The Application of the Ultrasound Technique in the Production of Rosé and Red Wines
Abstract
1. Introduction
2. Material and Methods
2.1. Microvinifications
2.2. Analytical Determinations
2.2.1. Physico-Chemical Parameters
2.2.2. Phenolic Parameters
2.2.3. Volatile Compounds
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of US Treatment on the Polyphenolic Composition of Bobal Wines
3.2. Effect of US Treatment on the Aromatic Composition of Bobal Wines
3.3. Effect of US Application Time on the Polyphenolic Composition of Bobal Wines
3.4. Effect of the Time of Application of US Treatment on the Aromatic Composition of Bobal Wines
3.5. PCA Applied to the Ultrasound-Treated Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OIV. Treatment of Crushed Grapes with Ultrasounds to Promote the Extraction of Their Compounds. In Resolution OIV-OENO 616-2019; OIV: Paris, France, 2019. [Google Scholar]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Valinger, D.; Denoya, G.I.; Barba, F.J.; Ganić, K.K. Effects of High Power Ultrasound Treatments on the Phenolic, Chromatic and Aroma Composition of Young and Aged Red Wine. Ultrason. Sonochem. 2019, 59, 104725. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of Prefermentative Maceration on the Phenolic and Volatile Compounds in Monastrell Red Wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V.; Aleixandre-Tudó, J.L. Effect of the Prefermentative Addition of Copigments on the Polyphenolic Composition of Tempranillo Wines after Malolactic Fermentation. Eur. Food Res. Technol. 2009, 228, 501–510. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in Analytical and Volatile Compositions of Red Wines Induced by Pre-Fermentation Heat Treatment of Grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of Ultrasound-Assisted Extraction of Phenolic Compounds from Wheat Bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Ferraretto, P.; Cacciola, V.; Batllo, I.F.; Celotti, E. Ultrasounds Application in Winemaking: Grape Maceration and Yeast Lysis. Ital. J. Food Sci. 2013, 25, 160. [Google Scholar]
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Zheng, H.; Lin, J.; Nie, G.; Fan, X.; García-Martín, J.F. The State-of-the-Art Research of the Application of Ultrasound to Winemaking: A Critical Review. Ultrason. Sonochem. 2023, 95, 106384. [Google Scholar] [CrossRef]
- Cocito, C.; Gaetano, G.; Delfini, C. Rapid Extraction of Aroma Compounds in Must and Wine by Means of Ultrasound. Food Chem. 1995, 52, 311–320. [Google Scholar] [CrossRef]
- Bates, D.; Patist, A. Industrial Applications of High Power Ultrasonics in the Food, Beverage and Wine Industry. In Case Studies in Novel Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2010; pp. 119–138. [Google Scholar]
- Yap, A.; Jiranek, V.; Grbin, P.; Barnes, M.; Bates, D. The Application of High Power Ultrasonics to Enhance Winemaking Processes and Wine Quality. Wine Indust. J. 2007, 22, 44–48. [Google Scholar]
- Ćurko, N.; Kelsin, K.; Rezek Jambrak, A.; Tomasevic, M.; Gracin, L.; Poturica, V.; Ruzman, E.; Kovacevic Ganic, K. The Effect of High Power Ultrasound on Phenolic Composition, Chromatic Characteristics, and Aroma Compounds of Red Wines. Croat. J. Food Sci. Technol. 2017, 9, 136–144. [Google Scholar] [CrossRef]
- García Martín, J.F.; Sun, D.-W. Ultrasound and Electric Fields as Novel Techniques for Assisting the Wine Ageing Process: The State-of-the-Art Research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Ferraretto, P.; Celotti, E. Preliminary Study of the Effects of Ultrasound on Red Wine Polyphenols. CYTA-J. Food 2016, 14, 529–535. [Google Scholar] [CrossRef]
- Plaza, E.G.; Jurado, R.; Iniesta, J.A.; Bautista-Ortín, A.B. High Power Ultrasounds: A Powerful, Non-Thermal and Green Technique for Improving the Phenolic Extraction from Grapes to Must during Red Wine Vinification. BIO Web. Conf. 2019, 12, 02001. [Google Scholar] [CrossRef]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis. In Organisation Internationale de la Vigne et du Vin (OIV); OIV: Paris, France, 2003. [Google Scholar]
- Lane, J.H.; Eynon, L. Methods for Determination of Reducing and Non-Reducing Sugars. J. Sci. 1923, 42, 32–37. [Google Scholar]
- Glories, Y. La Couleur Des Vins Rouges. 2e Partie: Mesure, Origine et Interprétation. OENO ONE 1984, 18, 253. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Le Dosage Des Tannins Du Vin Rouge et La Détermination de Leur Structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Le Dosage Des Anthocyanes Dans Le Vin Rouge. Bull. de la Société Chim. 1965, 9, 119–142. [Google Scholar]
- Vivas, N.; Glories, Y.; Lagune, L.; Cédric, S.; Augustin, M. Estimation du degré de polymérisation des procyanidines du Raisin et du vin par la méthode au p-dimethylaminocinnamaldéhyde. OENO ONE 1994, 28, 319. [Google Scholar] [CrossRef]
- Boido, E.; Alcalde-Eon, C.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging Effect on the Pigment Composition and Color of Vitis Vinifera L. Cv. Tannat Wines. Contribution of the Main Pigment Families to Wine Color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast Analysis of Important Wine Volatile Compounds. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal Projections to Latent Structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Gambacorta, G.; Trani, A.; Punzi, R.; Fasciano, C.; Leo, R.; Fracchiolla, G.; Faccia, M. Impact of Ultrasounds on the Extraction of Polyphenols during Winemaking of Red Grapes Cultivars from Southern Italy. Innov. Food Sci. Emerg. Technol. 2017, 43, 54–59. [Google Scholar] [CrossRef]
- Lee, H.; Feng, H. Effect of Power Ultrasound on Food Quality. In Ultrasound Technologies for Food and Bioprocessing; Springer: Berlin/Heidelberg, Germany, 2011; pp. 559–582. [Google Scholar]
- Souquet, J.-M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric Proanthocyanidins from Grape Skins. Phytochemistry 1996, 43, 509–512. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of High-Power Ultrasounds during Red Wine Vinification. Int. J. Food Sci. Technol. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- del Llaudy, M.C.; Canals, R.; Canals, J.M.; Zamora, F. Influence of Ripening Stage and Maceration Length on the Contribution of Grape Skins, Seeds and Stems to Phenolic Composition and Astringency in Wine-Simulated Macerations. Eur. Food Res. Technol. 2008, 226, 337–344. [Google Scholar] [CrossRef]
- Yacco, R.S.; Watrelot, A.A.; Kennedy, J.A. Red Wine Tannin Structure–Activity Relationships during Fermentation and Maceration. J. Agric. Food Chem. 2016, 64, 860–869. [Google Scholar] [CrossRef]
- Abi-Habib, E.; Vernhet, A.; Roi, S.; Carrillo, S.; Veran, F.; Ducasse, M.; Poncet-Legrand, C. Diffusion of Phenolic Compounds during a Model Maceration in Winemaking: Role of Flesh and Seeds. J. Sci. Food Agric. 2023, 103, 2004–2013. [Google Scholar] [CrossRef]
- Roman, T.; Tonidandel, L.; Nicolini, G.; Bellantuono, E.; Barp, L.; Larcher, R.; Celotti, E. Evidence of the Possible Interaction between Ultrasound and Thiol Precursors. Foods 2020, 9, 104. [Google Scholar] [CrossRef]
- Pozzatti, M.; Guerra, C.C.; Martins, G.; dos Santos, I.D.; Wagner, R.; Ferrão, M.F.; Manfroi, V. Effects of Winemaking on ‘Marselan’ Red Wines: Volatile Compounds and Sensory Aspects. Ciência Téc. Vitiv. 2020, 35, 63–75. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-Assisted Enzymatic Extraction and Identification of Anthocyanin Components from Mulberry Wine Residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef] [PubMed]
- Oliver Simancas, R.; Díaz-Maroto, M.C.; Alañón Pardo, M.E.; Pérez Porras, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Pérez-Coello, M.S. Effect of Power Ultrasound Treatment on Free and Glycosidically Bound Volatile Compounds and the Sensorial Profile of Red Wines. Molecules 2021, 26, 1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, H.; Cheng, S.; Xu, B.; Guo, P. Comparison of Ultrasound Type and Working Parameters on the Reduction of Four Higher Alcohols and the Main Phenolic Compounds. Sustainability 2021, 14, 417. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of Ultrasound in Analysis, Processing and Quality Control of Food: A Review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Labrador Fernández, L.; Díaz-Maroto, M.C.; Pérez Porras, P.; Bautista-Ortín, A.B.; Alañón, M.E.; Gómez-Plaza, E.; Pérez-Coello, M.S. Power Ultrasound Treatment of Viognier Grapes as a Tool to Increase the Aromatic Potential of Wines. J. Sci. Food Agric. 2023, 103, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, A.; Carrera, C.; Palma Lovillo, M.; García Barroso, C. Ultrasonic Treatments during the Alcoholic Fermentation of Red Wines: Effects on “Syrah” Wines. Vitis 2019, 58, 83–88. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Jurado, R.; Gómez-Plaza, E. A New Approach to the Reduction of Alcohol Content in Red Wines: The Use of High-Power Ultrasounds. Foods 2020, 9, 726. [Google Scholar] [CrossRef]
- Celotti, E.; Stante, S.; Ferraretto, P.; Román, T.; Nicolini, G.; Natolino, A. High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices. Foods 2020, 9, 1344. [Google Scholar] [CrossRef]
- Ferreira, V.; Escudero, A.; Campo, E.; CAcho, J.; Cacho, J. The Chemical Foundations of Wine Aroma: A role game aiming at wine quality, personality and varietal expresion. In Proceedings of the Thirteenth Australian Wine Industry Technical Conference, Adelaide, South Australia: Australian Wine Industry Technical Conference, Adelaide, SA, Australia, 29 July–2 August 2008; pp. 142–151. [Google Scholar]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile Components of Zalema White Wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]

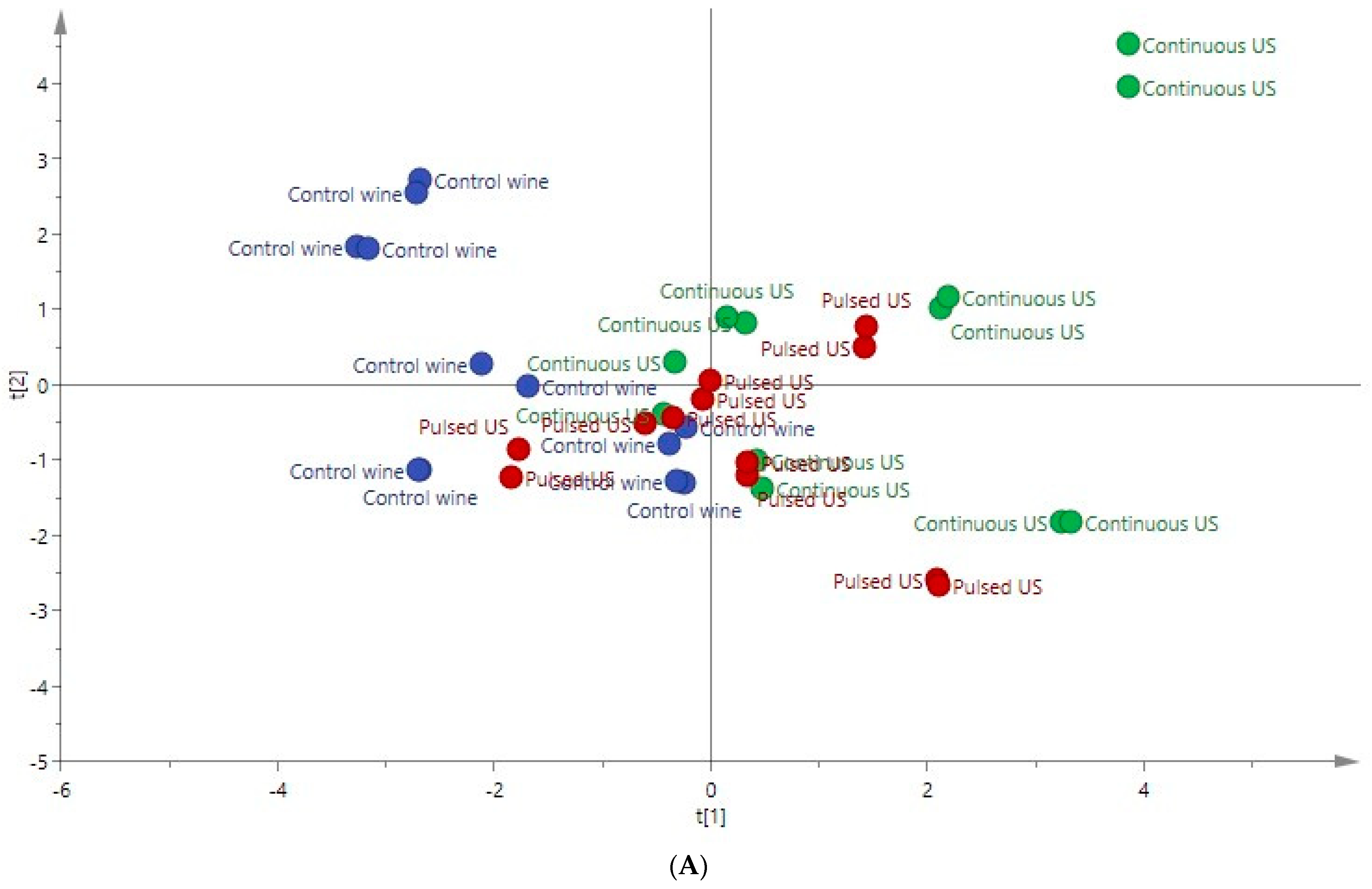
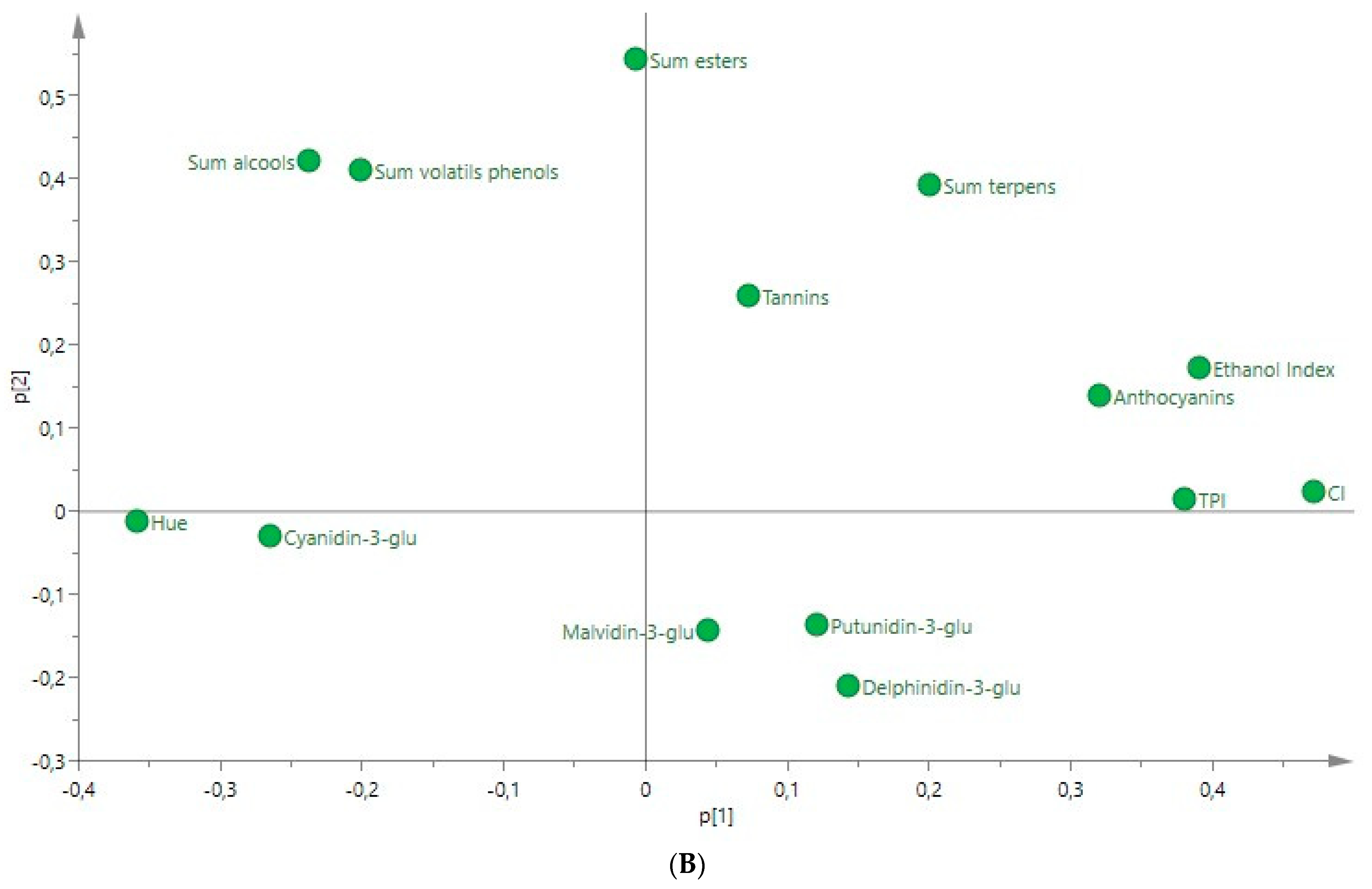
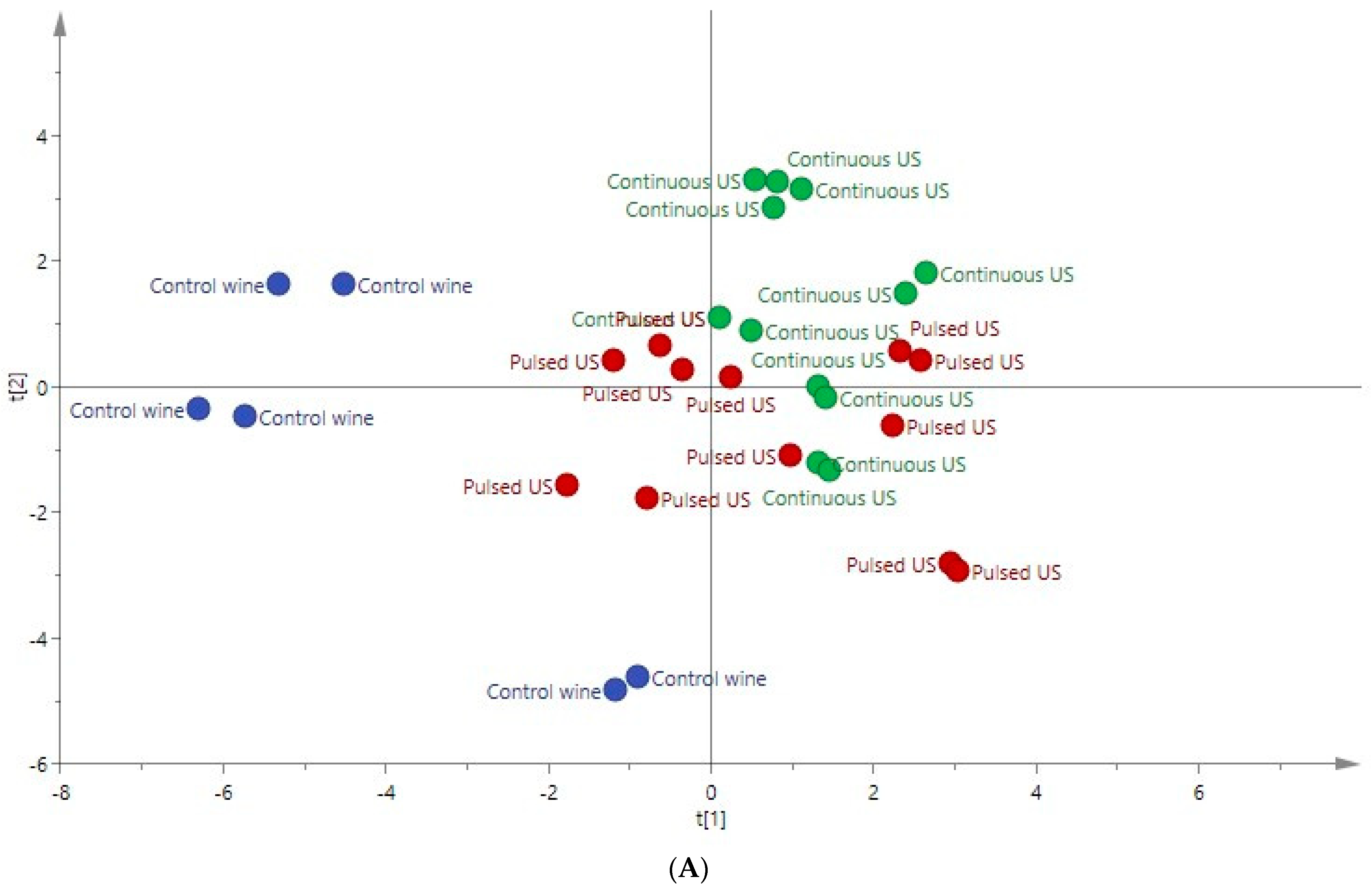
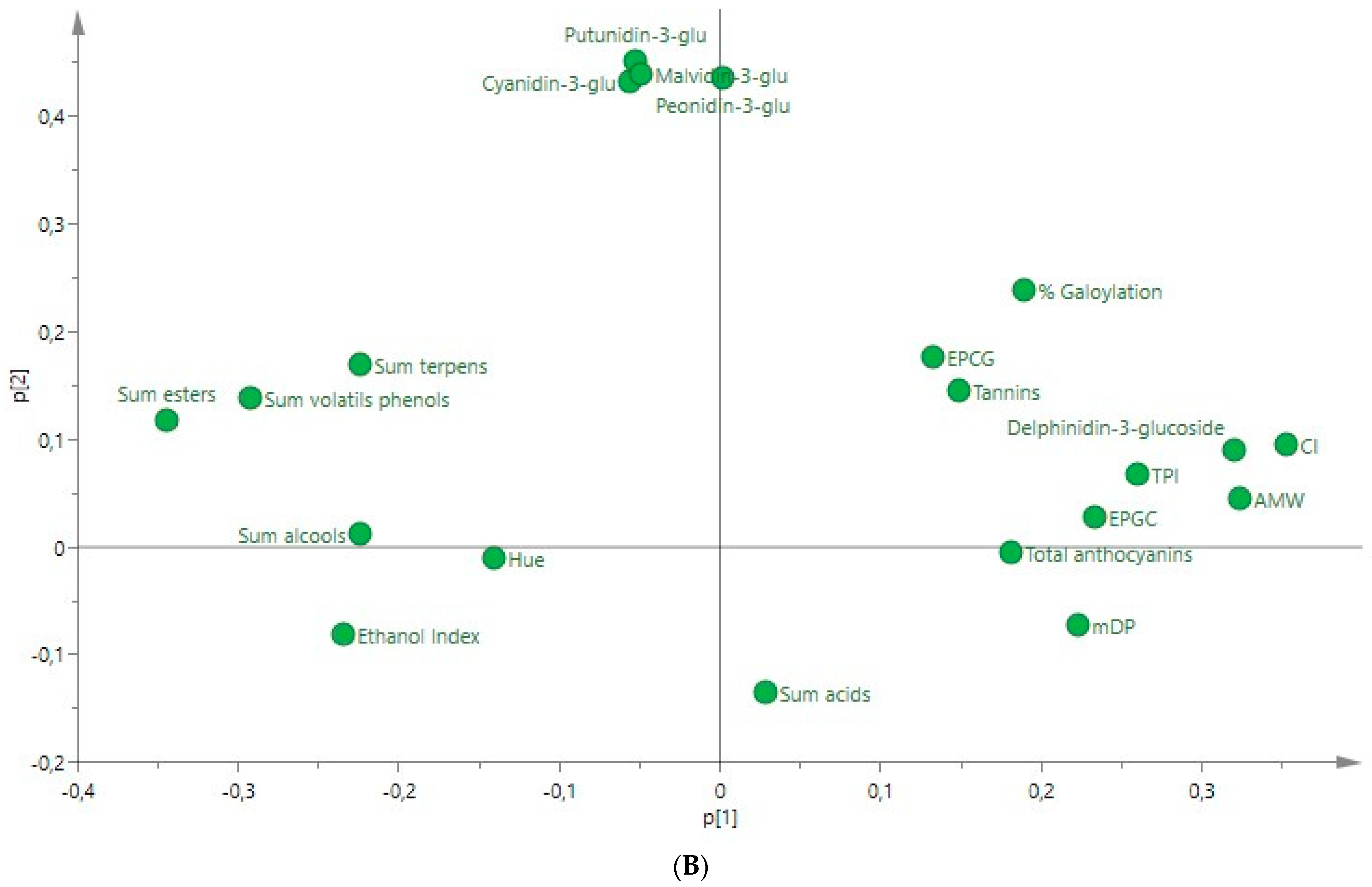
| Compounds | Rosé Wine | ANOVA F Ratio | Red Wine | ANOVA F Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Control Wine | Continuous | Pulsed | Treatment | Control Wine | Continuous | Pulsed | Treatment | |
| Color Density | 2.08 ± 0.2 a | 2.99 ± 0.41 c | 2.58 ± 0.3 b | 26.50 *** | 8.77 ± 0.3 a | 10.68 ± 0.3 b | 10.74 ± 0.6 b | 38.56 *** |
| Hue | 88.63 ± 4.6 a | 85.11 ± 3.3 a | 87.94 ± 5.1 a | 2.13 ns | 68.02 ± 2.9 a | 67.6 ± 1.3 a | 68.15 ± 2.5 a | 0.17 ns |
| Total Anthocyanes (mg/L) | 27.91 ± 2.9 a | 44.96 ± 10.1 b | 40.44 ± 7.8 b | 16.47 *** | 368.2 ± 22 a | 392.6 ± 26 ab | 400.46 ± 32 b | 12.58 ** |
| Delphinidin-3-glucoside | 0.28 ± 0.1 a | 0.31 ± 0.04 a | 0.36 ± 0.16 a | 1.68 ns | 14.09 ± 3.9 ab | 15.46 ± 2.5 b | 12.55 ± 1.7 a | 3.79 ** |
| Cyanidin-3-glucoside | 0.16 ± 0.1 a | 0.02 ± 0.01 a | 0.15 ± 0.07 a | 2.36 ns | 2.29 ± 1.5 a | 5.53 ± 0.4 b | 5.31 ± 0.7 b | 34.38 *** |
| Putunidin-3-glucoside | 0.45 ± 0.28 a | 0.50 ± 0.08 a | 0.61 ± 0.1 a | 0.95 ns | 23.80 ± 6.2 a | 26.46 ± 3.1 a | 22.65 ± 3.2 a | 2.58 ns |
| Peonidin-3-glucoside | n.d. | n.d. | n.d. | 9.91 ± 2.6 a | 12.00 ± 2.6 a | 10.21 ± 1.2 a | 2.95 ns | |
| Malvidin-3-glucoside | 4.03 ± 1.6 a | 5.24 ± 2.12 a | 4.02 ± 0.73 a | 2.27 ns | 98.3 ± 20.6 a | 110.3 ± 10.5 a | 102.6 ± 14.0 a | 1.65 ns |
| Condensed Tannins (g/L) | 0.40 ± 0.1 a | 0.41 ± 0.1 a | 0.43 ± 0.1 a | 0.45 ns | 1.14 ± 0.1 a | 1.36 ± 0.1 b | 1.26 ± 0.1 ab | 6.54 *** |
| P.T.I. | 9.61 ± 1.3 a | 11.09 ± 1.5 b | 10.39 ± 0.9 ab | 3.99 ** | 27.9 ± 2.2 a | 42.4 ± 1.1 b | 41.4 ± 3.4 b | 14.70 *** |
| Ethanol Index | 24.9 ± 1.8 a | 29.9 ± 6.9 a | 27.9 ± 4.6 a | 2.11 ns | 33.8 ± 1.0 ab | 36.3 ± 3.9 b | 30.6 ± 2.1 a | 3.49 *** |
| mDP | 5.44 ± 0.81 a | 5.74 ± 0.37 a | 5.58 ± 0.76 a | 0.46 ns | ||||
| % Galloylation | 3.57 ± 0.4 a | 4.42 ± 0.4 c | 3.98 ±0.4 b | 9.16 *** | ||||
| EGC (μM) | 35.41 ± 9.1 a | 52.33 ± 5.8 c | 44.6 ± 9.0 b | 9.64 *** | ||||
| EPCG (μM) | 35.7 ± 3.9 a | 43.8 ± 8.7 a | 40.2 ± 3.9 a | 0.01 ns | ||||
| AMW | 1988 ± 87 a | 2394 ± 141 b | 2317 ± 171 b | 15.89 *** | ||||
| Group | Aroma Compound | Rosé Wine | Red Wine | ||||
|---|---|---|---|---|---|---|---|
| Control Wine | Continuous | Pulsed | Control Wine | Continuous | Pulsed | ||
| Aldehydes | Diacetyl | 156.11 a | 104.7 a | 114.36 a | 204.54 b | 74.22 a | 72.59 a |
| Esters | Ethyl isobutyrate | 521.45 a | 677.71 a | 525.91 a | 596.92 b | 292.04 a | 319.62 a |
| Isoamyl acetate | 139.78 b | 68.16 a | 63.19 a | 94.61 a | 77.97 a | 126.70 a | |
| Ethyl hexanoate | 399.52 b | 299.46 a | 279.62 a | 462.00 b | 217.00 a | 263.00 a | |
| Hexyl acetate | 21.60 a | 34.87 a | 20.1 a | 15.00 a | 8.00 a | 24.00 a | |
| Ethyl lactate | 1014 b | 545.91 a | 412.04 a | 14.90 b | 7.89 a | 23.76 ab | |
| Ethyl-3- hydroxybutyrate | 382.81 a | 354.82 a | 334 a | 416.00 a | 339.00 a | 336.00 a | |
| Ethyl decanoate | 352.59 b | 313.16 ab | 266.18 a | 346.12 a | 213.79 a | 255.04 a | |
| Diethyl succinate | 382.55 a | 335.29 a | 993.85 a | 569.25 b | 388.53 a | 429.55 ab | |
| Ethyl laurate | 141.19 b | 142.2 b | 89.77 a | 147.44 b | 47.89 a | 104.31 ab | |
| Sum esters | 3355.49 | 2771.58 | 2984.66 | 2662.24 | 1592.11 | 1881.98 | |
| Alcohols | 1-2 propylene glycol | 76.96 a | 65.39 a | 57.51 a | 537.84 b | 243.67 a | 272.71 ab |
| Cis-3-hexenol | 667.53 b | 624.15 ab | 425 a | 54.40 b | 28.45 a | 42.82 a | |
| 2-Phenylethanol | 17,802.5 c | 17,376.67 b | 16,995 a | 19,677.17 c | 19,294 b | 18,694.5 a | |
| Isoamyl alcohol | 25,904.63 b | 16,720.61 a | 17,488.1 a | 14,861.97 b | 10,038.98 a | 11,944.21 ab | |
| Sum alcohols | 44,451.62 | 34,786.82 | 34,965.61 | 35,131.38 | 29,605.1 | 30,954.24 | |
| Terpens | α-pinen | 101.51 a | 202.04 a | 204.36 a | 54.09 ab | 41.35 a | 69.48 b |
| Linalol | 34.68 a | 68.67 b | 58.66 ab | 66.47 b | 41.76 a | 34.62 a | |
| Sum terpens | 136.19 | 270.71 | 263.02 | 120.56 | 83.11 | 104.1 | |
| Volatils phenols | 4-vinylphenol | 172.97 a | 156.68 a | 135.16 a | 101.67 b | 62.23 a | 72.95 ab |
| 2-metoxyphenol (guaiacol) | 769.81 b | 619.18 ab | 403.93 a | 351.38 a | 234.17 a | 286.37 a | |
| 4-ethylguaiacol | 161.27 b | 130.1 ab | 106.77 a | 98.34 a | 66.43 a | 66.87 a | |
| Eugenol | 239.99 a | 255.22 a | 98.06 a | 142.06 b | 106.29 ab | 88.45 a | |
| Sum v. phenols | 1344.04 | 1161.18 | 743.92 | 693.45 | 469.12 | 514.64 | |
| Acids | Decanoic acid | 274.05 a | 253.69 a | 172.76 a | 199.48 a | 165.81 a | 170.95 a |
| Octanoic acid | 805 a | 935.83 b | 903.33 b | 892.83 a | 1029.42 b | 993.67 b | |
| Hexanoic acid | 469.17 a | 713.83 c | 658.92 b | 498.67 a | 785.22 b | 724.81 c | |
| Butyric acid | 296.08 a | 519.17 c | 458.25 b | 317.53 a | 571.08 b | 504.08 c | |
| Isopentanoic acid | 234.37 a | 352.25 b | 339.67 b | 246.84 a | 387.48 b | 373.63 b | |
| Sum acids | 2078.67 | 2774.77 | 2532.93 | 2155.35 | 2939.01 | 2767.14 | |
| Others | Theaspirane | 237.39 b | 216.31 b | 141.25 a | 218.44 a | 173.14 a | 150.58 a |
| Naftalene | 181.48 ab | 145.63 a | 259.79 b | 207.11 a | 256.97 a | 203.35 a | |
| Compounds | Ultrasound Rosé Wines | ANOVA F ratio | |||||
|---|---|---|---|---|---|---|---|
| Continuous | Pulsed | Tr (Treatment) | T | Tr*T | |||
| 10 min | 20 min | 10 min | 20 min | (Time) | (Interaction) | ||
| Color Density | 2.60 ± 0.1 a | 3.38 ± 0.1 b | 2.44 ± 0.1 a | 2.73 ± 0.3 a | 32.97 ** | 57.67 ** | 12.69 ** |
| Hue | 86.87 ± 2.6 a | 83.35 ± 2.6 a | 88.58 ± 1.7 a | 87.30 ± 7.3 a | 2.60 ns | 1.86 ns | 0.41 ns |
| Total Anthocyanes (mg/L) | 38.10 ± 5.1 a | 51.81 ± 9.2 b | 37.0 ± 4.8 a | 43.90 ± 9.0 a | 2.28 ns | 11.92** | 1.29 ns |
| Delphinidin-3-glucoside | 0.32 ± 3.2 a | 0.29 ± 0.0 a | 0.34 ± 0.1 a | 0.39 ± 0.2 a | 1.38 ns | 0.00 ns | 0.73 ns |
| Cyanidin-3-glucoside | 0.04 ± 0.02 a | n.d. a | 0.08 ± 0.05 a | 0.22 ± 0.2 a | 5.89 ** | 0.76 ns | 2.68 ns |
| Putunidin-3-glucoside | 0.53 ± 0.1 a | 0.46 ± 0.0 a | 0.70 ± 0.25 a | 0.53 ± 0.3 a | 0.94 ns | 1.05 ns | 0.15 ns |
| Peonidin-3-glucoside | n.d. | n.d. | n.d. | n.d. | |||
| Malvidin-3-glucoside | 5.67 ± 1.7 a | 4.82 ± 2.5 a | 4.46 ± 0.8 a | 3.58 ± 0.27 a | 3.54 ns | 1.78 ns | 0.00 ns |
| Condensed Tannins (g/L) | 0.41 ± 0.1 a | 0.42 ± 0.1 a | 0.42 ± 0.1 a | 0.43 ± 0.1 a | 0.24 ns | 0.14 ns | 0.04 ns |
| P.T.I. | 10.12 ± 0.1 a | 12.06 ± 1.1 b | 10.42 ± 0.08 a | 10.34 ± 1.1 a | 12.53 ** | 34.34 ** | 5.43 ** |
| Ethanol Index | 26.96 ± 1.5 a | 29.89 ± 1.6 b | 26.04 ± 1.0 a | 29.74 ± 6.2 a | 3.60 ns | 3.43 ns | 2.52 ns |
| Compounds | Ultrasound Red Wines | ANOVA F Ratio | |||||
|---|---|---|---|---|---|---|---|
| Continuous | Pulsed | Tr (Treatment) | T | Tr*T | |||
| 10 min | 20 min | 10 min | 20 min | (Time) | (Interaction) | ||
| Color Density | 10.71 ± 0.2 a | 10.65 ± 0.4 a | 10.78 ± 0.6 a | 10.71 ± 0.7 a | 0.07 ns | 0.08 ns | 0.00 ns |
| Hue | 68.81 ± 0.6 a | 66.47 ± 0.5 a | 68.43 ± 2.5 a | 67.87 ± 2.9 a | 0.43 ns | 3.48 ns | 1.30 ns |
| Total Anthocyanes (mg/L) | 396.8 ± 33.1 a | 388.6 ± 20 a | 405.6 ± 29 a | 395.1 ± 38 a | 0.37 ns | 0.56 ns | 0.01 ns |
| Delphinidin-3-glucoside | 16.55 ± 3.2 a | 14.36 ± 0.9 aB | 11.63 ± 1.8 a | 13.7 ± 0.6 bA | 14.27 ** | 0.02 ns | 8.80 ** |
| Cyanidin-3-glucoside | 5.62 ± 0.5 a | 5.44 ± 0.2 a | 5.24 ± 0.9 a | 5.39 ± 0.7 a | 0.75 ns | 0.00 ns | 0.41 ns |
| Putunidin-3-glucoside | 27.85 ± 4.1 a | 25.08 ± 0.9 aB | 20.54 ± 3.3 a | 24.7 ± 1.0 bA | 12.10 ** | 0.44 ns | 10.19 ** |
| Peonidin-3-glucoside | 13.09 ± 3.48 a | 10.90 ± 0.4 aB | 9.05 ± 1.3 a | 11.3 ± 0.7 bA | 5.34 ** | 0.01 ns | 8.53 ** |
| Malvidin-3-glucoside | 114.8 ± 13.5 a | 105.8 ± 3.6 a | 93.8 ± 13.6 a | 111.5 ± 7.6 b | 3.21 ns | 1.05 ns | 9.81 ** |
| Condensed Tannins (g/L) | 1.26 ± 0.1 a | 1.45 ± 0.1 bB | 1.23 ± 0.1 a | 1.28 ± 0.1 aA | 6.43 ** | 9.20 ** | 3.09 ns |
| P.T.I. | 42.1 ± 0.52 a | 42.7 ± 1.4 a | 40.2 ± 4.7 a | 42.61 ± 0.7 a | 0.95 ns | 2.19 ns | 0.80 ns |
| Ethanol Index | 35.4 ± 5.6 a | 37.3 ± 0.7 aB | 29.8 ± 2.8 a | 31.4 ± 0.4 aA | 20.14 *** | 1.88 ns | 0.03 ns |
| mDP | 5.91 ± 0.22 a | 5.56 ± 0.42 a | 5.5 ± 1.1 a | 5.6 ± 0.2 a | 0.41 ns | 0.16 ns | 1.03 ns |
| % Galoylation | 4.30 ± 0.3 a | 4.54 ± 0.5 aB | 3.87 ±0.6 a | 4.09 ± 0.2 aA | 6.86 ** | 1.86 ns | 0.01 ns |
| EGC (μM) | 52.41 ± 6.1 a | 52.2 ± 6.0 aB | 44.8 ± 13.3 a | 44.4 ± 0.8 aA | 5.87 ** | 0.00 ns | 0.00 ns |
| EPCG (μM) | 39.0 ± 5.0 a | 48.6 ± 9.4 a | 38.2 ± 4.6 a | 42.2 ± 2.0 a | 0.54 ns | 0.68 ns | 0.51 ns |
| AMW | 2379 ± 94 a | 2410 ± 186 a | 2367 ± 217 a | 2268 ± 106 a | 1.39 ns | 0.27 ns | 1.00 ns |
| Group | Aroma Compound | Ultrasound Rosé Wines | ANOVA F Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Continuous | Pulsed | Tr | T | Tr*T | ||||
| 10 min | 20 min | 10 min | 20 min | |||||
| Aldehydes | Diacetyl | 134.89 a | 74.52 a | 159.95 a | 68.77 a | 0.06 ns | 3.69 ns | 0.15 ns |
| Esters | Ethyl isobutyrate | 673.11 a | 682.32 a | 489.09 a | 562.73 a | 3.54 ns | 0.26 ns | 0.16 ns |
| Isoamyl acetate | 68.97 a | 67.34 a | 73.86 b | 52.52 a | 0.56 ns | 2.80 ns | 2.04 ns | |
| Ethyl hexanoate | 318.13 a | 280.79 a | 306.64 a | 252.60 a | 0.33 ns | 172 ns | 0.06 ns | |
| Hexyl acetate | 38.16 a | 31.57 a | 29.68 a | 10.51 a | 2.83 ns | 2.17 ns | 0.51 ns | |
| Ethyl lactate | 531.27 a | 560.54 a | 584.66 a | 239.42 a | 0.85 ns | 1.18 ns | 1.66 ns | |
| Ethyl 3 hydroxybutyrate | 361.22 a | 348.42 a | 365.56 a | 302.43 a | 0.20 ns | 0,69 ns | 0,30 ns | |
| Ethyl decanoate | 330.86 a | 295.46 a | 291.56 a | 240.79 a | 2.13 ns | 1.78 ns | 0.06 ns | |
| Diethyl succinate | 311.29 a | 359.28 a | 320.54 a | 333.28 a | 0.03 ns | 0.41 ns | 0.14 ns | |
| Ethyl laurate | 139.45 a | 144.95 a | 91.53 a | 88.01 a | 4.67 ** | 0.00 ns | 0.04 ns | |
| Sum esters | 2772.46 | 2770.67 | 2553.12 | 2082.29 | ||||
| Alcohols | 1-2 propylene glycol | 57.79 a | 72.98 a | 73.40 a | 41.61 a | 0.76 ns | 0.79 ns | 6.49 ** |
| Cis-3-hexenol | 573.93 a | 674.36 a | 417.06 a | 432.86 a | 3.44 ns | 0.29 ns | 0.16 ns | |
| 2-Phenylethanol | 17,540.00 b | 17,213.33 a | 17,031.67 a | 16,958.33 a | 13.22 ** | 3.63 ns | 1.46 ns | |
| Isoamyl alcohol | 17,557.92 a | 15,883.29 a | 19,630.38 a | 15,345.82 a | 0.16 ns | 2.43 ns | 0.47 ns | |
| Sum alcohols | 35,729.64 | 33,843.96 | 37,152.51 | 32,778.62 | ||||
| Terpens | α-pinen | 247.47 a | 156.61 a | 333.81 a | 74.92 a | 0.00 ns | 3.67 ns | 0.85 ns |
| Linalol | 45.21 a | 92.12 b | 55.15 a | 62.17 a | 0.65 ns | 4.92 ** | 2.74 ns | |
| Sum terpens | 292.68 | 248.73 | 388.96 | 137.09 | ||||
| Volatils phenols | 4-vinylphenol | 161.87 a | 151.49 a | 145.19 a | 125.14 a | 1.49 ns | 0.73 ns | 0.07 ns |
| 2-metoxyphenol (guaiacol) | 640.73 a | 597.62 a | 470.67 a | 337.19 a | 5.30 ** | 0.89 ns | 0.23 ns | |
| 4-ethylguaiacol | 119.81 a | 140.39 a | 138.04 a | 75.50 a | 1.46 ns | 1.13 ns | 4.56 ** | |
| Eugenol | 419.60 b | 90.84 a | 105.91 a | 90.20 a | 9.33 ** | 11.24 ** | 9.25 ** | |
| Sum v. phenols | 1342.01 | 980.34 | 859.81 | 628.03 | ||||
| Acids | Decanoic acid | 157.58 a | 349.80 b | 145.41 a | 200.11 a | 4.39 ** | 10.25 ** | 3.17 ns |
| Octanoic acid | 930.00 a | 941.67 a | 885.00 a | 921.67 a | 3.59 ns | 1.98 ns | 0.53 ns | |
| Hexanoic acid | 739.00 b | 688.67 a | 628.67 a | 689.17 b | 29.72 ** | 0.25 ns | 30.27 ** | |
| Butyric acid | 502.17 a | 536.17 a | 447.17 a | 469.33 a | 22.14 ** | 4.71 ** | 0.21 ns | |
| Isopentanoic acid | 341.33 a | 363.17 a | 323.00 a | 356.33 b | 2.57 ns | 12.36 ** | 0.54 ns | |
| Sum acids | 2670.08 | 2879.48 | 2429.25 | 2636.61 | ||||
| Others | Theaspirane | 217.13 a | 215.50 a | 160.98 a | 121.52 a | 6.21 ** | 0.47 ns | 0.39 ns |
| Naftalene | 223.09 b | 68.17 a | 249.20 a | 270.38 a | 6.58 ** | 2.26 ns | 3.89 ns | |
| Group | Aroma Compound | Ultrasound Red Wines | ANOVA F Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Continuous | Pulsed | Tr | T | Tr*T | ||||
| 10 min | 20 min | 10 min | 20 min | |||||
| Aldehydes | Diacetyl | 40.93 a | 107.51 a | 66.18 a | 79 a | 0.00 ns | 2.69 ns | 1.21 ns |
| Esters | Ethyl isobutyrate | 270.68 a | 313.39 a | 167.3 a | 471.94 b | 0.14 ns | 5.63 ** | 3.20 ns |
| Isoamyl acetate | 50.7 a | 105.23 a | 136.31 a | 117.08 a | 2.71 ns | 0.36 ns | 1.56 ns | |
| Ethyl hexanoate | 216.59 a | 218.3 a | 259.31 a | 266.38 a | 1.36 ns | 0.01 ns | 0.01 ns | |
| Hexyl acetate | 6.6 a | 9.18 a | 33.17 a | 14.35 a | 3.24 ns | 0.84 ns | 1.43 ns | |
| Ethyl lactate | 289.64 a | 121.95 a | 236.86 a | 389.15 a | 1.67 ns | 0.01 ns | 3.72 ns | |
| Ethyl 3 hydroxybutyrate | 350.96 a | 326.59 a | 258.96 a | 412.65 a | 0.00 ns | 1.52 ns | 2.90 ns | |
| Ethyl decanoate | 207.65 a | 219.94 a | 259.17 a | 250.92 a | 1.61 ns | 0.00 ns | 0.10 ns | |
| Diethyl succinate | 358.74 a | 418.32 a | 446.43 a | 412.67 a | 0.43 ns | 0.04 ns | 0.55 ns | |
| Ethyl laurate | 64.78 a | 31.01 a | 126.66 a | 81.96 a | 3.19 ns | 1.54 ns | 0.03 ns | |
| Sum esters | 1816.34 | 1763.91 | 1924.17 | 2417.1 | ||||
| Alcohols | 1-2 propylene glycol | 28.26 a | 28.63 a | 32.35 a | 53.29 a | 4.89 ** | 2.77 ns | 2.52 ns |
| Cis-3-hexenol | 290.17 a | 197.17 a | 221.84 a | 323.58 a | 0.24 ns | 0.01 ns | 2.64 ns | |
| 2-Phenylethanol | 19,294 b | 18,934.67 a | 18,734.83 a | 18,654.17 a | 13.22 ** | 3.63 ns | 1.46 ns | |
| Isoamyl alcohol | 11,877.73 a | 8200.24 a | 10,226.29 a | 13,662.13 a | 1.33 ns | 0.01 ns | 4.62 ** | |
| Sum alcohols | 31,490.16 | 27,360.71 | 29,215.31 | 32,693.17 | ||||
| Terpens | α-pinen | 43.94 a | 38.75 a | 79.19 a | 59.77 a | 6.29 ** | 1.21 ns | 0.41 ns |
| Linalol | 35.69 a | 47.83 a | 41.74 a | 27.5 a | 0.64 ns | 0.01 ns | 2.32 ns | |
| Sum terpens | 79.63 | 86.58 | 120.93 | 87.27 | ||||
| Volatils phenols | 4-vinylphenol | 62.32 a | 62.15 a | 72.25 a | 73.64 a | 1.08 ns | 0.00 ns | 0.10 ns |
| 2-metoxyphenol (guaiacol) | 225.14 a | 243.21 a | 262.46 a | 310.27 a | 1.20 ns | 0.48 ns | 0.10 ns | |
| 4-ethylguaiacol | 78.46 a | 54.39 a | 58.81 a | 74.92 a | 0.00 ns | 0.06 ns | 1.66 ns | |
| Eugenol | 116.71 a | 95.88 a | 80.34 a | 96.55 a | 0.84 ns | 0.01 ns | 0.90 ns | |
| Sum v. phenols | 482.63 | 455.63 | 473.86 | 555.38 | ||||
| Acids | Decanoic acid | 149.29 a | 182.32 a | 206.78 a | 135.12 a | 0.02 ns | 0.29 ns | 2.12 ns |
| Octanoic acid | 1023 a | 1035.83 a | 973.5 a | 1013.83 a | 3.59 ns | 1.98 ns | 0.53 ns | |
| Hexanoic acid | 812.9 b | 757.53 a | 691.53 a | 758.08 b | 29.53 ** | 0.26 ns | 30.10 ** | |
| Butyric acid | 552.38 a | 589.78 a | 491.88 a | 516.27 a | 22.22 ** | 4.68 ** | 0.20 ** | |
| Isopentanoic acid | 375.47 a | 399.48 a | 355.3 a | 391.97 b | 2.57 ns | 12.56 ** | 0.53 ns | |
| Sum acids | 2913.04 | 2964.94 | 2718.99 | 2815.27 | ||||
| Others | Theaspirane | 146.22 a | 157.7 a | 200.06 a | 143.46 a | 0.30 ns | 0.38 ns | 0.88 ns |
| Naftalene | 40.93 a | 204.56 a | 299.31 a | 202.14 a | 2.12 ns | 3.61 ns | 2.41 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizama, V.; Álvarez, I.; García-Esparza, M.J. The Application of the Ultrasound Technique in the Production of Rosé and Red Wines. Fermentation 2024, 10, 164. https://doi.org/10.3390/fermentation10030164
Lizama V, Álvarez I, García-Esparza MJ. The Application of the Ultrasound Technique in the Production of Rosé and Red Wines. Fermentation. 2024; 10(3):164. https://doi.org/10.3390/fermentation10030164
Chicago/Turabian StyleLizama, Victoria, Inmaculada Álvarez, and María José García-Esparza. 2024. "The Application of the Ultrasound Technique in the Production of Rosé and Red Wines" Fermentation 10, no. 3: 164. https://doi.org/10.3390/fermentation10030164
APA StyleLizama, V., Álvarez, I., & García-Esparza, M. J. (2024). The Application of the Ultrasound Technique in the Production of Rosé and Red Wines. Fermentation, 10(3), 164. https://doi.org/10.3390/fermentation10030164







