Effect of High Altitude on Serum Biochemical Parameters, Immunoglobulins, and Rumen Metabolism of Sanhe Heifers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Regions, Animal, and Experimental Design
2.3. Collection and Analysis of Blood Samples
2.4. Ruminal Liquid Collection
2.5. Metabolite Extraction
2.6. LC–MS Analysis and Data Processing
2.7. Statistical Analysis
3. Results
3.1. Serum Immunoglobulins, Cytokines, and Biochemical Parameters
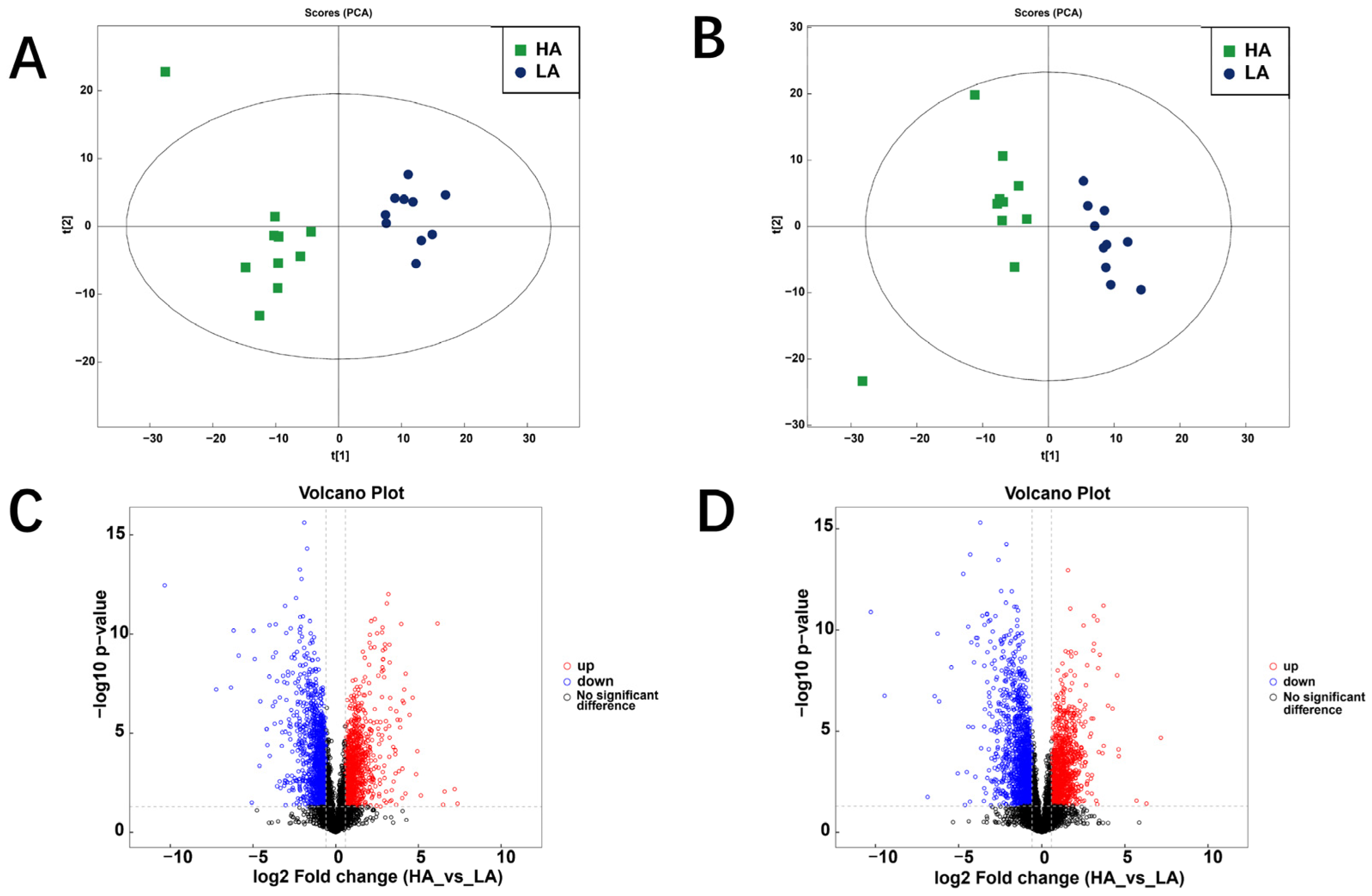
3.2. Rumen Metabolome of Sanhe Heifers from Different Altitudes
3.2.1. Differential Metabolites
3.2.2. KEGG Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T. The Qinghai-Tibetan Plateau: How high do Tibetans live? High Alt. Med. Biol. 2001, 2, 489–499. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Liu, Y.; Dong, Y.; Li, M.; An, Y.; Shi, F.; Beazley, R. Effects of the interaction among climate, terrain and human activities on biodiversity on the Qinghai-Tibet Plateau. Sci. Total Environ. 2021, 794, 148497. [Google Scholar] [CrossRef]
- Zha, R.; Sun, G.; Dong, Z.; Zhikang, Y.U. Assessment of atmospheric oxygen practical pressure and plateau reaction of tourists in the Qinghai-Tibet plateau. Ecol. Environ. Sci. 2016, 25, 92–98. (In Chinese) [Google Scholar]
- Cheviron, Z.A.; Brumfield, R.T. Genomic insights into adaptation to high-altitude environments. Heredity 2012, 108, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of functional ingredients in yak milk-derived food on health of Tibetan nomads living under high-altitude stress: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Rohm, I.; Ratka, J.; Pistulli, R.; Goebel, B.; Gecks, T.; Figulla, H.R.; Yilmaz, A.; Jung, C. Impact of systemic normobaric short-term hypoxia on pro-inflammatory and anti-inflammatory cytokines in healthy volunteers. Clin. Lab. 2015, 61, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S.; Child, A.; Butterfield, G.E.; Braun, B.; Rock, P.B.; Wolfel, E.E.; Zamudio, S.; Moore, L.G. Sympathoadrenal responses to submaximal exercise in women after acclimatization to 4300 meters. Metabolism 2000, 49, 1036–1042. [Google Scholar] [CrossRef]
- Cole, M.A.; Abd Jamil, A.H.; Heather, L.C.; Murray, A.J.; Sutton, E.R.; Slingo, M.; Sebag-Montefiore, L.; Tan, S.C.; Aksentijević, D.; Gildea, O.S.; et al. On the pivotal role of PPARα in adaptation of the heart to hypoxia and why fat in the diet increases hypoxic injury. FASEB J. 2016, 30, 2684–2697. [Google Scholar] [CrossRef]
- Kong, Z.; Li, B.; Zhou, C.; He, Q.; Zheng, Y.; Tan, Z. Comparative analysis of metabolic differences of Jersey cattle in different high-altitude areas. Front. Vet. Sci. 2021, 8, 713913. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Guan, L.L. Understanding host-microbial interactions in rumen: Searching the best opportunity for microbiota manipulation. J. Anim. Sci. Biotechnol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A structural and functional elucidation of the rumen microbiome influenced by various diets and microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J.; et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Bickhart, D.M.; Weimer, P.J.J. Symposium review: Host-rumen microbe interactions may be leveraged to improve the productivity of dairy cows. J. Dairy Sci. Symp. Rev. 2018, 101, 7680–7689. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e1. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Gaowa, N.; Panke-Buisse, K.; Wang, S.; Wang, H.; Cao, Z.; Wang, Y.; Yao, K.; Li, S. Brisket disease is associated with lower volatile fatty acid production and altered rumen microbiome in Holstein heifers. Animals 2020, 10, 1712. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, S.; Li, S.; Wang, W. Effects of altitude on the digestion performance, serum antioxidative characteristics, rumen fermentation parameters, and rumen bacteria of Sanhe heifers. Front. Microbiol. 2022, 13, 875323. [Google Scholar] [CrossRef] [PubMed]
- Ji-hui, F.; Qiao, L.; Yan, Z.; Gen-wei, C.; Xiang-de, F.; Wen-Ming, L. Dynamic variations and influencing factors of groundwater levels in Lhasa City. Wuhan Univ. J. Nat. Sci. 2005, 10, 665–673. [Google Scholar] [CrossRef]
- National Research Council. Nutrient requirements of dairy cattle. In Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Di Pietro, P.; Miano, M.; Cravana, C.; Ferlazzo, A.M. Effect of altitude on plasma serotonin levels in horses. In Trends in Veterinary Sciences: Current Aspects in Veterinary Morphophysiology, Biochemistry, Animal, Food Hygiene and Clinical Sciences; Boiti, C., Ferlazzo, A., Gaiti, A., Pugliese, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 9–13. [Google Scholar]
- Lemos, V.A.; dos Santos, R.V.; Lira, F.S.; Rodrigues, B.; Tufik, S.; de Mello, M.T. Can high altitude influence cytokines and sleep? Mediators Inflamm. 2013, 2013, 279365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Ganju, L. Influence of high altitude exposure on the immune system: A review. Immunol. Invest. 2010, 39, 219–234. [Google Scholar] [CrossRef]
- Song, T.T.; Bi, Y.H.; Gao, Y.Q.; Huang, R.; Hao, K.; Xu, G.; Tang, J.W.; Ma, Z.Q.; Kong, F.P.; Coote, J.H.; et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J. Neuroinflamm. 2016, 13, 63. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Duan, J.; Chen, J.; Wang, Q.; Liu, X.; Wang, C. Exploration of acute phase proteins and inflammatory cytokines in early stage diagnosis of acute mountain sickness. High Alt. Med. Biol. 2018, 19, 170–177. [Google Scholar] [CrossRef]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.W.; Radak, Z. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef]
- Seys, S.F.; Daenen, M.; Dilissen, E.; Van Thienen, R.; Bullens, D.M.; Hespel, P.; Dupont, L.J. Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax 2013, 68, 906–913. [Google Scholar] [CrossRef]
- Boos, C.J.; Woods, D.R.; Varias, A.; Biscocho, S.; Heseltine, P.; Mellor, A.J. High altitude and acute mountain sickness and changes in circulating endothelin-1, interleukin-6, and Interleukin-17a. High Alt. Med. Biol. 2016, 17, 25–31. [Google Scholar] [CrossRef]
- Kleger, G.R.; Bärtsch, P.; Vock, P.; Heilig, B.; Roberts, L.J., 2nd; Ballmer, P.E. Evidence against an increase in capillary permeability in subjects exposed to high altitude. J. Appl. Physiol. 1996, 81, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Kleger, G.R.; Holzgraefe, M.; Ballmer, P.E.; Bärtsch, P. Pathophysiological significance of peroxidative stress, neuronal damage, and membrane permeability in acute mountain sickness. J. Appl. Physiol. 2004, 96, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Fazio, E.; Cravana, C.; Ferlazzo, A.M. Effects of partial versus complete separation after weaning on plasma serotonin, tryptophan and pituitary-adrenal pattern of Anglo-Arabian foals. Livest. Sci. 2017, 198, 157–161. [Google Scholar] [CrossRef]
- Vargas, V.E.; Kaushal, K.M.; Monau, T.; Myers, D.A.; Ducsay, C.A. Long-term hypoxia enhances cortisol biosynthesis in near-term ovine fetal adrenal cortical cells. Reprod. Sci. 2011, 18, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, R.C.; Malhotra, A.S.; Singh, T. Glucoregulatory hormones in man at high altitude. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 286–291. [Google Scholar] [CrossRef]
- Harmon, D.L.; Swanson, K.C. Review: Nutritional regulation of intestinal starch and protein assimilation in ruminants. Animal 2020, 14, s17–s28. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Kristensen, N.B. Nitrogen recycling through the gut and the nitrogen economy of ruminants: An asynchronous symbiosis. J. Anim. Sci. 2008, 86, E293–E305. [Google Scholar] [CrossRef]
- Ogata, H.; Tokuyama, K.; Nagasaka, S.; Ando, A.; Kusaka, I.; Sato, N.; Goto, A.; Ishibashi, S.; Kiyono, K.; Struzik, Z.R.; et al. Long-range correlated glucose fluctuations in diabetes. Methods Inf. Med. 2007, 46, 222–226. [Google Scholar] [CrossRef]
- Venter, L.; Loots, D.T.; Mienie, L.J.; Jansen van Rensburg, P.J.; Mason, S.; Vosloo, A.; Lindeque, J.Z. Uncovering the metabolic response of abalone (Haliotis midae) to environmental hypoxia through metabolomics. Metabolomics 2018, 14, 49. [Google Scholar] [CrossRef]
- Ould Amer, Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. ABC family transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Tampé, R. Structural and mechanistic principles of ABC transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Zhang, R.; Jin, L.; Sun, X.; Wang, Z. Effects of high-altitude hypoxia on the metabolites and gut microbiota of Tibetan miniature pigs. Front. Microbiol. 2019, 10, 2879. [Google Scholar] [CrossRef]

| Items | Groups 1 | SEM | p-Value | |
|---|---|---|---|---|
| LA | HA | |||
| Immunoglobulin A (IgA, mg/mL) | 0.13 | 0.12 | 0.01 | 0.89 |
| Immunoglobulin G (IgG, mg/mL) | 2.39 | 2.41 | 0.17 | 0.96 |
| Immunoglobulin M (IgM, mg/mL) | 0.82 | 0.82 | 0.06 | 0.99 |
| Interleukin-2 (IL-2, ng/L) | 128.65 | 107.59 | 10.12 | 0.30 |
| Interleukin-6 (IL-6, ng/L) | 565.06 | 567.20 | 60.87 | 0.99 |
| Interleukin-10 (IL-10, ng/L) | 31.37 | 31.60 | 3.10 | 0.97 |
| Tumor necrosis factor-α (TNF-α, ng/L) | 180.33 | 66.73 | 10.36 | 0.53 |
| Items | Groups 1 | SEM | p Value | |
|---|---|---|---|---|
| LA | HA | |||
| Leptin (LEP, ng/mL) | 3.98 | 3.69 | 0.54 | 0.80 |
| Cortisol (Cor, ng/mL) | 15.35 | 32.46 | 3.17 | <0.01 |
| Glucose (GLU, mmol/L) | 3.65 | 4.03 | 0.09 | 0.04 |
| Blood urea nitrogen (BUN, mmol/L) | 3.81 | 7.23 | 0.43 | <0.01 |
| Total cholesterol (TC, mmol/L) | 2.63 | 2.68 | 0.08 | 0.79 |
| Nonesterified fatty acids (NEFA, mmol/L) | 0.43 | 0.47 | 0.04 | 0.54 |
| Ca2+ (mmol/L) | 2.05 | 0.86 | 0.21 | <0.01 |
| P5+ (mmol/L) | 2.08 | 1.99 | 0.08 | 0.59 |
| HMDB | Metabolite | VIP | Fold Change | p-Value | Direction |
|---|---|---|---|---|---|
| HMDB0000148 | Glutamic acid | 5.489 | 2.701 | <0.001 | ↑ |
| HMDB0033752 | 3-(2-hydroxyphenyl)propionic acid | 3.811 | 0.317 | <0.001 | ↓ |
| HMDB0000251 | Taurine | 1.099 | 0.224 | <0.001 | ↓ |
| HMDB0000440 | 3-hydroxyphenylacetic acid | 2.328 | 0.423 | <0.001 | ↓ |
| HMDB0001335 | Prostaglandin i2 | 4.766 | 2.500 | <0.001 | ↑ |
| HMDB0000058 | Adenosine 3′,5′-cyclic monophosphate | 5.007 | 0.086 | 0.005 | ↓ |
| HMDB0000159 | L-phenylalanine | 2.405 | 4.418 | <0.001 | ↑ |
| HMDB0003213 | Raffinose | 1.289 | 18.775 | <0.001 | ↑ |
| HMDB0001547 | Corticosterone | 3.482 | 0.315 | <0.001 | ↓ |
| HMDB0001830 | Progesterone | 3.423 | 0.362 | <0.001 | ↓ |
| HMDB0000158 | Tyrosine | 1.536 | 3.418 | <0.001 | ↑ |
| HMDB0000300 | Uracil | 8.893 | 2.225 | <0.001 | ↑ |
| HMDB0001314 | Guanosine 3′,5′-cyclic monophosphate | 1.690 | 0.099 | 0.006 | ↓ |
| HMDB0000020 | 4-hydroxyphenylacetic acid | 1.648 | 0.401 | <0.001 | ↓ |
| HMDB0000161 | L-alanine | 1.528 | 2.449 | <0.001 | ↑ |
| HMDB0000016 | 21-hydroxyprogesterone | 1.407 | 0.527 | <0.001 | ↓ |
| HMDB0000156 | L-malic acid | 1.666 | 2.686 | <0.001 | ↑ |
| HMDB0000191 | Aspartic acid | 4.091 | 2.684 | <0.001 | ↑ |
| HMDB0060475 | DL-glutamic acid | 5.339 | 2.259 | <0.001 | ↑ |
| HMDB0006483 | D-aspartic acid | 1.388 | 2.549 | <0.001 | ↑ |
| HMDB0000050 | Adenosine | 13.658 | 0.391 | <0.001 | ↓ |
| HMDB0001397 | Guanosine 5′-monophosphate | 1.202 | 2.575 | <0.001 | ↑ |
| HMDB0000273 | His-ser | 1.881 | 2.048 | 0.003 | ↑ |
| HMDB0002088 | N-oleoylethanolamine | 2.181 | 2.507 | <0.001 | ↑ |
| HMDB0000159 | Phenylalanine | 1.550 | 2.990 | 0.002 | ↑ |
| HMDB0000158 | DL-tyrosine | 1.080 | 2.336 | <0.001 | ↑ |
| HMDB0000156 | Malate | 2.853 | 3.128 | 0.002 | ↑ |
| HMDB0000259 | Serotonin | 1.459 | 0.224 | 0.003 | ↓ |
| HMDB0000022 | 3-methoxytyramine | 1.386 | 2.113 | <0.001 | ↑ |
| HMDB0000177 | Histidine | 1.422 | 0.589 | <0.001 | ↓ |
| HMDB0000767 | Pseudouridine | 1.599 | 3.272 | 0.003 | ↑ |
| HMDB0000228 | Phenol | 4.418 | 0.336 | <0.001 | ↓ |
| HMDB0000764 | 3-Phenylpropanoic acid | 22.624 | 0.697 | <0.001 | ↓ |
| HMDB0000011 | Beta-hydroxybutyrate | 1.583 | 0.629 | 0.002 | ↓ |
| HMDB0000172 | L-isoleucine | 1.143 | 0.254 | 0.002 | ↓ |
| D-glucose 6-phosphate | 1.316 | 0.206 | 0.034 | ↓ | |
| HMDB0000965 | Hypotaurine | 1.008 | 1.865 | 0.002 | ↑ |
| HMDB0060263 | Histamine | 3.487 | 1.904 | 0.019 | ↑ |
| HMDB0000148 | L-glutamate | 1.259 | 3.098 | 0.044 | ↑ |
| HMDB0062186 | L-aspartic acid | 1.314 | 2.114 | 0.010 | ↑ |
| HMDB0000089 | Cytidine | 5.894 | 3.292 | <0.001 | ↑ |
| HMDB0000669 | 2-hydroxyphenylacetic acid | 1.103 | 0.564 | <0.001 | ↓ |
| HMDB0000423 | 3,4-dihydroxyhydrocinnamic acid | 2.585 | 0.833 | 0.005 | ↓ |
| HMDB0000687 | Leucine | 4.389 | 4.818 | <0.001 | ↑ |
| HMDB0000301/HMDB0034174 | Urocanic acid | 1.904 | 6.727 | 0.001 | ↑ |
| HMDB0000210 | Pantothenic acid | 1.628 | 1.773 | 0.019 | ↑ |
| HMDB0000262 | Thymine | 4.986 | 3.304 | 0.002 | ↑ |
| HMDB0002434 | Hydroquinone | 1.518 | 0.596 | 0.007 | ↓ |
| HMDB0000167 | DL-threonine | 1.116 | 2.312 | 0.001 | ↑ |
| HMDB0000301 | Urocanate | 4.846 | 7.767 | 0.002 | ↑ |
| HMDB0001202 | 2′-deoxycytidine 5′-monophosphate | 1.442 | 1.567 | 0.029 | ↑ |
| HMDB0001870 | Benzoic acid | 1.111 | 0.527 | <0.001 | ↓ |
| HMDB0000162 | L-proline | 1.844 | 1.778 | 0.007 | ↑ |
| HMDB0000303 | Ala-Ala | 9.320 | 2.824 | 0.005 | ↑ |
| HMDB0000306 | Tyramine | 3.244 | 4.475 | 0.024 | ↑ |
| HMDB0000001 | 1-methylhistidine | 1.322 | 0.229 | 0.015 | ↓ |
| HMDB0004284 | 4-hydroxyphenethyl alcohol | 2.885 | 1.827 | 0.021 | ↑ |
| HMDB0000043 | Betaine | 3.599 | 0.384 | 0.016 | ↓ |
| HMDB0000375 | 3-(3-Hydroxyphenyl)propanoic acid | 1.547 | 0.617 | 0.028 | ↓ |
| HMDB0004063 | Metanephrine | 1.242 | 1.403 | 0.040 | ↑ |
| HMDB0000209 | Phenylacetic acid | 1.473 | 1.399 | 0.011 | ↑ |
| HMDB0002322 | 1,5-pentanediamine | 6.655 | 0.597 | <0.001 | ↓ |
| HMDB0001895 | Salicylic acid | 4.604 | 0.391 | <0.001 | ↓ |
| HMDB0000819 | Dl-normetanephrine | 2.686 | 1.941 | 0.005 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cao, Z.; Yang, H.; Wang, Y.; Wang, W.; Li, S. Effect of High Altitude on Serum Biochemical Parameters, Immunoglobulins, and Rumen Metabolism of Sanhe Heifers. Fermentation 2024, 10, 170. https://doi.org/10.3390/fermentation10030170
Zhang X, Cao Z, Yang H, Wang Y, Wang W, Li S. Effect of High Altitude on Serum Biochemical Parameters, Immunoglobulins, and Rumen Metabolism of Sanhe Heifers. Fermentation. 2024; 10(3):170. https://doi.org/10.3390/fermentation10030170
Chicago/Turabian StyleZhang, Xinyu, Zhijun Cao, Hongjian Yang, Yajing Wang, Wei Wang, and Shengli Li. 2024. "Effect of High Altitude on Serum Biochemical Parameters, Immunoglobulins, and Rumen Metabolism of Sanhe Heifers" Fermentation 10, no. 3: 170. https://doi.org/10.3390/fermentation10030170
APA StyleZhang, X., Cao, Z., Yang, H., Wang, Y., Wang, W., & Li, S. (2024). Effect of High Altitude on Serum Biochemical Parameters, Immunoglobulins, and Rumen Metabolism of Sanhe Heifers. Fermentation, 10(3), 170. https://doi.org/10.3390/fermentation10030170







