A Potential Diabetic-Friendly Food Material: Optimization, Nutritional Quality, Structural Characteristics, and Functional Properties of Oat and Purple Potato Fermented by Ganoderma lucidum Mycelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Culture and Fermentation Procedure
2.3. Sample Preparation
2.4. Single-Factor Experiments for Optimization
2.5. Response Surface Methodology for Optimization
2.6. Fermentation Time for Optimization
2.7. Determination of Nutritional and Bioactive Compositions
2.8. SEM, XRD, and FTIR Assays
2.9. Thermogravimetric Determination
2.10. DPPH, Hydroxyl, Superoxide Anion Radical Scavenging Ability Assays
2.11. α-Amylase and α-Glucosidase Inhibition Assays
2.12. Adsorption Capacity of Insoluble Dietary Fiber Assay
2.13. Animal Model for Type 2 Diabetes Mellitus
2.14. Animal Group and Diet
2.15. Biochemical and Morphological Analysis
2.16. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.17. Statistical Analysis
3. Results
3.1. Optimization of Fermentation Conditions
3.1.1. Single-Factor Experiments Results
3.1.2. Response Surface Methodology for Optimizing Fermentation Conditions
3.1.3. Determination of Fermentation Time
3.2. Nutritional Quality Analysis
3.2.1. Main Nutritional Composition Analysis
3.2.2. Amino Acid Analysis
3.2.3. Changes in the Contents of Polysaccharides, Flavonoids, and Total Phenols
3.3. Structural Analysis
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. X-Ray Diffraction (XRD)
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.4. Thermogravimetric Analysis
3.4. In Vitro Functional Properties Analysis
3.4.1. Antioxidant Activity Analysis
3.4.2. Hypoglycemic Activity Analysis
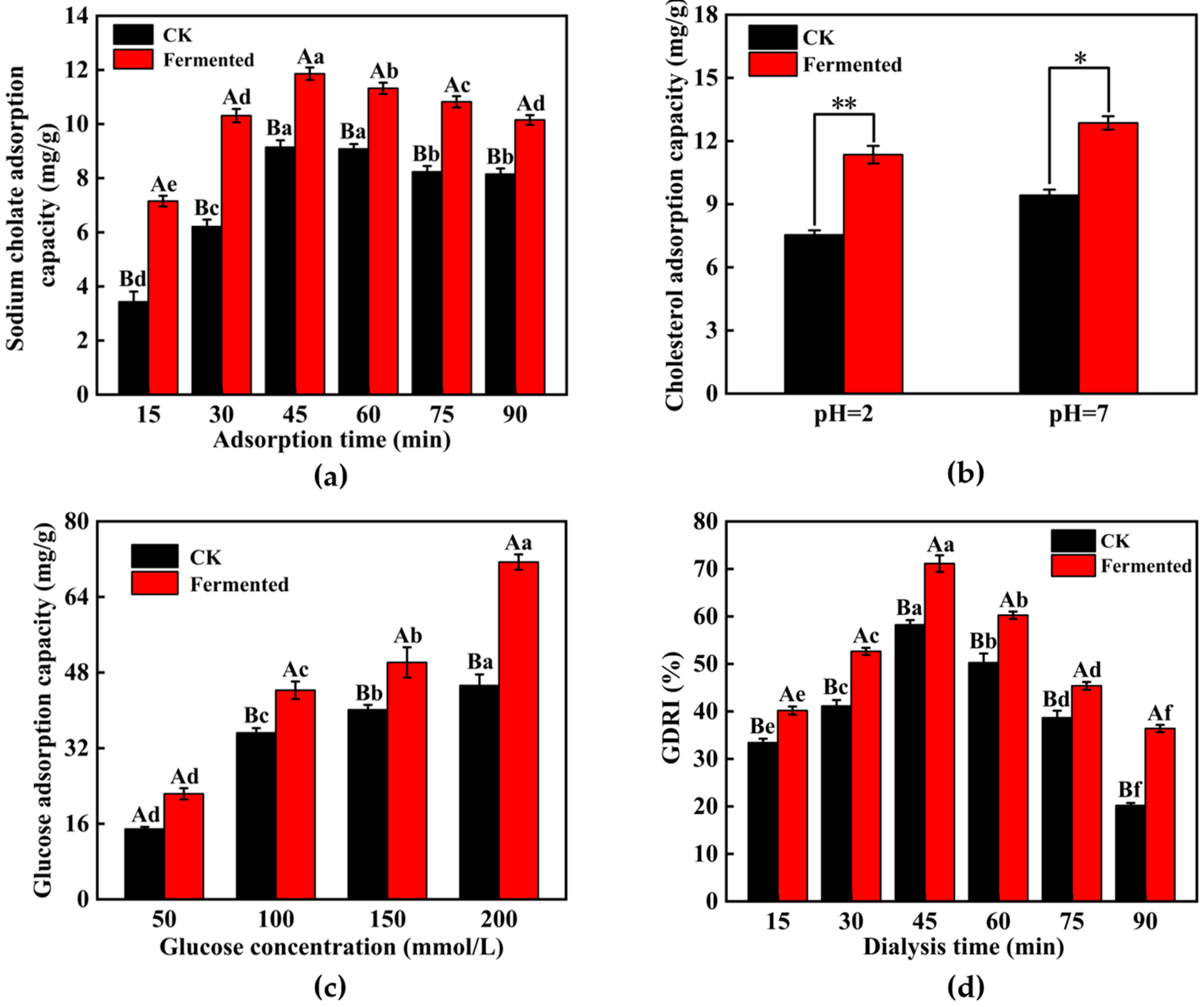
3.4.3. IDF Adsorption Capacity Analysis
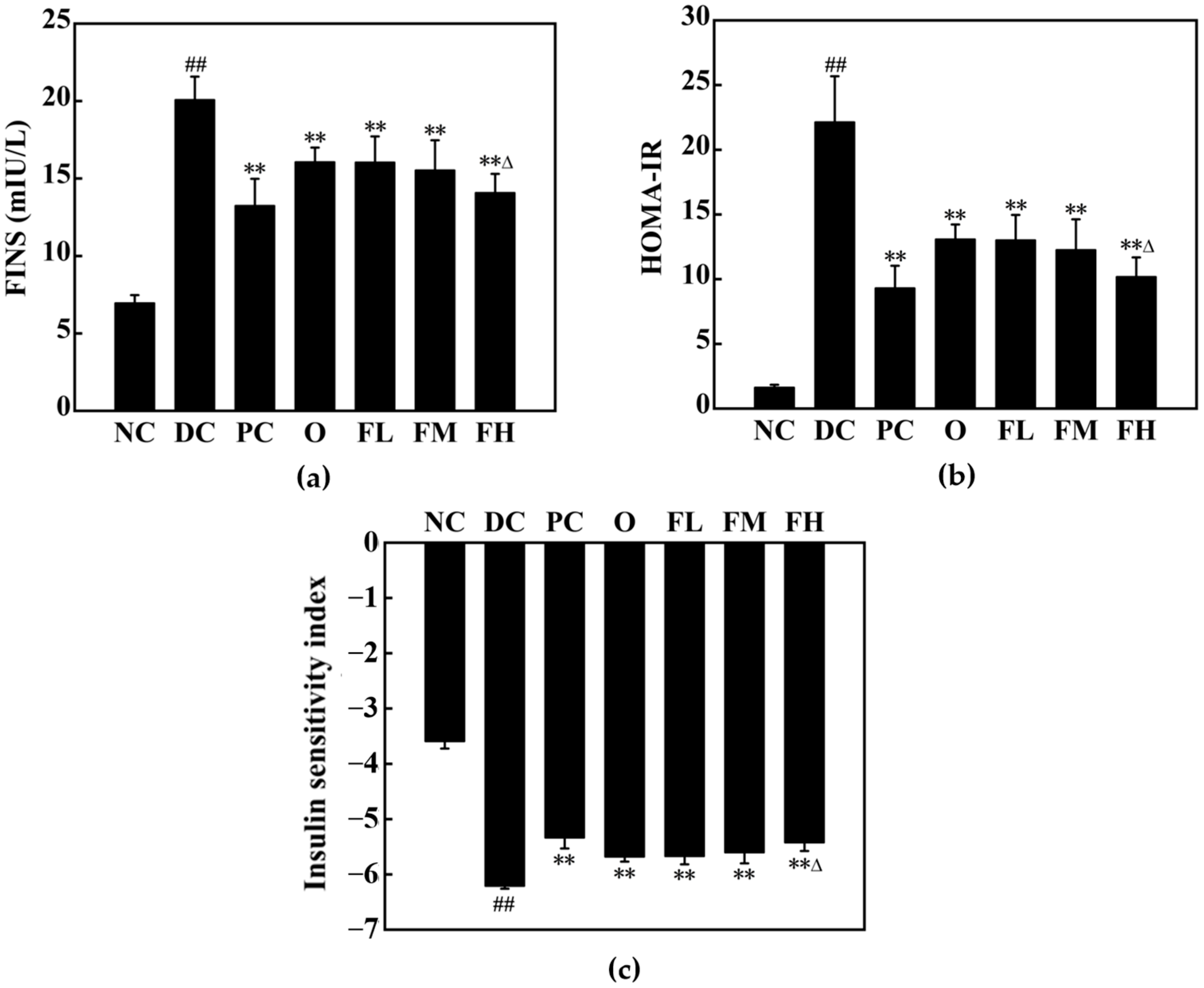
3.5. In Vivo Effect of F-OPPF on T2DM Rats
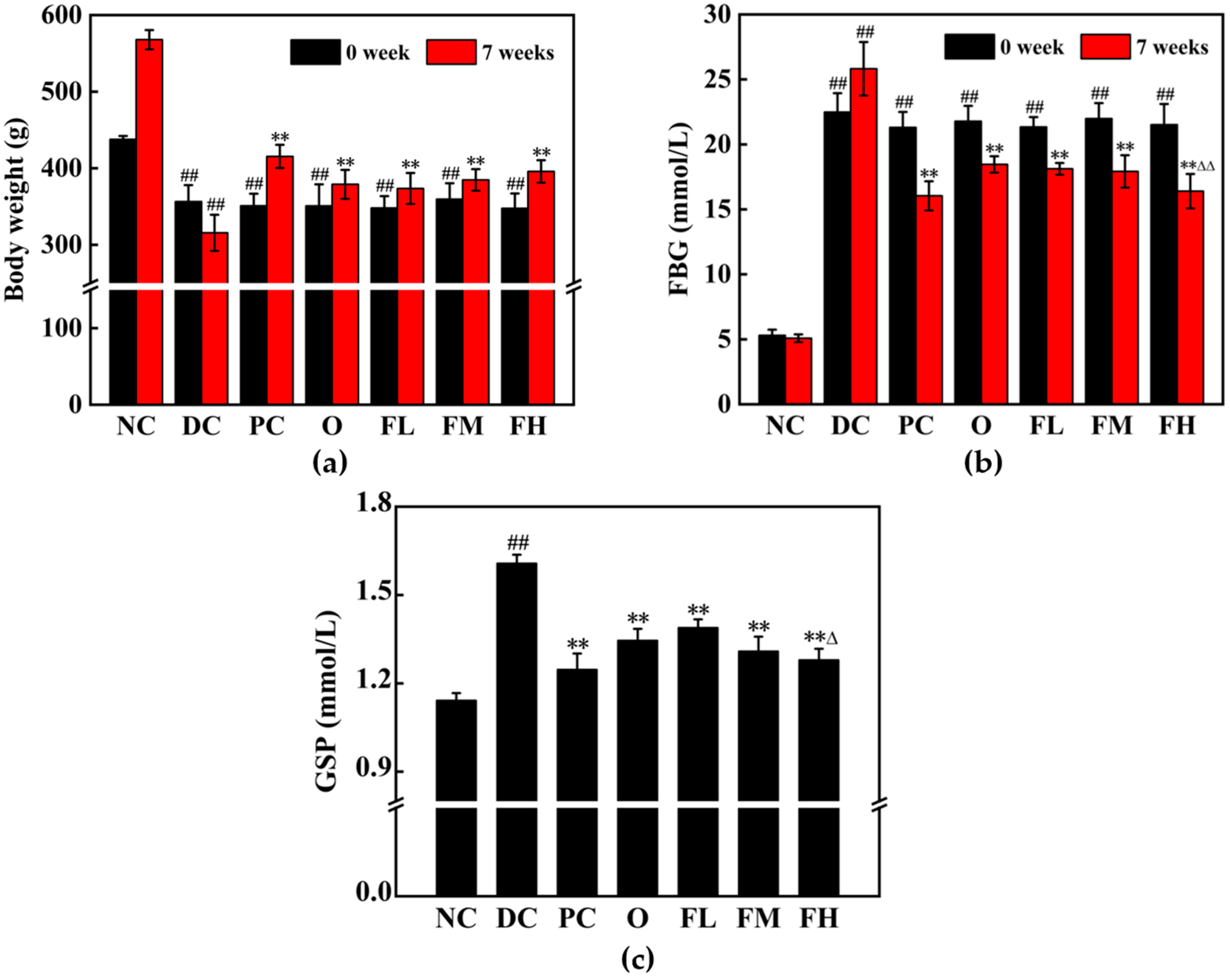
3.5.1. Effect of F-OPPF on Body Weight and Blood Glucose in T2DM Rats
3.5.2. Effect of F-OPPF on OGTT and ITT in T2DM Rats
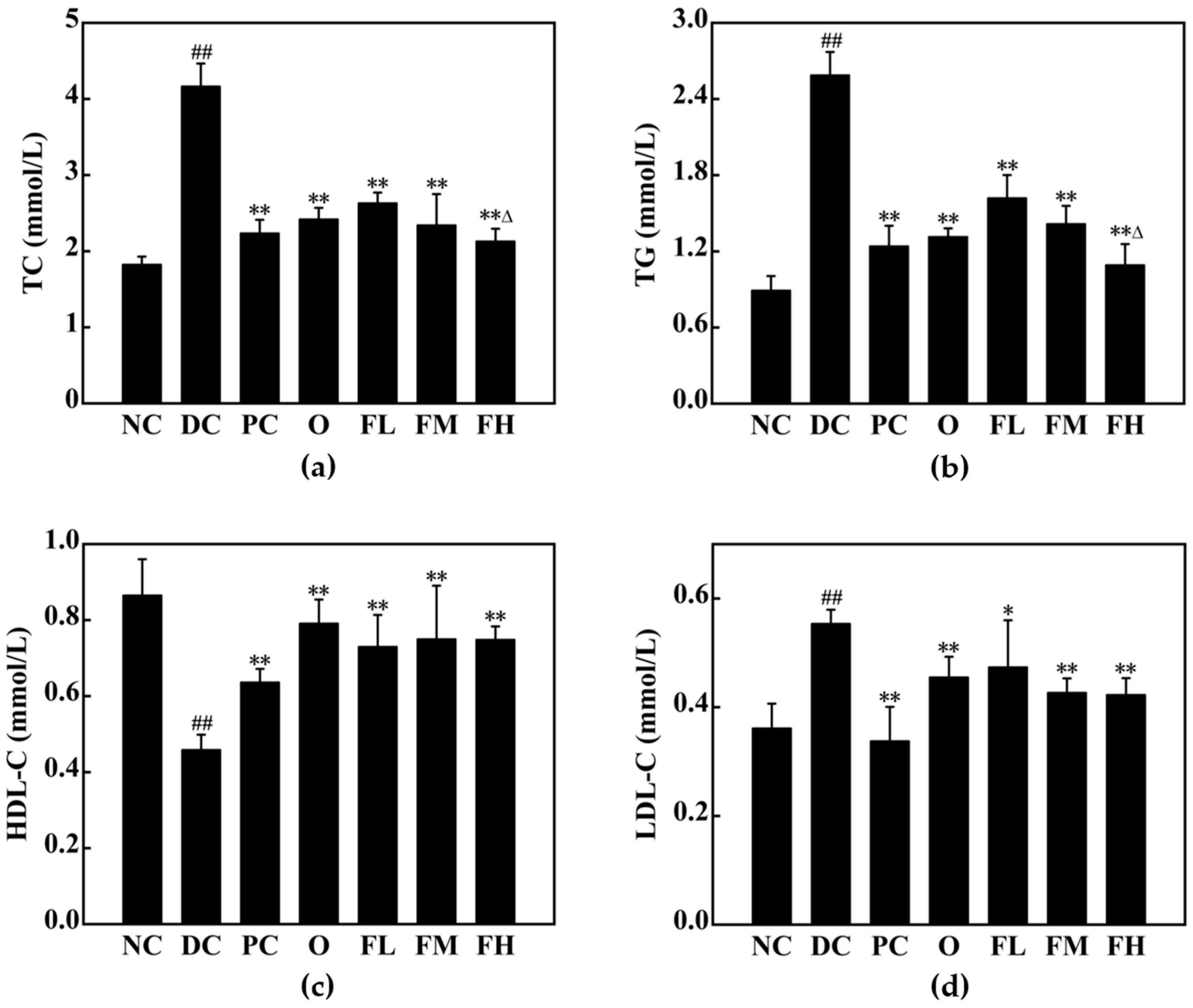
3.5.3. Effect of F-OPPF on Lipid Metabolism in T2DM Rats
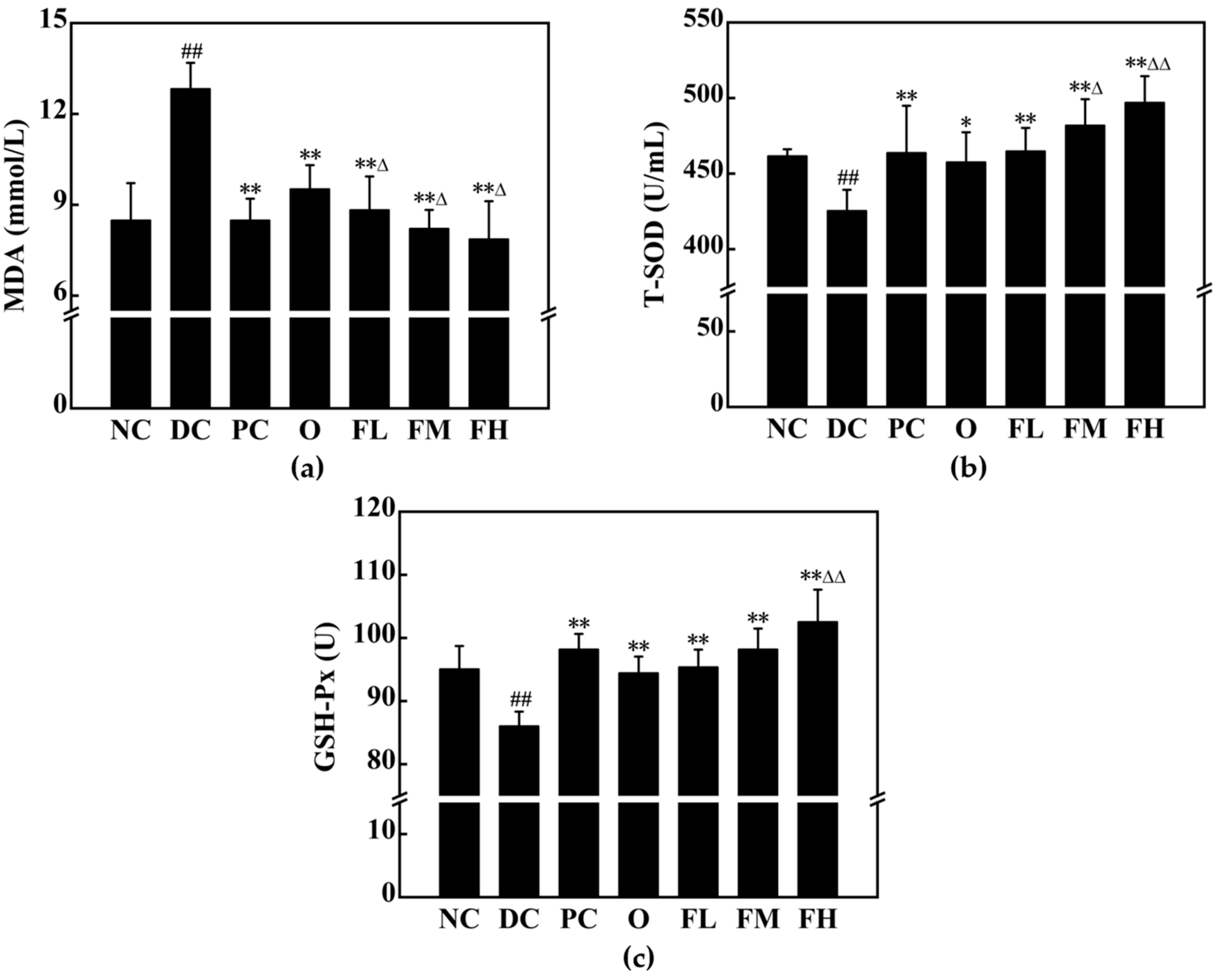
3.5.4. Effect of F-OPPF on Oxidative Stress in T2DM Rats
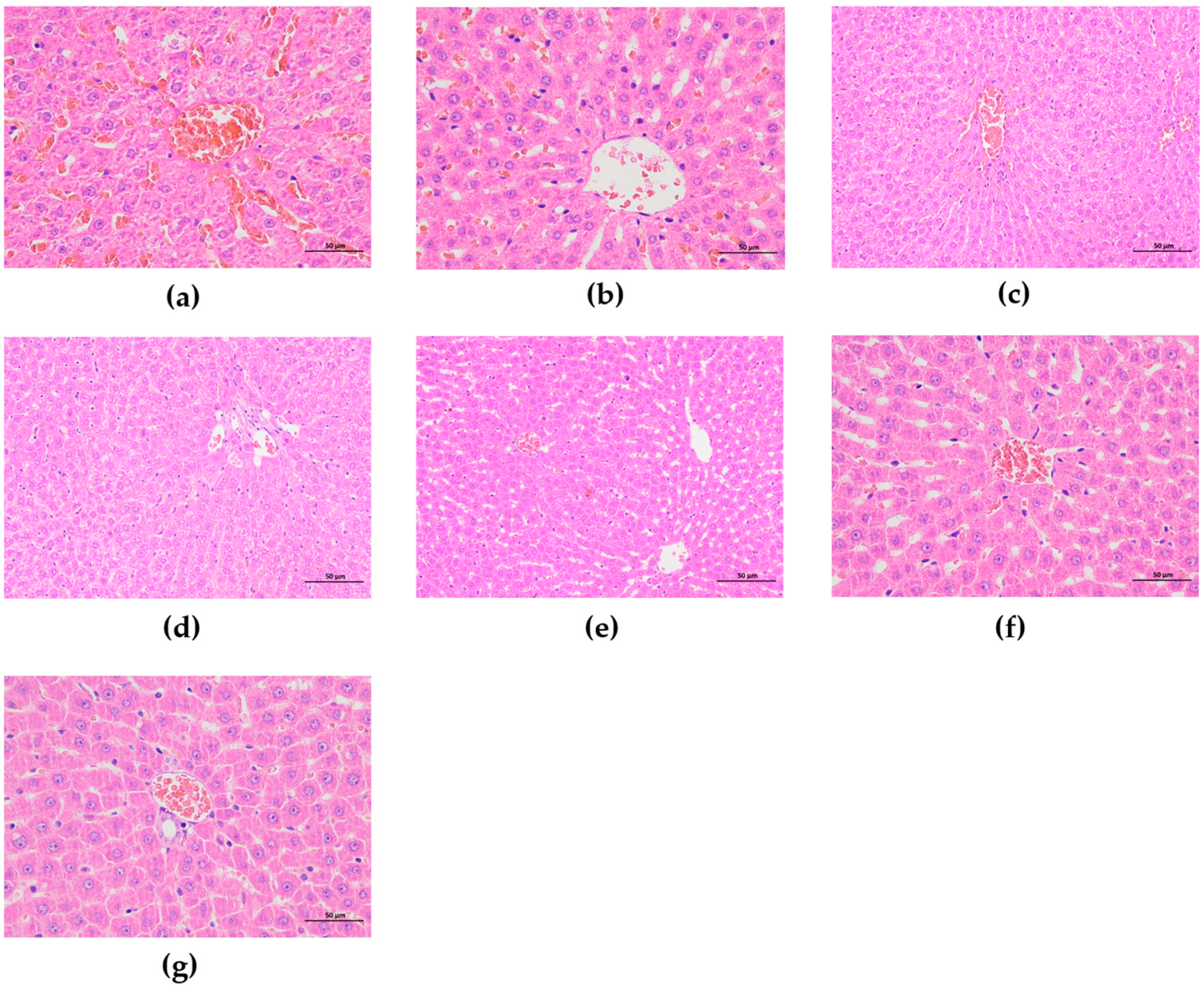
3.5.5. Effect of F-OPPF on Pancreatic Health in T2DM Rats
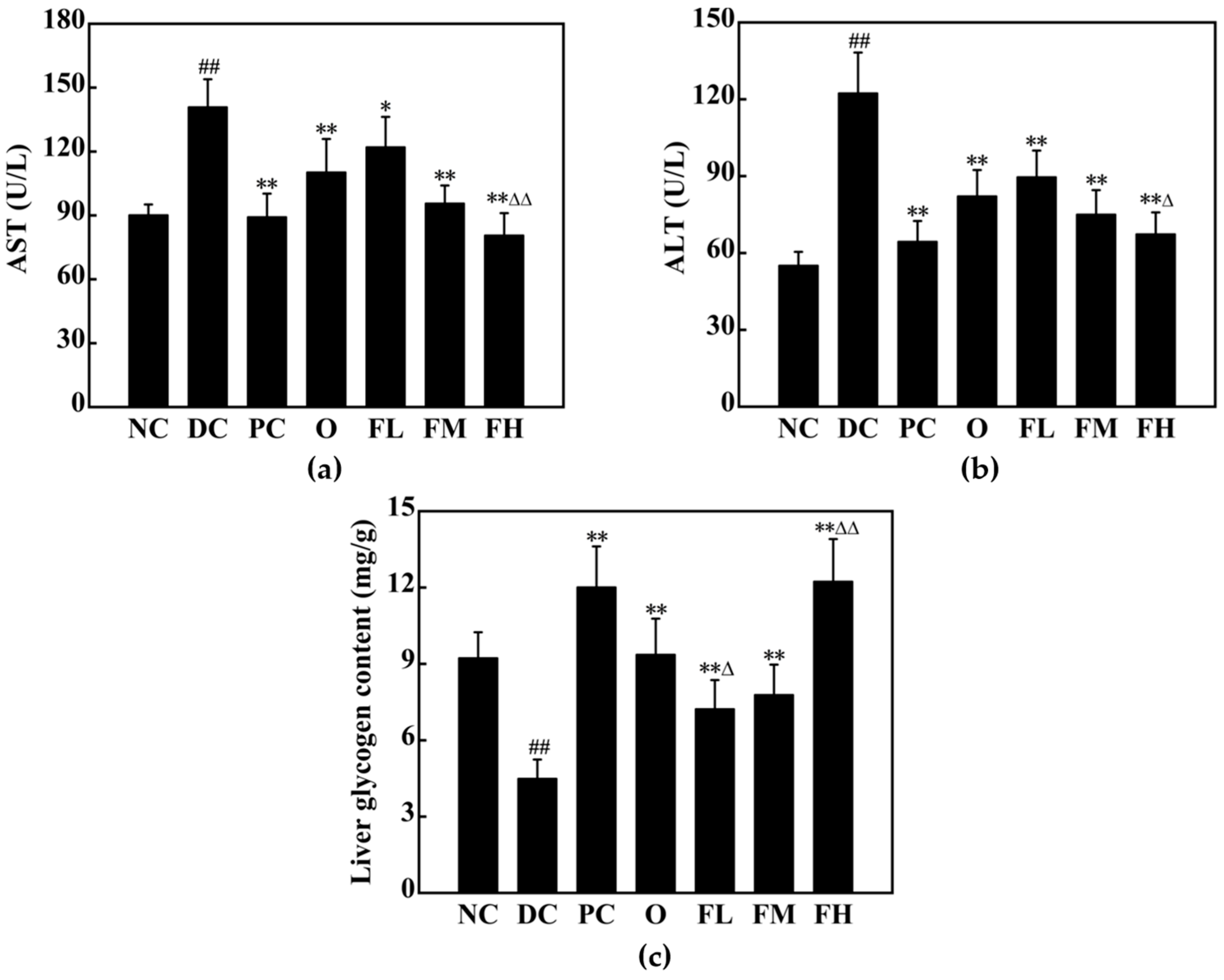
3.5.6. Effect of F-OPPF on Liver Health in T2DM Rats
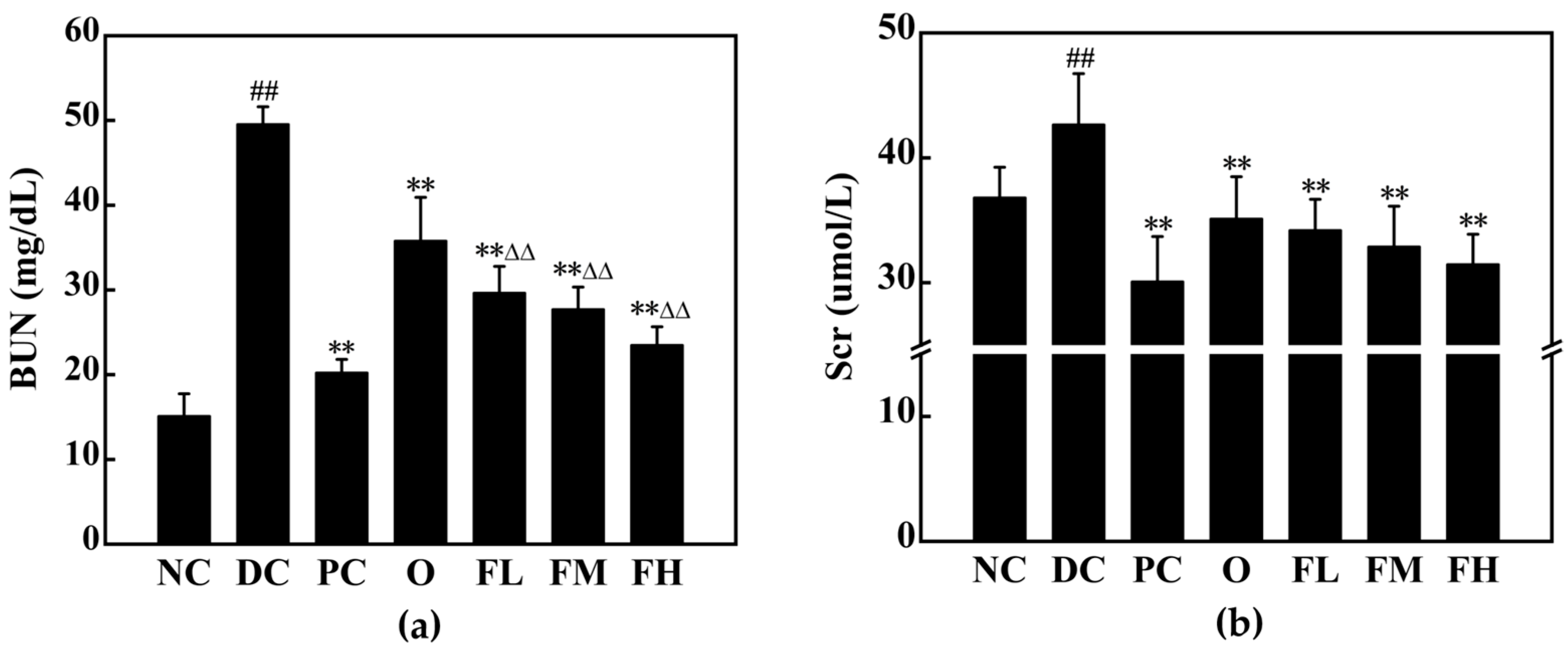
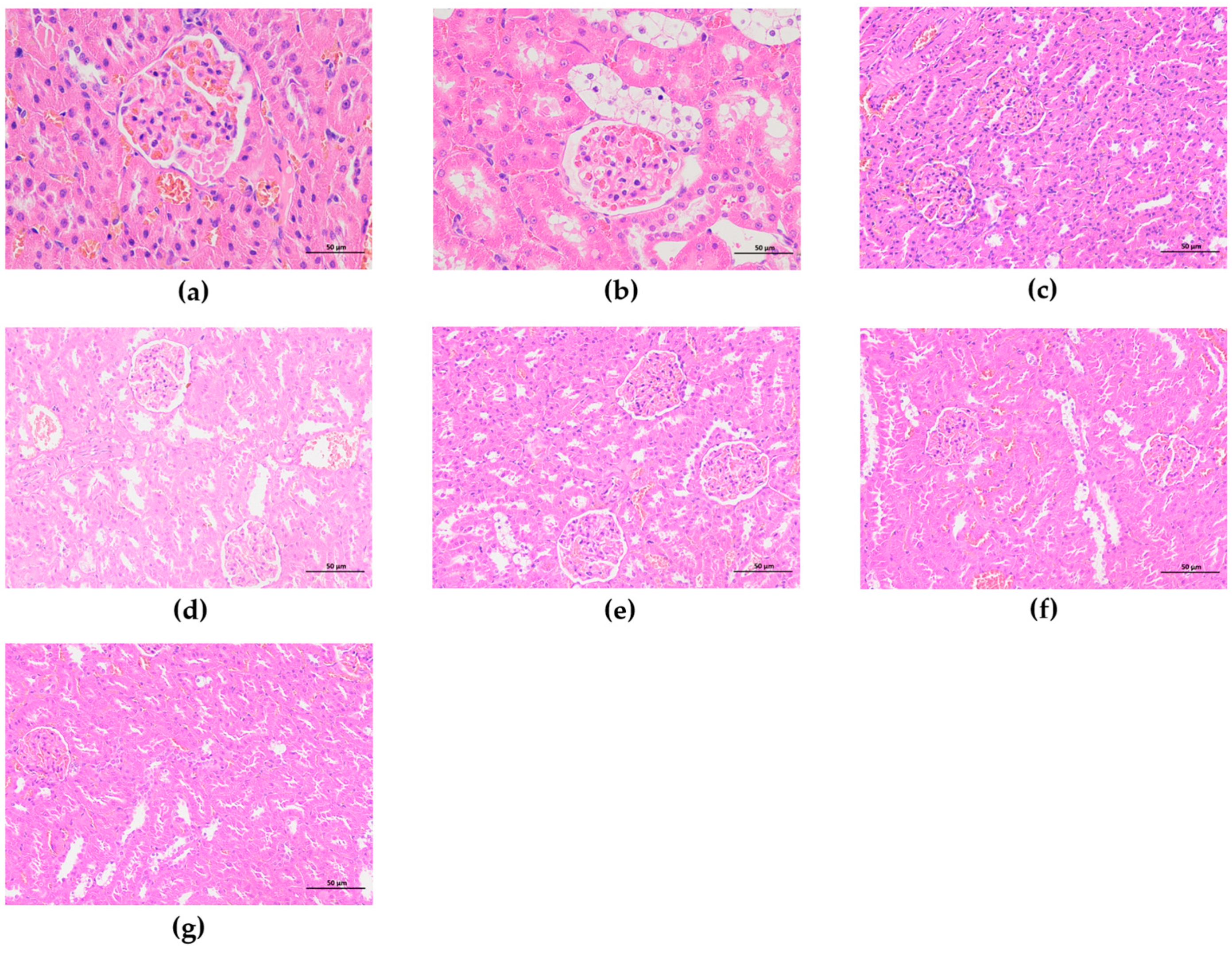
3.5.7. Effect of F-OPPF on Kidney Health in T2DM Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, Y.; Zhang, W.; Sun, M.; Zhang, Z. Hypoglycemic effect of inulin combined with ganoderma lucidum polysaccharides in T2DM rats. J. Funct. Foods 2019, 55, 381–390. [Google Scholar] [CrossRef]
- Janssen, J.-A.-M.-J.-L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.-W.; Curtus, T.-M.; Chen, M.; Medina, R.-J.; Mckay, G.-J.; Jenkins, A.; Lois, N. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.-H.; Micklesfield, L.-K. The effect of exercise on obesity, body fat distribution and risk for type 2 diabetes. Diabetes Phys. Act. 2014, 60, 82–93. [Google Scholar] [CrossRef]
- Sanders, T.-A. How important is the relative balance of fat and carbohydrate as sources of energy in relation to health? Proc. Nutr. Soc. 2016, 75, 147–153. [Google Scholar] [CrossRef]
- Baudet, M.; Daugareil, C.; Ferrieres, J. Cardiovascular disease prevention and life style modifications. Ann. Cardiol. d’Angéiologie 2012, 61, 93–98. [Google Scholar] [CrossRef]
- Ruxton, C.; Derbyshire, E. The health benefits of whole grains and fibre. Nutr. Food Sci. 2014, 44, 492–519. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J.; Yildiz, F. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent. Food Agric. 2016, 2, 1136015. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Wu, T.; Li, Q.; Meng, Y.; Zhang, M. Effects of ultrafine grinding and cellulase hydrolysis treatment on physicochemical and rheological properties of oat (Avena nuda L.) β-glucans. J. Cereal Sci. 2015, 65, 125–131. [Google Scholar] [CrossRef]
- Ma, X.; Gu, J.; Zhang, Z.; Jing, L.; Xu, M.; Dai, X.; Cai, X. Effects of Avena nuda L. on metabolic control and cardiovascular disease risk among Chinese patients with diabetes and meeting metabolic syndrome criteria: Secondary analysis of a randomized clinical trial. Eur. J. Clin. Nutr. 2013, 67, 1291–1297. [Google Scholar] [CrossRef]
- Nurdjanah, S.; Nurdin, S.-U.; Astuti, S.; Manik, V.-E. Chemical components, antioxidant activity, and glycemic response values of purple sweet potato products. Int. J. Food Sci. 2022, 2022, 7708172. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.-D.; Padhi, E.; Hawke, A.; Sivaramalingam, T.; Tsao, R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014, 5, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Palupi, E.; Delina, N.; Nurdin, N.-M.; Navratilova, H.-F.; Rimbawan, R.; Sulaeman, A. Kidney bean substitution ameliorates the nutritional quality of extruded purple sweet potatoes: Evaluation of chemical composition, glycemic index, and antioxidant capacity. Foods 2023, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.-S.; Katyal, P.; Beniwal, V.; Gupta, G.-K.; Sharma, A.-K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Structure-activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5900–5903. [Google Scholar] [CrossRef]
- Ren, L. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1961–1972. [Google Scholar] [CrossRef]
- Cen, Q.; Fan, J.; Zhang, R.; Chen, H.; Hui, F.; Li, J.; Qin, L. Impact of Ganoderma lucidum fermentation on the nutritional composition, structural characterization, metabolites, and antioxidant activity of Soybean, sweet potato and Zanthoxylum pericarpium residues. Food Chem. X 2024, 21, 101078. [Google Scholar] [CrossRef]
- GB 5009.88-2023; National Food Safety Standard–Determination of Dietary Fiber in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2023.
- GB 5009.9-2023; National Food Safety Standard–Determination of Starch in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2023.
- GB 5009.5-2016; National Food Safety Standard–Determination of Protein in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard–Determination of Fat in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB/T 15672-2009; Determination of Total Sugar Contents in Edible Fungi. National Standardization Administration of China: Beijing, China, 2009.
- GB/T 6434-2022; Determination of Crude Fiber Content in Feed. National Standardization Administration of China: Beijing, China, 2022.
- GB 5009.4-2016; National Food Safety Standard–Determination of Ash in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.268-2016; National Food Safety Standard–Determination of Multielement in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.124-2016; National Food Safety Standard–Determination of Amino Acids in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Zhai, F.-H.; Wang, Q.; Han, J.-R. Nutritional components and antioxidant properties of seven kinds of cereals fermented by the basidiomycete Agaricus blazei. J. Cereal Sci. 2015, 65, 202–208. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.-D.; Autolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- NY/T 3676-2020; Determination of Total Triterpene Contents in Ganoderma lucidum–Spectrophotometric Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- Tang, M.; Wang, L.; Cheng, X.; Wu, Y.-W.; Jie, O. Non-starch constituents influence the in vitro digestibility of naked oat (Avena nuda L.) starch. Food Chem. 2019, 297, 124953. [Google Scholar] [CrossRef]
- Jia, M.; Chen, J.; Liu, Z.; Xie, Y.; Nie, P.; Chen, Y.; Xie, J.; Yu, Q. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibers: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef] [PubMed]
- GB/T 34791-2017; Quality Control Requirements for Experimental Animals. National Standardization Administration of China: Beijing, China, 2017.
- Li, W.; Liu, Y.; Wang, Z.; Han, Y.; Tian, Y.-H.; Zhang, G.-S.; Sun, Y.-S.; Wang, Y.-P. Platycodin D isolated from the aerial parts of Platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. 2015, 6, 1418–1427. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Zhu, C.; Meng, Y.; Wang, J.; Chen, X.; Huang, J. Optimization of fermentation process of wheat germ protein by Aspergillus niger and analysis of antioxidant activity of peptide. Fermentation 2024, 10, 121. [Google Scholar] [CrossRef]
- Chen, H.; Fang, L.; Yu, X. Influence of different cultivation methods on ganoderic acid synthesis and solid–state fermentation condition optimization. Sci. Technol. Food Ind. 2017, 38, 167–172. [Google Scholar] [CrossRef]
- Lou, H.; Yang, C.; Gong, Y.; Li, Y.; Li, Y.; Tian, S.; Zhao, Y.; Zhao, R. Edible fungi efficiently degrade aflatoxin B1 in cereals and improve their nutritional composition by solid-state fermentation. J. Hazard. Mater. 2023, 451, 131139. [Google Scholar] [CrossRef]
- Yin, L.; Liu, Z.; Lu, X.; Cheng, J.; Lu, G.; Sun, J.; Yang, H.; Guan, Y.; Pang, L. Analysis of the nutritional properties and flavor profile of sweetpotato residue fermented with Rhizopus oligosporus. LWT 2023, 174, 114401. [Google Scholar] [CrossRef]
- Yang, L.; Fu, T.; Yang, F. Biovalorization of soybean residue (okara) via fermentation with Ganoderma lucidum and Lentinus edodes to attain products with high anti-osteoporotic effects. J. Biosci. Bioeng. 2020, 129, 514–518. [Google Scholar] [CrossRef]
- GB 2762-2022; National Food Safety Standard–Limit of Pollutants in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2022.
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of treatment methods on the formation of resistant starch in purple sweet potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.-A.; Gani, A.; Ashwar, B. Dual enzyme modified oat starch: Structural characterisation, rheological properties, and digestibility in simulated GI tract. Int. J. Biol. Macromol. 2018, 106, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Snape, C.-E.; Morrison, W.-R.; Maroto-Valer, M.-M.; Karkalas, J.; Pethrick, R.-A. Solid state13C NMR investigation of lipid ligands in V-amylose inclusion complexes. Carbohyd. Polym. 1998, 36, 225–237. [Google Scholar] [CrossRef]
- Gupta, R.; Gaur, S. Investigating the effect of natural fermentation in modifying the physico-functional, structural and thermal characteristics of pearl and finger millet starch. J. Sci. Food Agric. 2023, 104, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, X.; Zhu, R.; Ma, Z.; Liu, L.; Wang, X.; Hu, X. Effect of natural fermentation on the structure and physicochemical properties of wheat starch. Carbohyd. Polym. 2019, 218, 163–169. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Li, L.-T.; Min, W.-H.; Wang, F.; Tatsumi, E. The effects of natural fermentation on the physical properties of rice flour and the rheological characteristics of rice noodles. Int. J. Food Sci. Technol. 2005, 40, 985–992. [Google Scholar] [CrossRef]
- Shaikh, F.; Ali, T.-M.; Mustafa, G.; Hasnain, A. Comparative study on effects of citric and lactic acid treatment on morphological, functional, resistant starch fraction and glycemic index of corn and sorghum starches. Int. J. Biol. Macromol. 2019, 135, 314–327. [Google Scholar] [CrossRef]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, J.-D.; Cai, Z.-H.; Huang, H.; Zhang, S.; Fu, L.-N.; Zhao, P.-Q.; Yan, X.-Y.; Fu, Y.-J. Improved physicochemical and functional properties of dietary fiber from Rosa roxburghii pomace fermented by Bacillus natto. Food Biosci. 2022, 50, 102030. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, H.; Zhang, H.; Li, R.; Zhang, Q.; Luo, D.; Li, M. Serum oxidized low density lipoprotein serves as a mediator for the inverse relationship between serum D-ribose and cognitive performance in type 2 diabetic patients. Free. Radic. Biol. Med. 2021, 171, 91–98. [Google Scholar] [CrossRef]
- Zampieri, M.; Karpach, K.; Salerno, G.; Raguzzini, A.; Barchetta, I.; Cimini, F.-A.; Dule, X.; Reale, A. PAR level mediates the link between ROS and inflammatory response in patients with type 2 diabetes mellitus. Redox Biol. 2024, 75, 103243. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, F.; Harrat, N.-I.; Affane, F.; Chekkal, H.; Lamri-Senhadii, M. Incorporation of whole oat, especially bran, into a high-fat diet, improves cardio–metabolic risk factors in type 2 diabetic rats. Nutr. Food Sci. 2018, 49, 600–616. [Google Scholar] [CrossRef]
- Lazar, M.-A. How obesity causes diabetes: Not a tall tale. Science 2005, 307, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Attaf, D.; Mailfert, A.-S.; Nasu, M.; Joubet, R. Maillard reaction, mitochondria and oxidative stress: Potential role of antioxidants. Pathol. Biol. 2010, 58, 220–225. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Zhong, Z. Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011, 12, 6135–6145. [Google Scholar] [CrossRef]
- Harris, K.-A.; Kris-Etherton, P.-M. Effects of whole grains on coronary heart disease risk. Curr. Atheroscler. Rep. 2010, 12, 368–376. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, J.; Ding, J.; Li, S.; Xia, J. Ganoderic acid a improves high fat diet-induced obesity, lipid accumulation and insulin sensitivity through regulating srebp pathway. Chem.-Biol. Interact. 2018, 290, 77–87. [Google Scholar] [CrossRef]
- Hajjaj, H.; Mace, C.; Roberts, M.; Niederberger, P.; Fay, L.-B. Effect of 26-oxygenosterols from ganoderma lucidum and their activity as cholesterol synthesis inhibitors. Appl. Environ. Microb. 2005, 71, 3653–3658. [Google Scholar] [CrossRef]
- Tsopmo, A. Processing and Impact on Active Components in Food; Academic Press: San Diego, CA, USA, 2015; pp. 361–368. [Google Scholar] [CrossRef]
- Zhao, R.; Li, N.; Liu, W.; Liu, Q.; Zhang, L.; Peng, X.; Zhao, R.; Hu, H. Low glycemic index potato biscuits alleviate physio-histological damage and gut dysbiosis in rats with type-2 diabetes mellitus induced by high-sugar and high-fat diet and streptozotocin. J. Nutr. Biochem. 2023, 119, 109401. [Google Scholar] [CrossRef]
- Li, L.; Li, R.-C.; Song, Y.-H.; Wu, W.-Y.; Yin, S.-H.; Fu, W.-W.; Li, W. Effects of a Ganoderma atrum polysaccharide against pancreatic damage in streptozotocin-induced diabetic mice. Food Funct. 2019, 10, 7227–7238. [Google Scholar] [CrossRef]
- Adeyi, A.-O.; Awosanya, S.-A.; Adeyi, O.-E.; James, A.-S.; Adenipekun, C.-O. Ganoderma lucidum ethanol extract abrogates metabolic syndrome in rats: In vivo evaluation of hypoglycemic, hypolipidemic, hypotensive and antioxidant properties. Obes. Med. 2021, 22, 100320. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Fu, H.-Y.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, doubleblind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef]

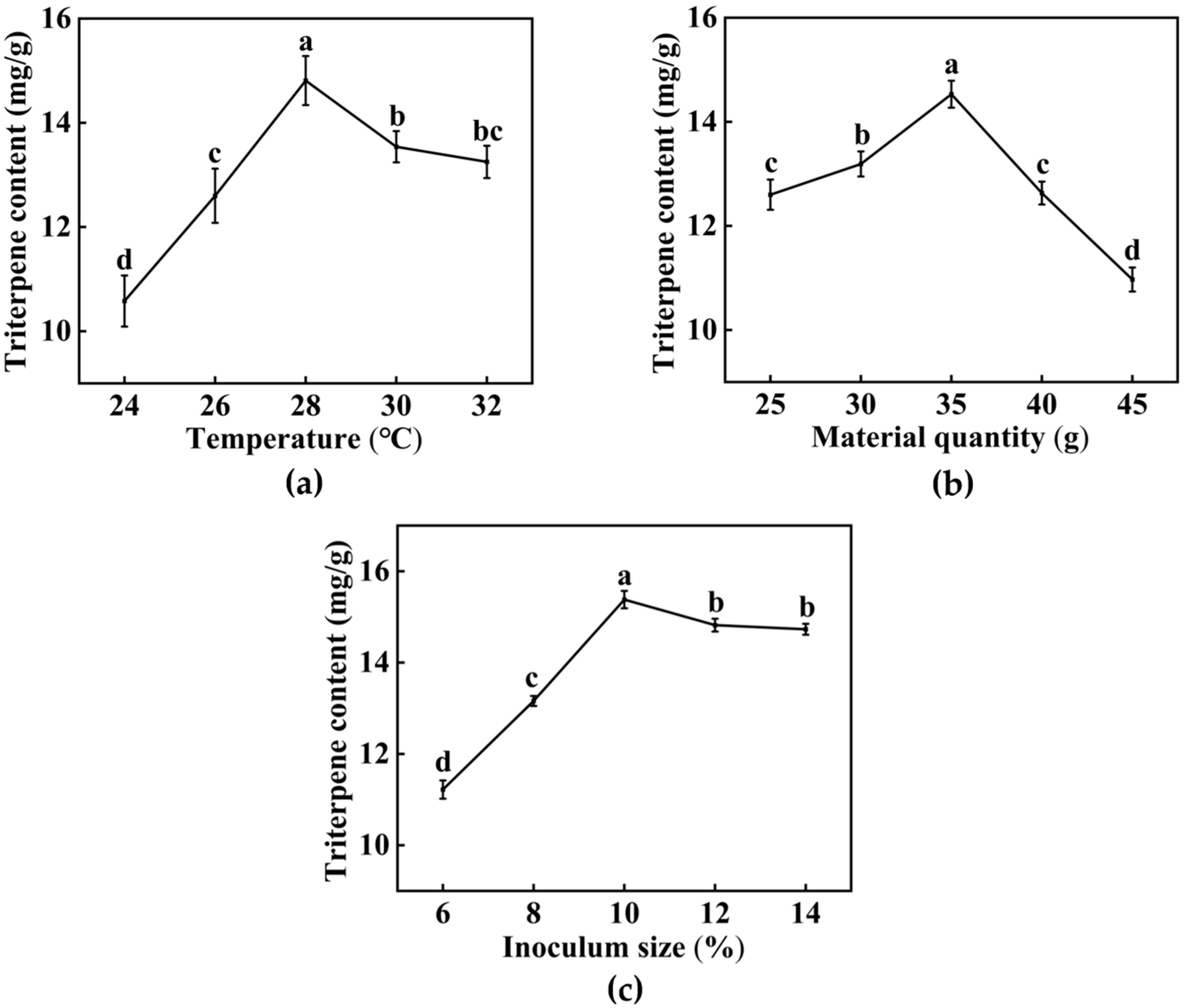
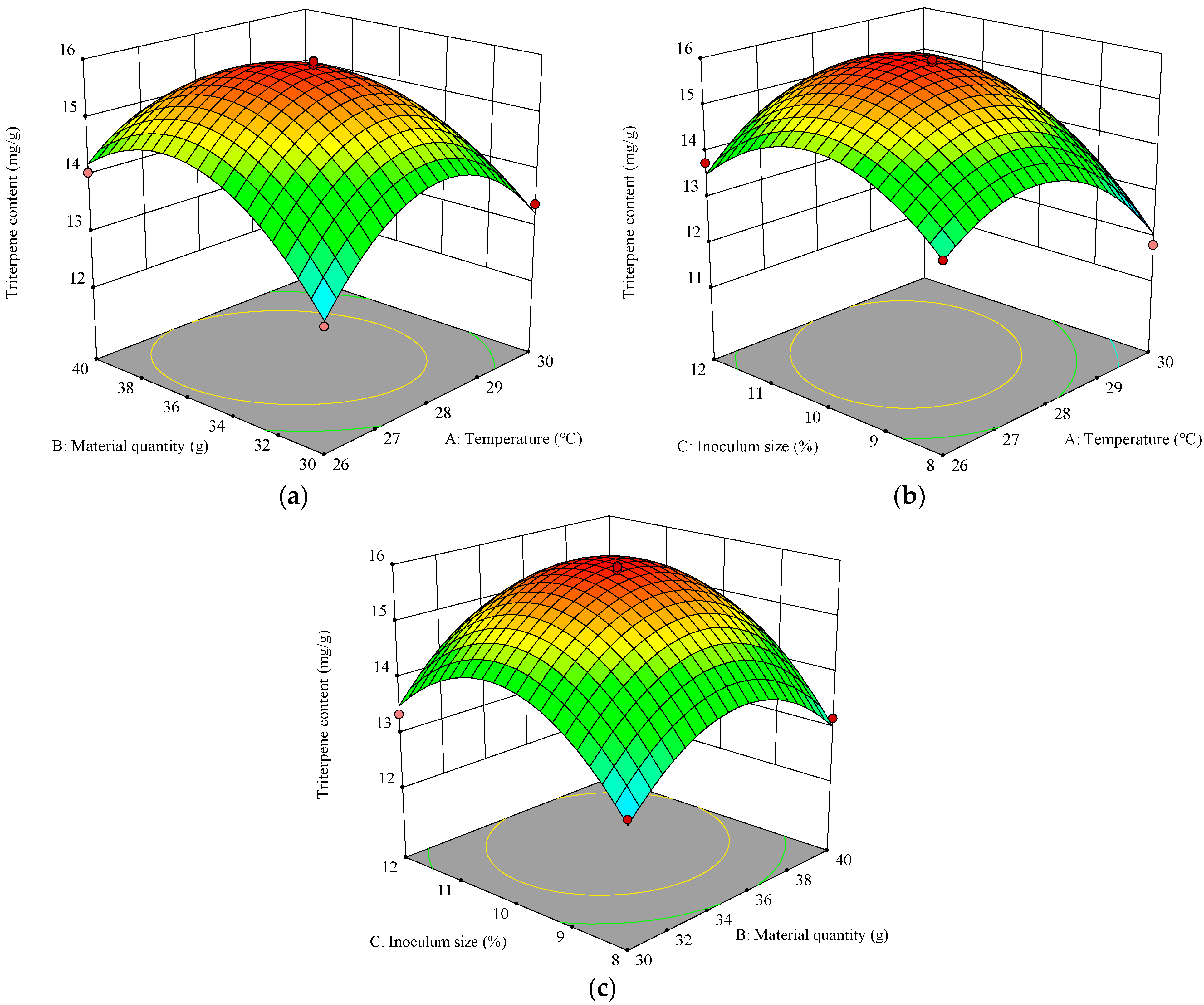



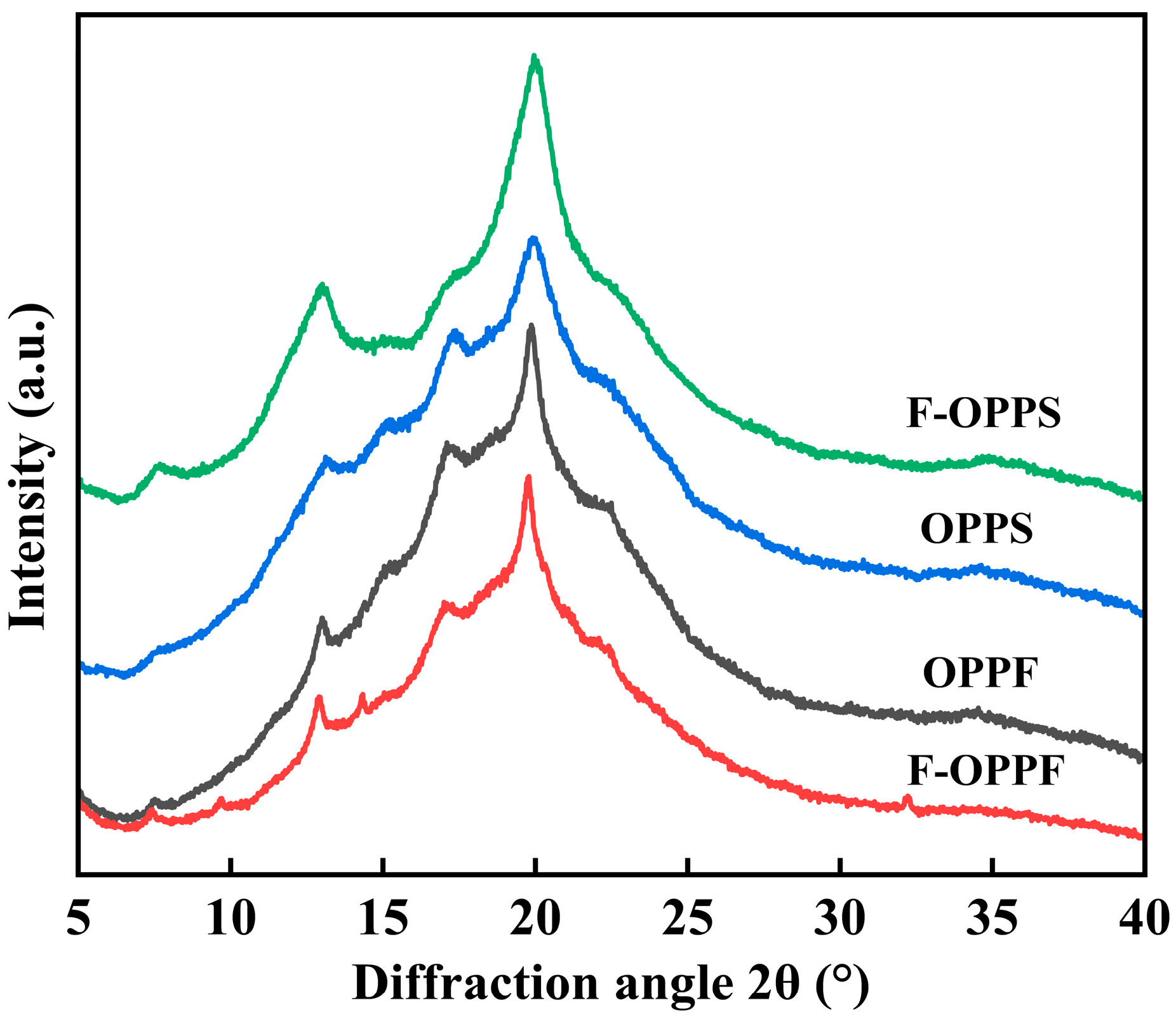



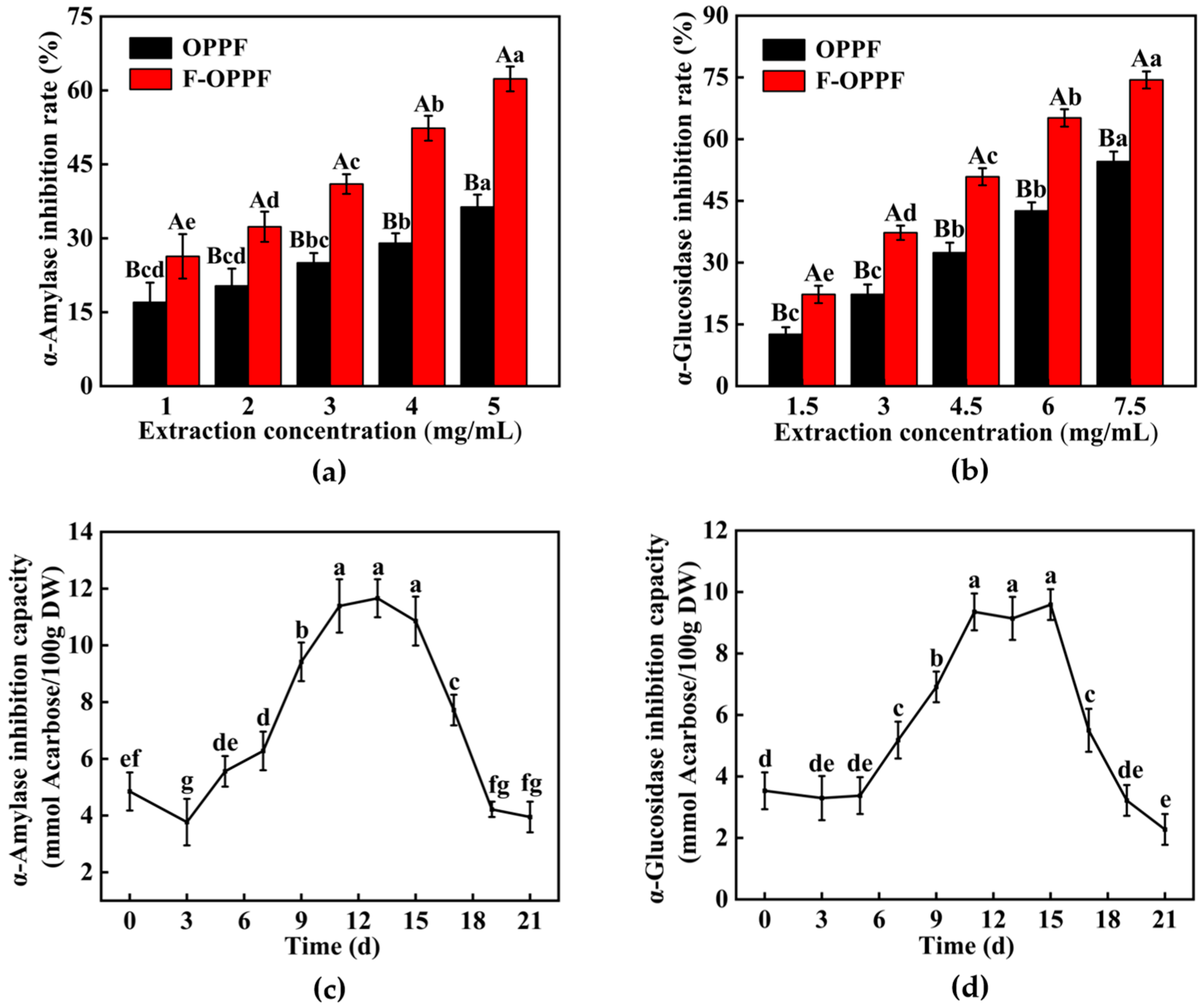


| Level | A—Temperature/(°C) | B—Material Quantity/(g) | C—Inoculum Size/% |
|---|---|---|---|
| −1 | 26 | 30 | 8 |
| 0 | 28 | 35 | 10 |
| 1 | 30 | 40 | 12 |
| Runs | A—Temperature/°C | B—Material Quantity/g | C—Inoculum Size/% | Triterpene Content /(mg/g) |
|---|---|---|---|---|
| 1 | 26 | 30 | 10 | 12.78 ± 0.78 ef |
| 2 | 30 | 30 | 10 | 13.39 ± 0.47 bcde |
| 3 | 26 | 40 | 10 | 14.05 ± 0.62 bc |
| 4 | 30 | 40 | 10 | 13.04 ± 0.66 de |
| 5 | 26 | 35 | 8 | 13.29 ± 1.01 bcde |
| 6 | 30 | 35 | 8 | 11.81 ± 0.36 f |
| 7 | 26 | 35 | 12 | 13.76 ± 1.04 bcde |
| 8 | 30 | 35 | 12 | 13.84 ± 0.28 bcd |
| 9 | 28 | 30 | 8 | 12.88 ± 0.38 de |
| 10 | 28 | 40 | 8 | 13.17 ± 0.56 cde |
| 11 | 28 | 30 | 12 | 13.35 ± 0.34 bcde |
| 12 | 28 | 40 | 12 | 14.22 ± 0.73 b |
| 13 | 28 | 35 | 10 | 15.93 ± 0.67 a |
| 14 | 28 | 35 | 10 | 15.87 ± 0.58 a |
| 15 | 28 | 35 | 10 | 15.86 ± 0.54 a |
| 16 | 28 | 35 | 10 | 15.91 ± 0.58 a |
| 17 | 28 | 35 | 10 | 15.51 ± 0.42 a |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 26.79 | 9 | 2.98 | 55.93 | <0.0001 |
| A-Temperature | 0.405 | 1 | 0.405 | 7.61 | 0.0282 |
| B-Material Quantity | 0.5408 | 1 | 0.5408 | 10.16 | 0.0153 |
| C-Inoculum Size | 2.02 | 1 | 2.02 | 37.95 | 0.0005 |
| AB | 0.6561 | 1 | 0.6561 | 12.33 | 0.0098 |
| AC | 0.6084 | 1 | 0.6084 | 11.43 | 0.0117 |
| BC | 0.0841 | 1 | 0.0841 | 1.58 | 0.2491 |
| A2 | 7.85 | 1 | 7.85 | 147.51 | <0.0001 |
| B2 | 5.43 | 1 | 5.43 | 102.00 | <0.0001 |
| C2 | 6.85 | 1 | 6.85 | 128.7 | <0.0001 |
| Residual | 0.3726 | 7 | 0.0532 | ||
| Lack of Fit | 0.2523 | 3 | 0.0841 | 2.8 | 0.173 |
| Pure Error | 0.1203 | 4 | 0.0301 | ||
| Cor Total | 27.17 | 16 |
| Sample | Total Starch | Protein | Fat | Soluble Protein | Total Sugar | Crude Fiber | Ash |
|---|---|---|---|---|---|---|---|
| OPPF | 76.03 ± 1.47 a | 10.90 ± 0.46 b | 5.28 ± 0.07 a | 8.31 ± 0.07 b | 68.59 ± 1.36 a | 1.73 ± 0.09 b | 1.71 ± 0.07 b |
| F-OPPF | 54.60 ± 1.05 b | 12.21 ± 0.35 a | 5.42 ± 0.05 a | 13.12 ± 0.12 a | 58.42 ± 1.06 b | 3.10 ± 0.06 a | 2.31 ± 0.11 a |
| Index | OPPF | F-OPPF |
|---|---|---|
| Na | 95.25 ± 0.08 b | 137.82 ± 0.14 a |
| Mg | 896.59 ± 0.08 b | 1323.90 ± 2.57 a |
| K | 5198.41 ± 47.34 a | 5163.30 ± 0.08 b |
| Ca | 294.56 ± 1.57 b | 458.11 ± 3.34 a |
| V | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Mn | 25.69 ± 0.02 b | 40.23 ± 0.03 a |
| Fe | 74.03 ± 3.62 a | 53.59 ± 0.28 b |
| Cu | 3.04 ± 0.00 b | 4.23 ± 0.22 a |
| Zn | 12.28 ± 0.56 b | 16.67 ± 0.12 a |
| Se | 0.01 ± 0.00 b | 0.02 ± 0.01 a |
| Sr | 2.97 ± 0.01 b | 4.56 ± 0.04 a |
| Cr | 0.63 ± 0.06 b | 0.70 ± 0.03 a |
| Ni | 1.73 ± 0.06 a | 2.66 ± 0.29 a |
| As | 0.10 ± 0.00 a | 0.05 ± 0.03 a |
| Cd | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Hg | <0.00 ± 0.00 a | <0.00 ± 0.00 a |
| Pb | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Index | OPPF | F-OPPF |
|---|---|---|
| Thr | 0.40 ± 0.03 b | 0.45 ± 0.03 a |
| Val | 0.56 ± 0.04 b | 0.56 ± 0.08 a |
| Ile | 0.45 ± 0.03 b | 0.46 ± 0.03 a |
| Met | 0.04 ± 0.01 b | 0.15 ± 0.02 a |
| Leu | 0.80 ± 0.06 a | 0.77 ± 0.04 b |
| Lys | 0.43 ± 0.03 a | 0.37 ± 0.03 a |
| Phe | 0.61 ± 0.04 a | 0.56 ± 0.04 b |
| Tyr | 0.34 ± 0.03 b | 0.36 ± 0.03 a |
| Asp | 0.95 ± 0.05 b | 0.98 ± 0.06 a |
| Ser | 0.53 ± 0.03 b | 0.56 ± 0.03 a |
| Glu | 2.11 ± 0.08 a | 1.69 ± 0.08 a |
| Gly | 0.51 ± 0.04 a | 0.55 ± 0.03 a |
| Ala | 0.52 ± 0.02 b | 0.54 ± 0.02 a |
| His | 0.26 ± 0.03 b | 0.26 ± 0.03 a |
| Arg | 0.67 ± 0.03 b | 0.67 ± 0.04 a |
| Pro | 0.52 ± 0.05 b | 0.53 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Zhao, T.; Zhang, A.; Zhang, D.; Huang, X.; Fang, X.; Geng, J.; Gang, J. A Potential Diabetic-Friendly Food Material: Optimization, Nutritional Quality, Structural Characteristics, and Functional Properties of Oat and Purple Potato Fermented by Ganoderma lucidum Mycelium. Fermentation 2024, 10, 618. https://doi.org/10.3390/fermentation10120618
Guan Y, Zhao T, Zhang A, Zhang D, Huang X, Fang X, Geng J, Gang J. A Potential Diabetic-Friendly Food Material: Optimization, Nutritional Quality, Structural Characteristics, and Functional Properties of Oat and Purple Potato Fermented by Ganoderma lucidum Mycelium. Fermentation. 2024; 10(12):618. https://doi.org/10.3390/fermentation10120618
Chicago/Turabian StyleGuan, Yingxian, Tong Zhao, Anrong Zhang, Di Zhang, Xiaoxiao Huang, Xiao Fang, Jiajun Geng, and Jie Gang. 2024. "A Potential Diabetic-Friendly Food Material: Optimization, Nutritional Quality, Structural Characteristics, and Functional Properties of Oat and Purple Potato Fermented by Ganoderma lucidum Mycelium" Fermentation 10, no. 12: 618. https://doi.org/10.3390/fermentation10120618
APA StyleGuan, Y., Zhao, T., Zhang, A., Zhang, D., Huang, X., Fang, X., Geng, J., & Gang, J. (2024). A Potential Diabetic-Friendly Food Material: Optimization, Nutritional Quality, Structural Characteristics, and Functional Properties of Oat and Purple Potato Fermented by Ganoderma lucidum Mycelium. Fermentation, 10(12), 618. https://doi.org/10.3390/fermentation10120618





