obtained.
It has been stressed that the oxygen atoms present in the glycidic moiety of the glycolipid P. cepacia CCT6659 biosurfactant [20] contributes to the formation of a protective band on the metal surface, thanks to the presence of lone electron pairs that can form attractive interactions with the metal. In addition, it is known that the nonpolar moiety of natural surfactants can act by repelling contaminants that could trigger undesired electrochemical reactions. Thus, the adsorption of biosurfactants on metal surfaces is essential to protect materials against corrosion, preventing the electrochemical reactions that lead to their wear [41].
Although the use of bacterial biosurfactants to prevent metal corrosion has already been explored in previous studies, this research presents an unprecedented contribution by evaluating the biosurfactant produced by P. cepacia CCT 6659 as a corrosion inhibitor on metal surfaces using advanced methodologies, such as the accelerated corrosion chamber. Unlike the work of Faccioli et al. [40], which addressed the use of a biosurfactant against corrosion in seawater focusing on biological factors and antimicrobial properties that inhibit the formation of biofilms, this study explored biosurfactant application for protection against corrosion caused by atmospheric factors. In addition, comparative tests were performed with matrices composed of biosurfactant and commercial coatings, representing significant methodological advances. According to Soares da Silva et al. [20], the target biosurfactant was identified as a rhamnolipid, a glycolipid whose molecular properties demonstrate high efficacy. In this work, the reduction in mass loss proves its potential as a sustainable alternative to protect metals against atmospheric corrosion. According to Wang and Yan [42], the presence of heteroatoms (such as nitrogen, phosphorus, and oxygen), as well as double and triple bonds, favors the chemical adsorption of biosurfactants on the metal surface. This process occurs through strong interactions between the functional groups of the biomolecule and the metal surface, forming a protective layer that isolates the material against corrosive agents. This layer is especially effective in aggressive conditions, such as saline environments, and under extreme pH conditions, including acidic and alkaline situations, highlighting the potential of these molecules as sustainable and efficient corrosion inhibitors.
4. Conclusions
This study confirmed the feasibility of incorporating a biosurfactant into an alternative coating made from biodegradable raw materials, which proved to be competitive with commercial corrosion inhibitors. The natural surfactant produced by Pseudomonas cepacia CCT6659 showed excellent properties, indicating its potential as a support for emerging technologies that are being implemented in the industrial sector.
The experiments performed in an accelerated corrosion chamber allowed for the evaluation of the corrosion resistance of metallic materials, protected or not, under critical atmospheric conditions. The results not only confirmed the viability of the bacterial surfactant as an additive in an alternative biodegradable coating but also demonstrated its potential as a corrosion inhibitor on metallic surfaces compared to commercial counterparts. Furthermore, they demonstrated the benefits of incorporating the P. cepacia biosurfactant as a biological additive in commercial paints. The significant reduction in mass loss observed in all tests performed in this study reinforces the effectiveness of the biosurfactant as a viable solution for metal protection.
In conclusion, this study sought to value increasingly relevant ecological solutions in improving anticorrosive coating formulations. Currently, environmentally safe corrosion inhibitors are being developed in forward-looking countries that, though not facing the same corrosion challenges as Brazil, recognize that high temperature and salinity increase corrosive potential. Therefore, ecological solutions may become more prominent and gain space in this market.
Author Contributions
All authors contributed to this work. Conceptualization, R.d.C.F.S.d.S. and L.A.S.; methodology, Y.E.S.F., Y.K.S., A.A.P.S.F. and R.d.C.F.S.d.S.; validation, R.d.C.F.S.d.S. and L.A.S.; formal analysis, R.d.C.F.S.d.S. and L.A.S.; investigation, R.d.C.F.S.d.S.; resources, L.A.S.; data curation, R.d.C.F.S.d.S. and L.A.S.; writing—original draft preparation, R.d.C.F.S.d.S., Y.E.S.F., A.A.P.S.F., K.W.O., G.P.A., N.M.P.R.e.S., A.C. and L.A.S.; writing—review and editing, R.d.C.F.S.d.S., L.A.S. and A.C.; visualization, R.d.C.F.S.d.S., L.A.S. and A.C.; supervision, R.d.C.F.S.d.S.; project administration, L.A.S.; funding acquisition, L.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Brazilian fostering agencies Fundação de Amparo à Ciência do Estado de Pernambuco (FACEPE (State of Pernambuco Science Assistance Foundation)), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES (Coordination for the Advancement of Higher Education Personnel); finance code 001), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq (National Council of Scientific and Technological Development)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the laboratories of the Catholic University of Pernambuco (UNICAP) and the Advanced Institute of Technology and Innovation (IATI), Brazil.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could constitute a potential conflict of interest.
References
- Zehra, S.; Mobin, M.; Aslam, R. Application of biosurfactants as anti-corrosive agents. In Advancements in Biosurfactants Research; Aslam, R., Mobin, M., Aslam, J., Zehra, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 171–189. [Google Scholar] [CrossRef]
- Cheng, X.; He, Y.; Yan, S.; Yan, L.; Li, Z.; Xu, S.; Wei, K.; Fan, Y.; Chen, Q.; Peng, W. Exploration of the mechanism of wear and seawater erosion resistance of modified MXene-reinforced Ni-Cu alloy composite coatings. Tribol. Int. 2024, 200, 110080. [Google Scholar] [CrossRef]
- Sales, D.E.L.; Takenobu, G.M.U.; Alves, L.W.S.; Perícoli, V.E.N.A. Análise da corrosão em aço 1020 utilizando meio de proteção com anticorrosivo e mistura de cloro com água como agente corrosivo. In Anais do Curso de Engenharia Mecânica da UniEvangélica, Anápolis, Brazil, 4–6 June 2018; UniEvangélica: Anápolis, Brazil, 2018; Volume II, pp. 1–9. [Google Scholar]
- Okyere, M.S. Corrosion Protection for the Oil and Gas Industry: Pipelines, Subsea Equipment, and Structures, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2019; pp. 1–186. ISBN 978-04-2905-645-1. [Google Scholar] [CrossRef]
- Danaee, I.; Rameshkumar, S.; Rashvandavei, M.; Vijayan, M. Electrochemical and quantum chemical studies on corrosion inhibition performance of 2,2′-(2-Hydroxyethylimino) bis[N-(alphaalpha-dimethylphenethyl)-N-methylacetamide] on mild steel corrosion in 1 M HCl solution. Mater. Res. 2020, 23, 2. [Google Scholar] [CrossRef]
- Gómez Botero, M.A.; Sepúlveda, J.A.T.; González, J.G.C.; Osorio, F.J.B.; García, P.M.; Cruz, D.; Torres, J.A.M. Evaluación de metodologías para la aplicación de sistemas de protección contra la corrosión en el interior de tanques de lastre en enbarcaciones marinas. Ing. Desarro. 2017, 35, 174–197. [Google Scholar] [CrossRef]
- PROPEQ. Projeto e Pesquisa em Engenharia Química. Corrosão: O Que é e Como Evitá-La em sua Indústria. O Que é a Oxidação e sua Ligação com Processos Corrosivos; Como a Corrosão Afeta o Meio Industrial; Métodos de Proteção à Corrosão. Available online: https://propeq.com/post/corrosao-o-que-e-e-como-evitar/ (accessed on 21 September 2023).
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Adv. Mater. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly eco-friendly and innovative biocides against biocorrosion. Int. J. Biol. Macromol. 2020, 21, 2152. [Google Scholar] [CrossRef]
- Lavanya, M.; Machado, A.A. Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: A comprehensive review. Sci. Total Environ. 2024, 908, 168407. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R.C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural surfactants and their applications for heavy oil removal in industry natural surfactants and their applications for heavy oil removal in industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Shaikhah, D.; Loise, V.; Angelico, R.; Porto, M.; Calandra, P.; Abe, A.A.; Testa, F.; Bartucca, C.; Rossi, C.O.; Caputo, P. New trends in biosurfactants: From renewable origin to green enhanced oil recovery applications. Molecules 2024, 29, 301. [Google Scholar] [CrossRef]
- Sivakumar, D.; Ramasamy, R.; Thiagarajan, Y.R.; Thirumalairaj, B.; Krishnamoorthy, U.; Haque Siddiqui, M.I.; Lakshmaiya, N.; Kumar, A.; Shah, M.A. Biosurfactants in biocorrosion and corrosion mitigation of metals: An overview. Open Chem. 2024, 22, 20240036. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, W.; Yuan, J.; Tan, X.; Liu, S.; Wu, G.; Liu, K. Equivalent relationship of accelerated corrosion based on the chloride ion diffusion property in calcium sulfoaluminate cement-based pastes. Int. Commun. Heat Mass Transf. 2024, 152, 107283. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; He, Y.; Xu, Q.; Fan, X.; Liu, C. Study on the correlation between indoor accelerated corrosion testing of concrete epoxy polysiloxane coating and real-sea environmental testing in the East China Sea. Case Stud. Constr. Mater. 2024, 20, e02905. [Google Scholar] [CrossRef]
- Bass Equipamentos. Available online: https://www.bass.com.br/ (accessed on 14 March 2023).
- Equilam. Ensaio de Névoa Salina. Available online: https://equilam.com.br/ (accessed on 22 December 2023).
- TESTEX Instrument LTD. Available online: https://www.testextextile.com/pt/produto/testador-de-spray-de-sal-tu380/ (accessed on 23 December 2023).
- Soares da Silva, R.C.F.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.B.; Rufino, R.D.; Sarubbo, L.A. Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Soares da Silva, R.C.F.; Almeida, F.C.G.; Santos, V.A.; Sarubbo, L.A. Removal of heavy oil from contaminated surfaces with a detergent formulation containing biosurfactants produced by Pseudomonas spp. PeerJ 2021, 9, e12518. [Google Scholar] [CrossRef] [PubMed]
- Assis, S.L. Estudo Comparativo de Ensaios Acelerados Para Simulação da Corrosão Atmosférica. Master’s Thesis, IPEN, Autarquia Associada à Universidade de São Paulo, São Paulo, Brazil, 2000. [Google Scholar]
- ASTM B117-11; Standard Practice for Operating Salt Spray (Fog) Apparatus. American Society for Testing and Materials (S.I.): West Conshohocken, PA, USA, 2011; pp. 1–12.
- Souza, D.O.; Hammel, N.P.; Santos, W.I.A.; Ramirez, A.H.; Rojo, N.; Costa, I. Investigação da corrosividade do ensaio de névoa salina segundo norma ISO 9227. In Proceedings of the Encontro e Exposição Brasileira de Tratamento de superfície III INTERFINISH Latino Americano, Taboão da Serra, Brazil, 11–13 April 2012; pp. 360–367. [Google Scholar]
- Gentil, V. Corrosão, 6th ed.; LTC—Livros Técnicos e Científicos: Rio de Janeiro, Brazil, 2012; pp. 1–376. ISBN 978-852-161-804-1. [Google Scholar]
- Rodrigues, L.M. Desenvolvimento de Equipamento Para Realização de Ensaios Com Névoa Salina Para Avaliar a Resistência à Corrosão em Metais. Master’s Thesis, Centro Universitário de Volta Redonda, Volta Redonda, Brazil, 2017. [Google Scholar]
- Associação Brasileira de Normas Técnicas. Material Metálico Revestido e Não Revestido: Corrosão Por Exposição à Névoa Salina; NBR 8094; ABNT: Rio de Janeiro, Brazil, 1983. [Google Scholar]
- Dantas, E. Geração de Vapor e Água de Refrigeração, 2nd ed.; Ecolab Química Ltd.a: Rio de Janeiro, Brazil, 1988; pp. 1–305. [Google Scholar]
- Oliveira, S.H. Estudo da Utilização da Xantana e Hipoclorito de Sódio Como Estratégia Para Controle da Biocorrosão. Ph.D. Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2010. [Google Scholar]
- Acevedo, M.S.; Puentes, C.; Carreño, K.; León, J.G.; Stupak, M.; García, M.; Pérez, M.; Blustein, G. Antifouling paints based on marine natural products from Colombian Caribbean. Int. Biodeterior. Biodegrad. 2013, 83, 97–104. [Google Scholar] [CrossRef]
- Pontes, J.F.R.; Bendinelli, E.V.; Amorim, C.C.; Sá, M.M.; Ordine, A.P. Effect of corrosion inhibitor used in surface treatment on the anticorrosive performance of an epoxy paint system. Mater. Sci. Appl. 2016, 7, 593–609. [Google Scholar] [CrossRef]
- Qian, X.; Jin, K.; Lu, S.; Lv, L. Research on salt spray test of power facilities based on standardized laboratory construction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 032013. [Google Scholar] [CrossRef]
- ISO 9227:2017; Corrosion Tests in Artificial Atmospheres-Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: www.iso.org (accessed on 20 October 2024).
- Ostroski, V.C. Avaliação da velocidade de corrosão nos equipamentos portuários. Rev. Gestão Sustentabilidade Ambient. 2019, 8, 848–880. [Google Scholar] [CrossRef][Green Version]
- Santos, F.R.; Panossian, Z. Comparative study of the corrosion resistance of zinc electrodeposits obtained in chloride bath with additives and in sulfate/chloride bath without additives part 2: Corrosion resistance of the electrodeposits. Tecnol. Metal. Mater. Miner. 2018, 15, 137–143. [Google Scholar] [CrossRef]
- Hemapriya, V.; Prabakaran, M.; Chitra, S.; Swathika, M.; Kim, S.; Chung, I. Utilization of biowaste as an eco-friendly biodegradable corrosion inhibitor for mild steel in 1 mol/L HCl solution. Arab. J. Chem. 2020, 13, 8684–8696. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Mello, M.V.P.; Santos Júnior, N.E.; Souza, A.M.T.; Lanznaster, M.; Ponzio, E.A. Theoretical and experimental studies of a new aniline derivative corrosion inhibitor for mild steel in acid medium. Mater. Corros. 2020, 71, 280–291. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alfakeer, M.; Altass, H.M.; Althagafi, I.I.; El-ossaily, Y.A. Performance of unprecedented synthesized biosurfactants as green inhibitors for the corrosion of mild steel-37-2 in neutral solutions: A mechanistic approach. Green Chem. Lett. Rev. 2021, 14, 488–499. [Google Scholar] [CrossRef]
- Fawzy, A.; Al Bahir, A.; Alqarni, N.; Toghan, A.; Khider, M.; Ibrahim, I.M.; Abulreesh, H.H.; Elbanna, K. Evaluation of synthesized biosurfactants as promising corrosion inhibitors and alternative antibacterial and antidermatophytes agents. Sci. Rep. 2023, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Faccioli, Y.E.S.; Oliveira da Silva, G.; Soares da Silva, R.C.F.; Sarubbo, L.A. Application of a biosurfactant from Pseudomonas cepacia CCT 6659 in bioremediation and metallic corrosion inhibition processes. J. Biotechnol. 2022, 351, 109–121. [Google Scholar] [CrossRef]
- Semeniuk, I.; Karpenko, O.; Banya, A.; Lubenets, V.; Shapovalenko, S.; Pengxiang, G. New environmentally friendly compositions as inhibiors of metal corrosion. Sci. Study Res. Chem. C. 2023, 24, 41–48, ISSN 1582-540X. [Google Scholar]
- Wang, Q.; Yan, Z. Potential of biosurfactants in corrosion inhibition. In Industrial Applications of Biosurfactants and Microorganisms: Green Technology Avenues from Lab to Commercialization; Aslam, R., Aslam, J., Hussain, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 277–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).