Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Bacterial Strains
2.2. Optimization of Carbon and Nitrogen Source in Culture Medium
2.3. Batch and Repeated Batch Fermentation
2.4. EAES System Construction
2.5. COS Production in EAES System
2.6. Analysis of Antioxidant Activity of CHOS Produced by EAES System
2.7. Analytical Methods and Statistical Analysis
3. Results and Discussion
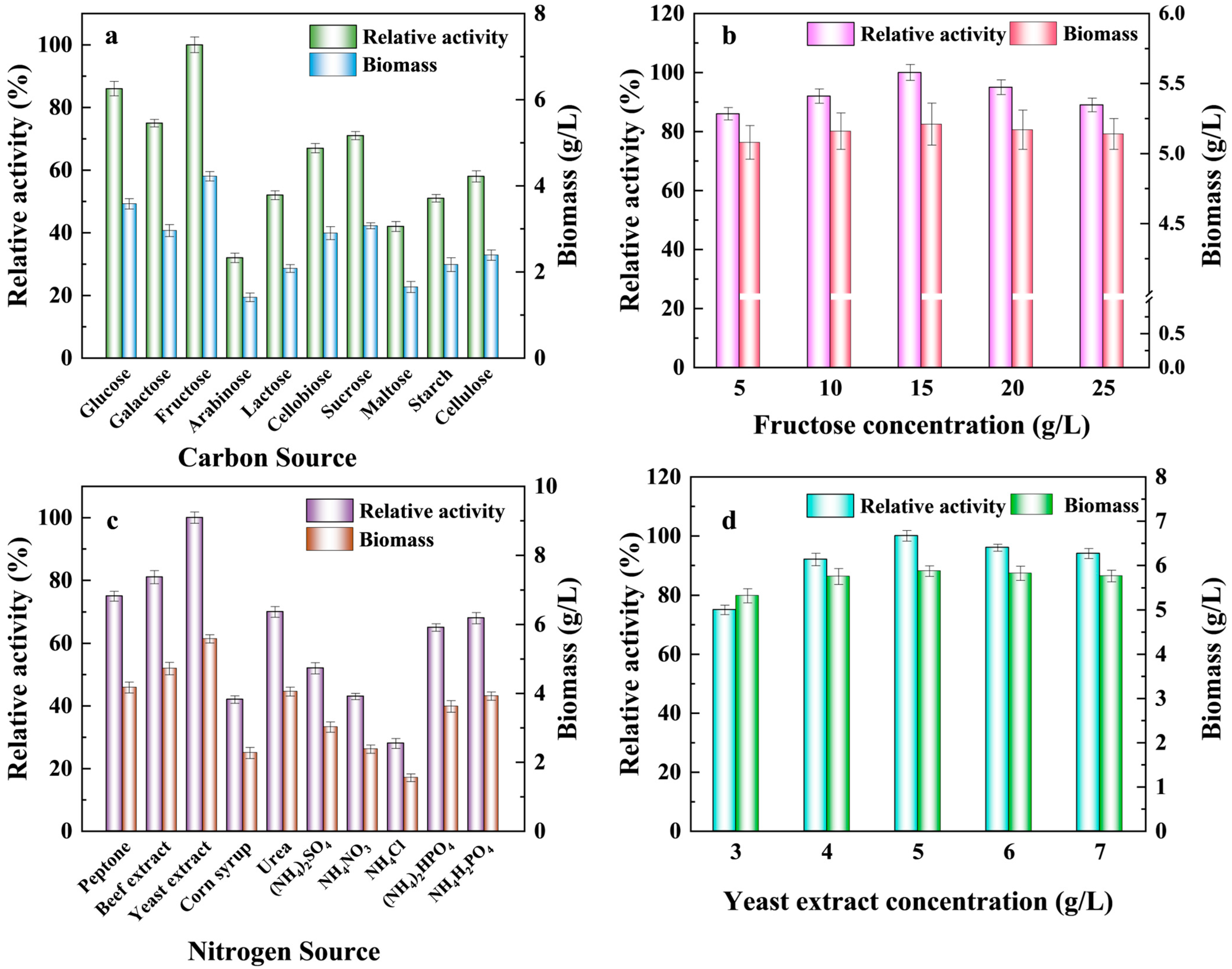
3.1. Optimization of Carbon and Nitrogen Sources in Culture Medium
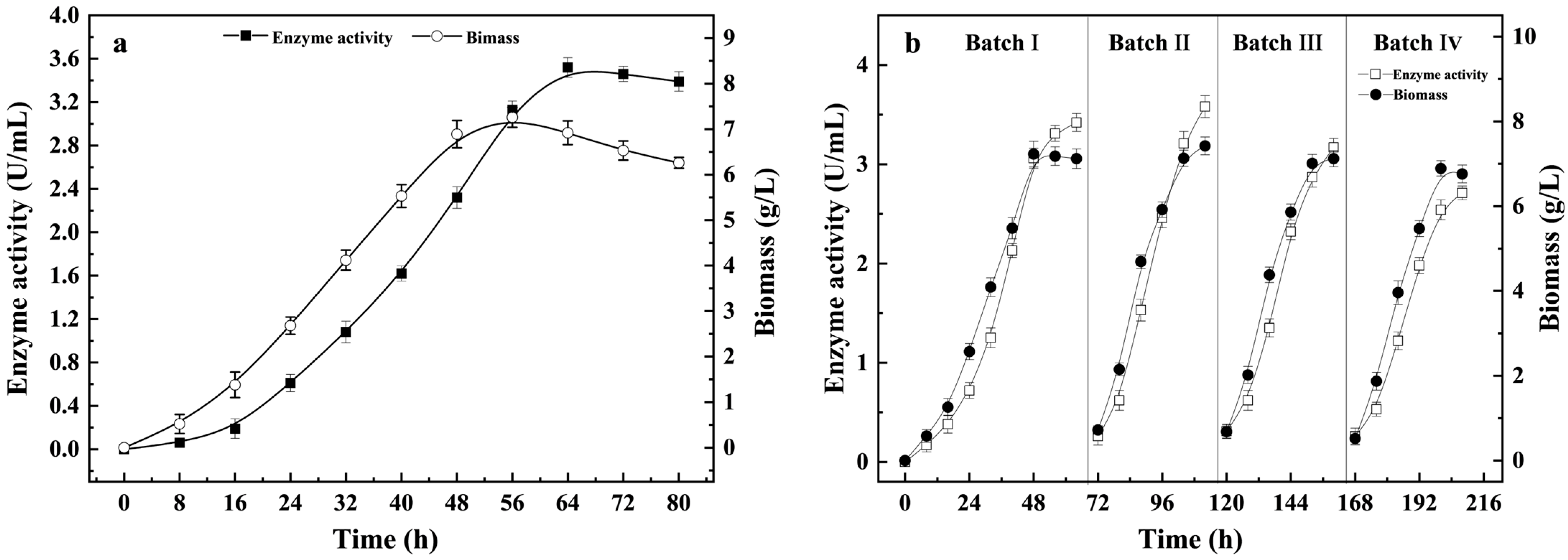
3.2. Production of Chitin Enzymes by Batch and Repeat Batch Processes
3.3. Optimization of Operating Conditions for HLP Adsorption of Chitin Enzyme
3.4. Optimization of Ultrafiltration Conditions for CHOS Separation
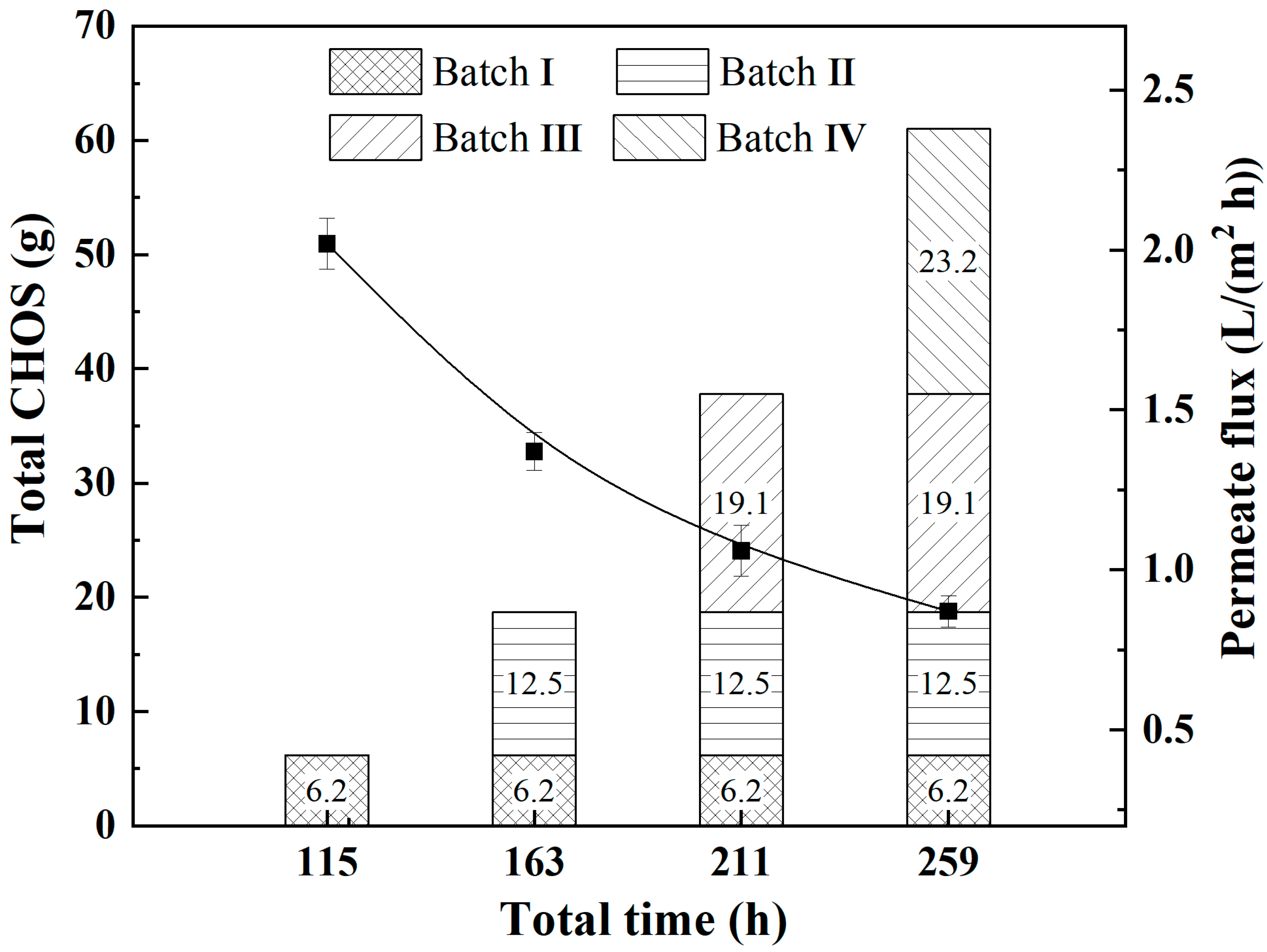
3.5. EAES System Continuously Produces CHOS
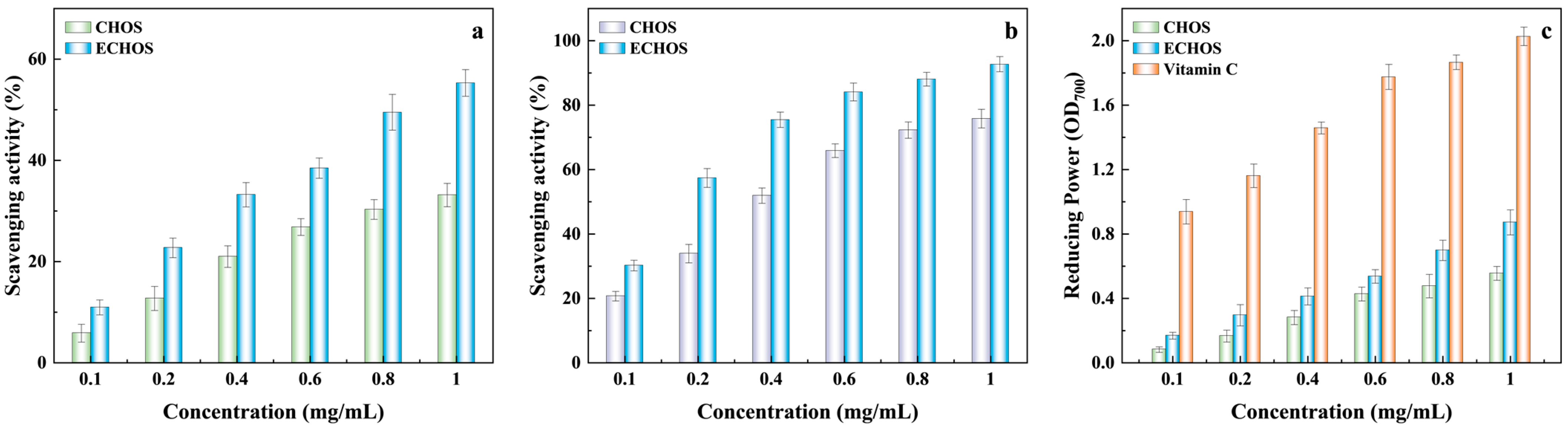
3.6. Antioxidant Activity of Enzymolysis Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.-W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ye, X.; Zhong, H.; Wang, T.; Jin, F. Sustainable Nitrogen-Containing Chemicals and Materials from Natural Marine Resources Chitin and Microalgae. Mol. Catal. 2021, 505, 111517. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Rovaletti, A.; De Gioia, L.; Fantucci, P.; Greco, C.; Vertemara, J.; Zampella, G.; Arrigoni, F.; Bertini, L. Recent Theoretical Insights into the Oxidative Degradation of Biopolymers and Plastics by Metalloenzymes. Int. J. Mol. Sci. 2023, 24, 6368. [Google Scholar] [CrossRef]
- Pan, D.; Xiao, P.; Li, F.; Liu, J.; Zhang, T.; Zhou, X.; Zhang, Y. High Degree of Polymerization of Chitin Oligosaccharides Produced from Shrimp Shell Waste by Enrichment Microbiota Using Two-Stage Temperature-Controlled Technique of Inducing Enzyme Production and Metagenomic Analysis of Microbiota Succession. Mar. Drugs 2024, 22, 346. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Q.; Yi, Z.; Zhang, K.; Shi, J.; Zhu, L.; Chen, Y.; Jin, J.; Zhao, L. Chitin Oligosaccharides for the Food Industry: Production and Applications. Syst. Microbiol. Biomanuf. 2022, 3, 49–74. [Google Scholar] [CrossRef]
- Ngo, D.-N.; Kim, M.-M.; Kim, S.-K. Chitin Oligosaccharides Inhibit Oxidative Stress in Live Cells. Carbohydr. Polym. 2008, 74, 228–234. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, Antioxidant, and Antimicrobial Properties of Chitooligosaccharides Produced Using Three Different Enzyme Treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Rahman, H.; Shovan, L.R.; Hjeljord, L.G.; Aam, B.B.; Eijsink, V.G.H.; Sørlie, M.; Tronsmo, A. Inhibition of Fungal Plant Pathogens by Synergistic Action of Chito-Oligosaccharides and Commercially Available Fungicides. PLoS ONE 2014, 9, e93192. [Google Scholar] [CrossRef]
- KIM, S.; RAJAPAKSE, N. Enzymatic Production and Biological Activities of Chitosan Oligosaccharides (COS): A Review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural Polysaccharides Exhibit Anti-Tumor Activity by Targeting Gut Microbiota. Int. J. Biol. Macromol. 2018, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Tian, G.; Chen, D.W.; Yao, Y.; He, J.; Zheng, P.; Mao, X.B.; Yu, J.; Huang, Z.Q.; Yu, B. Involvement of PKA Signalling in Anti-inflammatory Effects of Chitosan Oligosaccharides in IPEC-J2 Porcine Epithelial Cells. J. Anim. Physiol. Anim. Nutr. 2017, 102, 252–259. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-Inflammatory Effects of Low-Molecular Weight Chitosan Oligosaccharides in IgE-Antigen Complex-Stimulated RBL-2H3 Cells and Asthma Model Mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef]
- Li, Q.; Shi, W.-R.; Huang, Y.-L. Comparison of the Protective Effects of Chitosan Oligosaccharides and Chitin Oligosaccharide on Apoptosis, Inflammation and Oxidative Stress. Exp. Ther. Med. 2024, 28, 310. [Google Scholar] [CrossRef]
- Shang, W.; Si, X.; Zhou, Z.; Wang, J.; Strappe, P.; Blanchard, C. Studies on the Unique Properties of Resistant Starch and Chito-Oligosaccharide Complexes for Reducing High-Fat Diet-Induced Obesity and Dyslipidemia in Rats. J. Funct. Foods 2017, 38, 20–27. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-Obese Effect of Glucosamine and Chitosan Oligosaccharide in High-Fat Diet-Induced Obese Rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Pangestuti, R.; Bak, S.-S.; Kim, S.-K. Attenuation of Pro-Inflammatory Mediators in LPS-Stimulated BV2 Microglia by Chitooligosaccharides via the MAPK Signaling Pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef]
- Eom, T.-K.; Ryu, B.; Lee, J.-K.; Byun, H.-G.; Park, S.-J.; Kim, S.-K. β-Secretase Inhibitory Activity of Phenolic Acid Conjugated Chitooligosaccharides. J. Enzym. Inhib. Med. Chem. 2012, 28, 214–217. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Karadeniz, F.; Kim, S.-K. Anti-HIV Activities of Novel Synthetic Peptide Conjugated Chitosan Oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Wang, Z.; Wang, H.; Chen, L.; Yang, L.; Qiao, R.; Hu, L.; Li, Z. Effect of Bond Linkage on in Vitro Drug Release and Anti-HIV Activity of Chitosan-Stavudine Conjugates. Macromol. Res. 2012, 20, 358–365. [Google Scholar] [CrossRef]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Fluoroalkenyl-Grafted Chitosan Oligosaccharide Derivative: An Exploration for Control Nematode Meloidogyne incognita. Int. J. Mol. Sci. 2022, 23, 2080. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, R.; Wang, X.; Li, P. The Bioactivity of New Chitin Oligosaccharide Dithiocarbamate Derivatives Evaluated against Nematode Disease (Meloidogyne incognita). Carbohydr. Polym. 2019, 224, 115155. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, L.; Qin, Y.; Li, P. Activity of Chitin/Chitosan/Chitosan Oligosaccharide against Plant Pathogenic Nematodes and Potential Modes of Application in Agriculture: A Review. Carbohydr. Polym. 2023, 306, 120592. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and Biological Activities of Chitosan Oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Li, B.; Cui, J.; Xu, T.; Xu, Y.; Long, M.; Li, J.; Liu, M.; Yang, T.; Du, Y.; Xu, Q. Advances in the Preparation, Characterization, and Biological Functions of Chitosan Oligosaccharide Derivatives: A Review. Carbohydr. Polym. 2024, 332, 121914. [Google Scholar] [CrossRef]
- Shi, J.; Deng, C.; Zhang, C.; Quan, S.; Fan, L.; Zhao, L. Combinatorial Metabolic Engineering of Escherichia coli for de Novo Production of Structurally Defined and Homogeneous Amino Oligosaccharides. Synth. Syst. Biotechnol. 2024, 9, 713–722. [Google Scholar] [CrossRef]
- Yamabhai, M.; Khamphio, M.; Min, T.T.; Soem, C.N.; Cuong, N.C.; Aprilia, W.R.; Luesukprasert, K.; Teeranitayatarn, K.; Maneedaeng, A.; Tuveng, T.R.; et al. Valorization of Shrimp Processing Waste-Derived Chitosan into Anti-Inflammatory Chitosan-Oligosaccharides (CHOS). Carbohydr. Polym. 2024, 324, 121546. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.U.G.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; Messaoudi, N.E.; et al. Eco-Friendly and Safe Alternatives for the Valorization of Shrimp Farming Waste. Environ. Sci. Pollut. Res. 2023, 31, 38960–38989. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Bioconversion of Chitin to Bioactive Chitooligosaccharides: Amelioration and Coastal Pollution Reduction by Microbial Resources. Mar. Biotechnol. 2018, 20, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Balaraman, D.; Panangal, M.; Nageswara Rao, T.; Perumal, E.; R, A.; Kumarappan, A.; Sampath Renuga, P.; Arumugam, S.; Thirunavukkarasu, R.; et al. Bioconversion of Chitin Waste through Stenotrophomonas maltophilia for Production of Chitin Derivatives as a Seabass Enrichment Diet. Sci. Rep. 2022, 12, 4792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Z.; Wang, C.; Jiang, Z. N-Acetyl-d-Glucosamine-Based Oligosaccharides from Chitin: Enzymatic Production, Characterization and Biological Activities. Carbohydr. Polym. 2023, 315, 121019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Madhuprakash, J.; Balan, V.; Kumar Singh, A.; Vivekanand, V.; Pareek, N. Chemoenzymatic Production of Chitooligosaccharides Employing Ionic Liquids and Thermomyces lanuginosus Chitinase. Bioresour. Technol. 2021, 337, 125399. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, B.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Advances in the Preparation and Assessment of the Biological Activities of Chitosan Oligosaccharides with Different Characteristics. Food Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Tan, H.; Chi, N.; Zhang, Q.; Du, Y.; Yin, H. Characterisation of a Chitinase from Pseudoalteromonas Sp. DL-6, a Marine Psychrophilic Bacterium. Int. J. Biol. Macromol. 2014, 70, 455–462. [Google Scholar] [CrossRef]
- Zhang, A.; Mo, X.; Wei, G.; Zhou, N.; Yang, S.; Chen, J.; Wang, Y.; Chen, K.; Ouyang, P. The Draft Genome Sequence and Analysis of an Efficiently Chitinolytic Bacterium Chitinibacter Sp. Strain GC72. Curr. Microbiol. 2020, 77, 3903–3908. [Google Scholar] [CrossRef]
- Lv, C.; Gu, T.; Xu, K.; Gu, J.; Li, L.; Liu, X.; Zhang, A.; Gao, S.; Li, W.; Zhao, G. Biochemical Characterization of a β-N-Acetylhexosaminidase from Streptomyces alfalfae and Its Application in the Production of N-Acetyl-d-Glucosamine. J. Biosci. Bioeng. 2019, 128, 135–141. [Google Scholar] [CrossRef]
- Mukherjee, S.; Behera, P.K.; Madhuprakash, J. Efficient Conversion of Crystalline Chitin to N-Acetylglucosamine and N,N’-Diacetylchitobiose by the Enzyme Cocktail Produced by Paenibacillus Sp. LS1. Carbohydr. Polym. 2020, 250, 116889. [Google Scholar] [CrossRef]
- Ren, X.-B.; Dang, Y.-R.; Liu, S.-S.; Huang, K.-X.; Qin, Q.-L.; Chen, X.-L.; Zhang, Y.-Z.; Wang, Y.-J.; Li, P.-Y. Identification and Characterization of Three Chitinases with Potential in Direct Conversion of Crystalline Chitin into N,N′-Diacetylchitobiose. Mar. Drug 2022, 20, 165. [Google Scholar] [CrossRef]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef] [PubMed]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2020, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Son, Y.-J.; Hwang, I.-K.; Nho, C.W.; Kim, S.M.; Kim, S.H. Determination of Carbohydrate Composition in Mealworm (Tenebrio molitor L.) Larvae and Characterization of Mealworm Chitin and Chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of Insect Chitin Isolated from Beetle Larva Cuticle and Silkworm (Bombyx mori) Pupa Exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and Characterization of α-Chitin and Chitosan from Six Different Aquatic Invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2015, 48, 40–50. [Google Scholar] [CrossRef]
- Zhang, A.-J.; Qin, Q.-L.; Zhang, H.; Wang, H.-T.; Li, X.; Miao, L.; Wu, Y.-J. Preparation and Characterisation of Food Grade Chitosan from Housefly Larvae. Czech J. Food Sci. 2011, 29, 616–623. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, Antifungal and Antiviral Activities of Chitosan from the Larvae of Housefly, Musca domestica L. Food Chem. 2011, 132, 493–498. [Google Scholar] [CrossRef]
- Pădureţ, S.; Ghinea, C.; Prisacaru, A.E.; Leahu, A. Physicochemical, Textural, and Antioxidant Attributes of Yogurts Supplemented with Black Chokeberry: Fruit, Juice, and Pomace. Foods 2024, 13, 3231. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Ji, L.; Du, X.; Sang, Q.; Chen, F. Enzymatic Single-Step Preparation and Antioxidant Activity of Hetero-Chitooligosaccharides Using Non-Pretreated Housefly Larvae Powder. Carbohydr. Polym. 2017, 172, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sánchez, J.P.; Morales-Oyervides, L.; Giuffrida, D.; Dufossé, L.; Montañez, J.C. Production of Pigments under Submerged Culture through Repeated Batch Fermentation of Immobilized Talaromyces atroroseus GH2. Fermentation 2023, 9, 171. [Google Scholar] [CrossRef]
- Broos, W.; Wittner, N.; Geerts, J.; Dries, J.; Vlaeminck, S.E.; Gunde-Cimerman, N.; Richel, A.; Cornet, I. Evaluation of Lignocellulosic Wastewater Valorization with the Oleaginous Yeasts R. kratochvilovae EXF7516 and C. oleaginosum ATCC 20509. Fermentation 2022, 8, 204. [Google Scholar] [CrossRef]
- Kaur, S.; Guleria, P.; Yadav, S.K. Evaluation of Fermentative Xylitol Production Potential of Adapted Strains of Meyerozyma caribbica and Candida tropicalis from Rice Straw Hemicellulosic Hydrolysate. Fermentation 2023, 9, 181. [Google Scholar] [CrossRef]
- Thurn, A.-L.; Schobel, J.; Weuster-Botz, D. Photoautotrophic Production of Docosahexaenoic Acid- and Eicosapentaenoic Acid-Enriched Biomass by Co-Culturing Golden-Brown and Green Microalgae. Fermentation 2024, 10, 220. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Shen, Y.; Zhang, X.; Zan, Z.; Xia, M.; Luo, J.; Wang, M. Efficient Repeated Batch Production of Androstenedione Using Untreated Cane Molasses by Mycobacterium Neoaurum Driven by ATP Futile Cycle. Bioresour. Technol. 2020, 309, 123307. [Google Scholar] [CrossRef]
- Kidibule, P.E.; Costa, J.; Atrei, A.; Plou, F.J.; Fernandez-Lobato, M.; Pogni, R. Production and Characterization of Chitooligosaccharides by the Fungal Chitinase Chit42 Immobilized on Magnetic Nanoparticles and Chitosan Beads: Selectivity, Specificity and Improved Operational Utility. RSC Adv. 2021, 11, 5529–5536. [Google Scholar] [CrossRef]
- Mechri, S.; Jabeur, F.; Bessadok, B.; Moumnassi, S.; Idrissi Yahyaoui, M.; Mannani, N.; Asehraou, A.; Mensi, F.; Vita, S.; D’Amore, P.; et al. Production of a New Chitinase from Nocardiopsis halophila TN-X8 Utilizing Bio-Waste from the Blue Swimming Crab: Enzyme Characterization and Immobilization. Environ. Sci. Pollut. Res. Int. 2024, 31, 45217–45233. [Google Scholar] [CrossRef]
- Cheba, B.A.; Zaghloul, T.I.; EL-Mahdy, A.R.; EL-Massry, M.H. Affinity Purification and Immobilization of Chitinase from Bacillus Sp.R2. Procedia Technol. 2015, 19, 958–964. [Google Scholar] [CrossRef]
- Seo, D.-J.; Jang, Y.-H.; Park, R.-D.; Jung, W.-J. Immobilization of Chitinases from Streptomyces griseus and Paenibacillus illinoisensis on Chitosan Beads. Carbohydr. Polym. 2011, 88, 391–394. [Google Scholar] [CrossRef]
- Charoenpol, A.; Crespy, D.; Schulte, A.; Suginta, W. Immobilized Chitinase as Effective Biocatalytic Platform for Producing Bioactive Di-N-Acetyl Chitobiose from Recycled Chitin Food Waste. Bioresour. Technol. 2024, 406, 130945. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Mini, K.D.; Antony, A.C.; Mathew, J. Deproteinization of Shrimp Shell Waste by Kurthia gibsonii Mb126 Immobilized Chitinase. J. Pure Appl. Microbiol. 2022, 16, 909–923. [Google Scholar] [CrossRef]
- Pan, D.; Liu, J.; Xiao, P.; Xie, Y.; Zhou, X.; Zhang, Y. Research Progress of Lytic Chitin Monooxygenase and Its Utilization in Chitin Resource Fermentation Transformation. Fermentation 2023, 9, 754. [Google Scholar] [CrossRef]
- Kumar, M.; Chakdar, H.; Pandiyan, K.; Thapa, S.; Shahid, M.; Singh, A.; Srivastava, A.K.; Saxena, A.K. Bacterial Chitinases: Genetics, Engineering and Applications. World J. Microbiol. Biotechnol. 2022, 38, 252. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Zhuang, N.; Zhang, A.; Chen, K.; Xu, N.; Xin, F.; Zhang, W.; Dong, W.; Jiang, M. Immobilization and Purification of Enzymes With the Novel Affinity Tag ChBD-AB from Chitinolyticbacter meiyuanensis SYBC-H1. Front. Bioeng. Biotechnol. 2020, 8, 579. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Deng, C.; Ma, C.; Zhang, C.; Zhao, L. Bioconversion of Chitin into Chitin Oligosaccharides Using a Novel Chitinase with High Chitin-Binding Capacity. Int. J. Biol. Macromol. 2023, 244, 125241. [Google Scholar] [CrossRef]
- Gede Wenten, I.; Friatnasary, D.L.; Khoiruddin, K.; Setiadi, T.; Boopathy, R. Extractive Membrane Bioreactor (EMBR): Recent Advances and Applications. Bioresour. Technol. 2019, 297, 122424. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and Biological Activities of Chitosan from the Larvae of Housefly, Musca Domestica. Carbohydr. Polym. 2007, 72, 419–423. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The Synthesis and Antioxidant Activity of the Schiff Bases of Chitosan and Carboxymethyl Chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Preparation of Chitosan-Rosmarinic Acid Derivatives with Enhanced Antioxidant and Anti-Inflammatory Activities. Carbohydr. Polym. 2022, 296, 119943. [Google Scholar] [CrossRef]
- MIYASHITA, K.; FUJII, T.; SAITO, A. Induction and Repression of a Streptomyces lividans Chitinase Gene Promoter in Response to Various Carbon Sources. Biosci. Biotechnol. Biochem. 2000, 64, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Fujii, T.; Sawada, Y. Molecular Cloning and Characterization of Chitinase Genes from Streptomyces lividans 66. J. Gen. Microbiol. 1991, 137, 2065–2072. [Google Scholar] [CrossRef]
- Ni, X.; Westpheling, J. Direct Repeat Sequences in the Streptomyces chitinase-63 Promoter Direct Both Glucose Repression and Chitin Induction. Proc. Natl. Acad. Sci. USA 1997, 94, 13116–13121. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.E.; Gevers, D.; Vahora, N.M.; Polz, M.F. Conservation of the Chitin Utilization Pathway in the Vibrionaceae. Appl. Environ. Microbiol. 2007, 74, 44–51. [Google Scholar] [CrossRef]
- Zhang, A.; Mo, X.; Zhou, N.; Wang, Y.; Wei, G.; Hao, Z.; Chen, K. Identification of Chitinolytic Enzymes in Chitinolyticbacter meiyuanensis and Mechanism of Efficiently Hydrolyzing Chitin to N-Acetyl Glucosamine. Front. Microbiol. 2020, 11, 572053. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Succinic Acid Biosynthesis from Cane Molasses under Low pH by Actinobacillus succinogenes Immobilized in Luffa Sponge Matrices. Bioresour. Technol. 2018, 268, 45–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Xu, H.; Yao, Z.; Ouyang, P. ε-Poly-L-Lysine Production by Immobilized Cells of Kitasatospora Sp. MY 5-36 in Repeated Fed-Batch Cultures. Bioresour. Technol. 2010, 101, 5523–5527. [Google Scholar] [CrossRef]
- Colson, S.; van Wezel, G.P.; Craig, M.; Noens, E.E.E.; Nothaft, H.; Mommaas, A.M.; Titgemeyer, F.; Joris, B.; Rigali, S. The Chitobiose-Binding Protein, DasA, Acts as a Link between Chitin Utilization and Morphogenesis in Streptomyces coelicolor. Microbiology 2008, 154, 373–382. [Google Scholar] [CrossRef]
- López-García, C.L.; Guerra-Sánchez, G.; Santoyo-Tepole, F.; Olicón-Hernández, D.R. Chitinase Induction in Trichoderma harzianum: A Solid-State Fermentation Approach Using Shrimp Waste and Wheat Bran/Commercial Chitin for Chitooligosaccharides Synthesis. Prep. Biochem. Biotechnol. 2024, 54, 1040–1050. [Google Scholar] [CrossRef]
- Curcio, S.; De Luca, G.; Saha, K.; Chakraborty, S. Advance Membrane Separation Processes for Biorefineries. In Membrane Technologies for Biorefining; Woodhead Publishing: Sawston, UK, 2016; pp. 3–28. [Google Scholar]
- Garg, S.; Behera, S.; Ruiz, H.A.; Kumar, S. A Review on Opportunities and Limitations of Membrane Bioreactor Configuration in Biofuel Production. Appl. Biochem. Biotechnol. 2022, 195, 5497–5540. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, X.; Jiang, Y.; Yao, J.; Jiang, Y.; Li, Z.; Dai, F. Chemical Structure and Antioxidant Activity of a Neutral Polysaccharide from Asteris Radix et Rhizoma. Carbohydr. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wei, X.-Y.; Xie, Y.-Y.; Zhou, T. A Novel Chitosan Oligosaccharide Derivative: Synthesis, Antioxidant and Antibacterial Properties. Carbohydr. Polym. 2022, 291, 119608. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and Their Biological Activities: A Comprehensive Review. Carbohydr. Polym. 2017, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.-W.; Huang, C.-T.; Dzung, N.; Wang, S.-L. Squid Pen Chitin Chitooligomers as Food Colorants Absorbers. Mar. Drugs 2015, 13, 681–696. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Huang, Y.; Liu, Y.; Pan, D.; Zhang, Y. Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System. Fermentation 2024, 10, 634. https://doi.org/10.3390/fermentation10120634
Zhou X, Huang Y, Liu Y, Pan D, Zhang Y. Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System. Fermentation. 2024; 10(12):634. https://doi.org/10.3390/fermentation10120634
Chicago/Turabian StyleZhou, Xiuling, Yang Huang, Yuying Liu, Delong Pan, and Yang Zhang. 2024. "Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System" Fermentation 10, no. 12: 634. https://doi.org/10.3390/fermentation10120634
APA StyleZhou, X., Huang, Y., Liu, Y., Pan, D., & Zhang, Y. (2024). Continuous Production of Chitin Oligosaccharides Utilizing an Optimized Enzyme Production-Adsorption-Enzymolysis-Product Separation (EAES) System. Fermentation, 10(12), 634. https://doi.org/10.3390/fermentation10120634





