Sourdough Fermentation of Oat and Barley Flour with Bran and Its Application in Flatbread Made with No-Time and Dough Retardation Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients
2.2. Chemical Analyses of Flour/Bran and Flatbread
2.3. Sourdough Fermentation and Characterization
Measurement of pH, Total Titratable Acidity and Viable Cell Counts in Sourdough
2.4. Modelling of Sourdough Fermentation Kinetics
2.5. Experimental Design
2.6. Breadmaking
2.7. Evaluation of Dough and Bread Physical Properties
2.8. Statistical Analyses
3. Results and Discussion
3.1. Enzymatic Activity and Bioactive Components of Flour and Bran
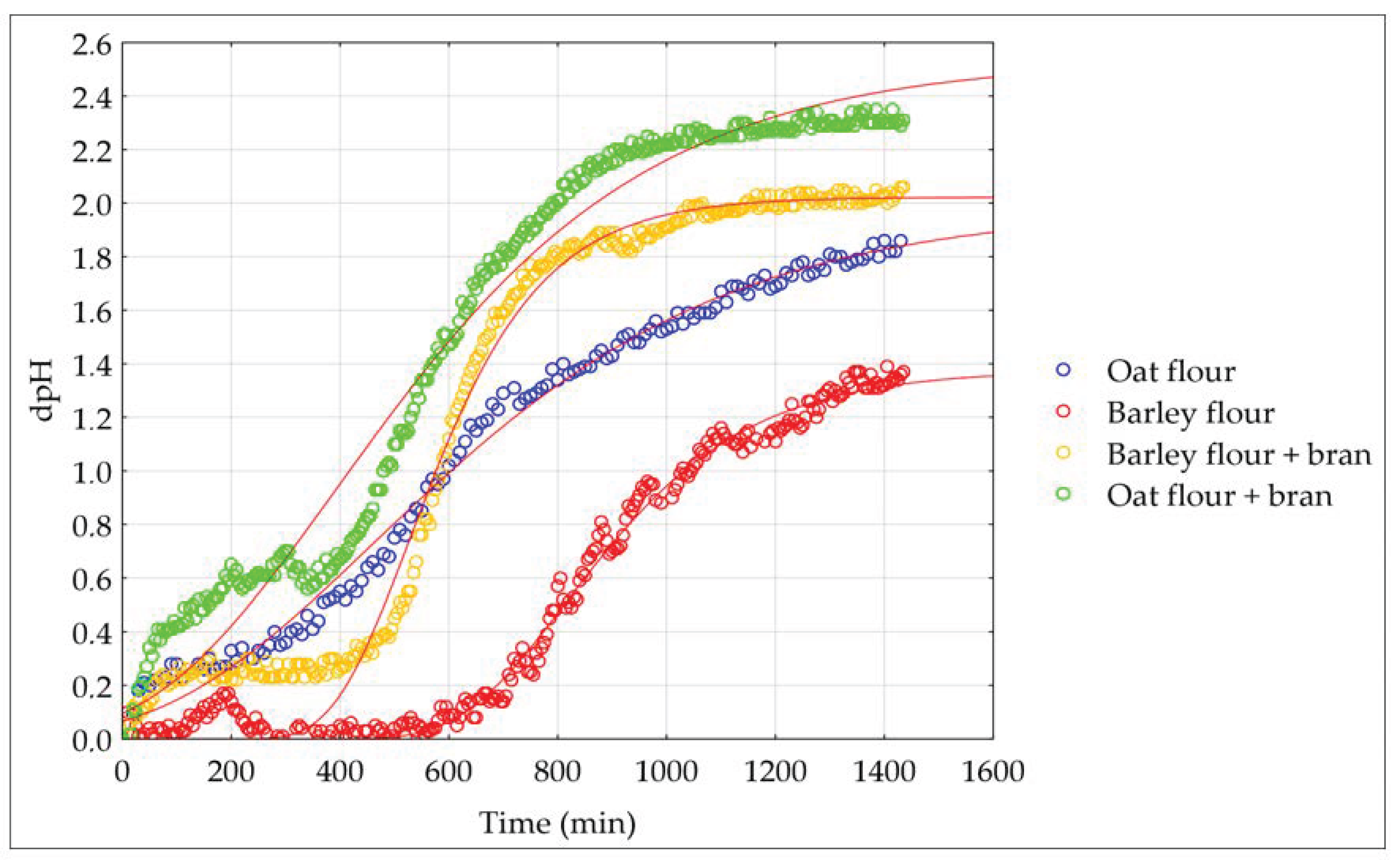
3.2. Fermentation Kinetics of Oat and Barley Sourdough
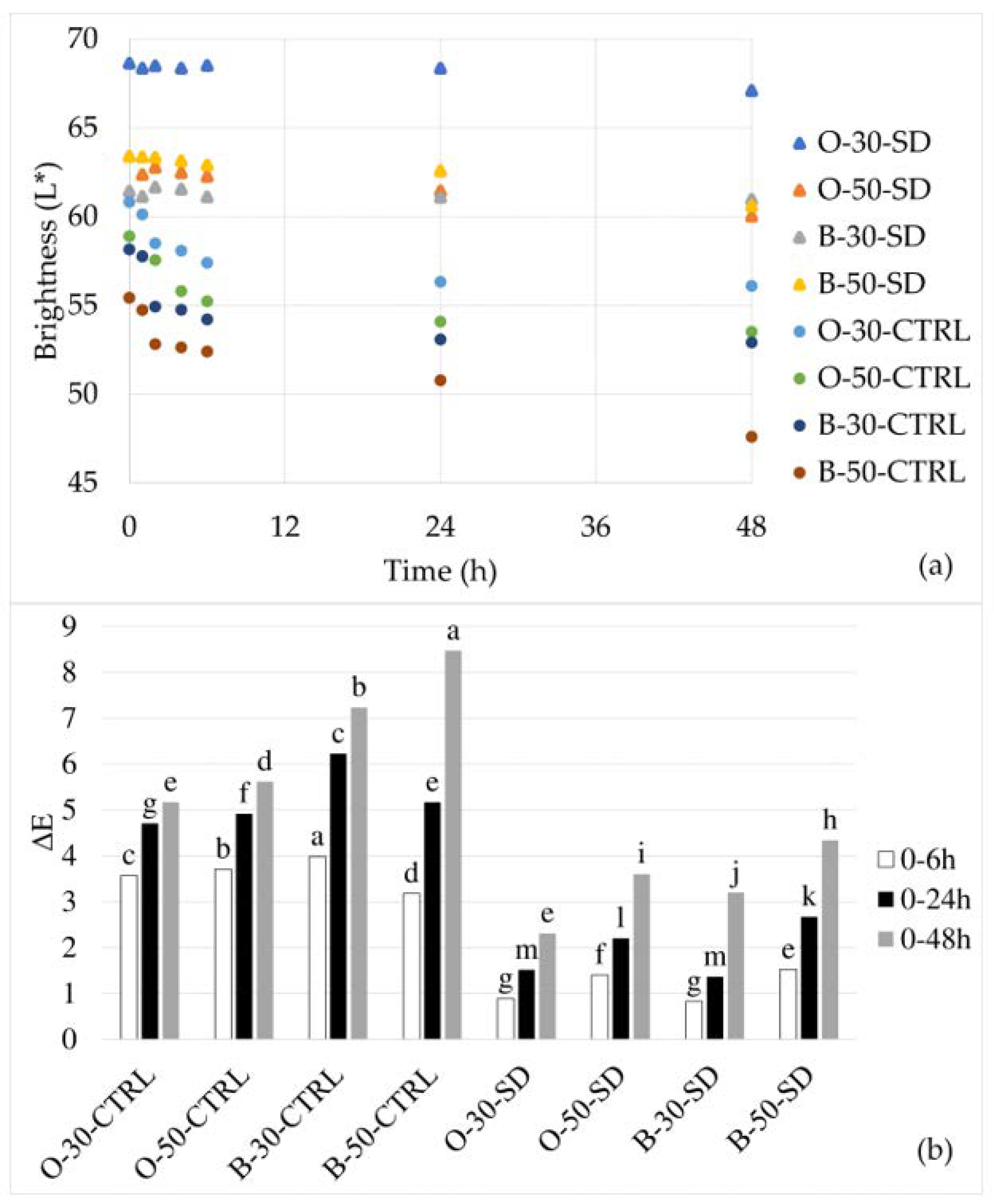
3.3. Changes in Color, pH, and Weight of the Dough during the Retardation Process
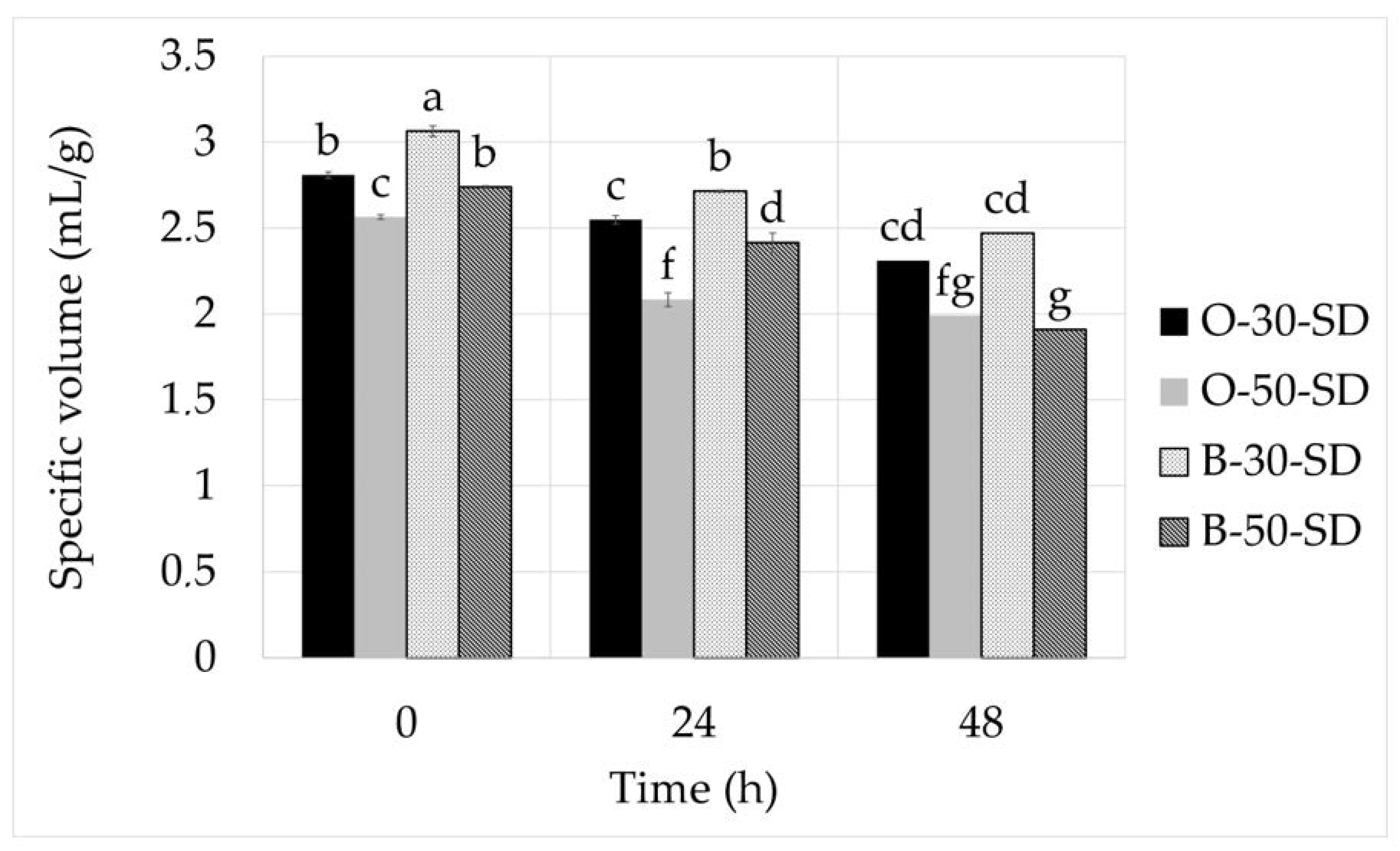
3.4. The Physical Properties of Flatbread
3.5. Nutritive Value of Bread
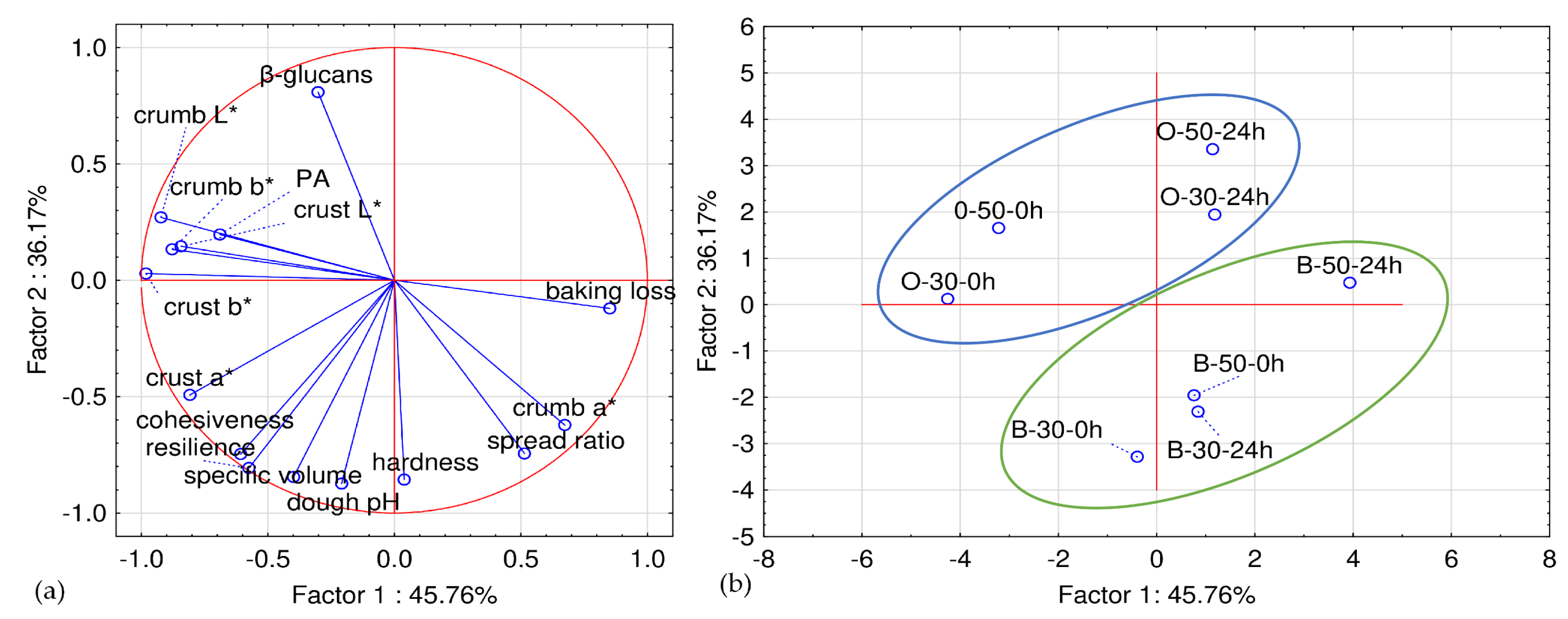
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dymchenko, A.; Geršl, M.; Gregor, T. Trends in bread waste utilisation. Trends Food Sci. Technol. 2023, 132, 93–102. [Google Scholar] [CrossRef]
- Pasqualone, A.; Vurro, F.; Summo, C.; Abd-El-Khalek, M.H.; Al-Dmoor, H.H.; Grgić, T.; Ruiz, M.; Magro, C.; Deligeorgakis, C.; Helou, C.; et al. The Large and Diverse Family of Mediterranean Flat Breads: A Database. Foods 2022, 11, 2326. [Google Scholar] [CrossRef]
- Kumar, A. Chapatis and related products. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 724–734. [Google Scholar] [CrossRef]
- Pasqualone, A. Traditional flat breads spread from the Fertile Crescent: Production process and history of baking systems. J. Ethn. Foods 2018, 5, 10–19. [Google Scholar] [CrossRef]
- Garzon, R.; Gasparre, N.; Pasqualone, A.; Papageorgiou, M.; Grgic, T.; Le-Bail, P.; Mínguez Pablos, I.; El Tomb, C.; Magro, C.; Rosell, C.M. Flatbreads on the rise, what about their nutritional quality? The current state of the Mediterranean market. Med. Res. Arch. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Avena-Bustillos, R.J.; Brichta, J.L.; Kahlon, A.K. High-protein nutritious flatbreads and an option for gluten-sensitive individuals. Foods 2019, 8, 591. [Google Scholar] [CrossRef]
- Boukid, F. Flatbread—A canvas for innovation: A review. Appl. Food Res. 2022, 2, 100071. [Google Scholar] [CrossRef]
- Boers, H.M.; MacAulay, K.; Murray, P.; Dobriyal, R.; Mela, D.J.; Spreeuwenberg, M.A.M. Efficacy of fibre additions to flatbread flour mixes for reducing post-meal glucose and insulin responses in healthy Indian subjects. Br. J. Nutr. 2017, 117, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kaur, C.; Jambh, H.K. Rheological, textural, and technological modifications in wheat unleavened flatbread substituted with extruded finger millet. J. Texture Stud. 2021, 52, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mehfooz, T.; Ali, T.M.; Arif, S.; Hasnain, A. Effect of barley husk addition on rheological, textural, thermal and sensory characteristics of traditional flat bread (chapatti). J. Cereal Sci. 2018, 79, 376–382. [Google Scholar] [CrossRef]
- Bhavya, S.N.; Prakash, J. Nutritional properties of iron fortified flatbreads enriched with greens and legumes. J. Food Process. Preserv. 2021, 45, e15495. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel of dietetic products, nutrition and allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 1–21. [Google Scholar] [CrossRef]
- Pejcz, E.; Czaja, A.; Wojciechowicz-Budzisz, A.; Gil, Z.; Spychaj, R. The potential of naked barley sourdough to improve the quality and dietary fibre content of barley enriched wheat bread. J. Cereal Sci. 2017, 77, 97–101. [Google Scholar] [CrossRef]
- Koksel, H.; Tekin-Cakmak, Z.H.; Oruc, S.; Kilic, G.; Ozkan, K.; Cetiner, B.; Sagdic, O.; Sestili, F.; Jilal, A. A New Functional Wheat Flour Flatbread (Bazlama) Enriched with High-β-Glucan Hull-Less Barley Flour. Foods 2024, 13, 236. [Google Scholar] [CrossRef]
- Mansoor, R.; Ali, T.M.; Arif, S.; Moin, A.; Hasnain, A. Effects of barley flour on dough rheology, texture, sensory and glycemic index of traditional unleavened flat bread (Roti). Cereal Chem. 2019, 96, 1170–1179. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Hosseininezhad, M. Improving the quality of traditional Iranian bread by using sourdough and optimizing the fermentation conditions. SN Appl. Sci. 2022, 4, 148. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, P.; Gill, B.S.; Kaur, S. Effect of incorporating hydrothermal, kilned and defatted oats on antioxidant and chapatti making properties of wheat flour. Food Chem. 2013, 138, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Weckx, S.; Van Kerrebroeck, S.; De Vuyst, L. Omics approaches to understand sourdough fermentation processes. Int. J. Food Microbiol. 2019, 302, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Venturi, M.; Guerrini, S.; Blandino, M.; Luti, S.; Pazzagli, L.; Granchi, L. Antioxidant properties of sourdoughs made with whole grain flours of hull-less barley or conventional and pigmented wheat and by selected lactobacilli strains. Foods 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Rieder, A.; Holtekjølen, A.K.; Sahlstrøm, S.; Moldestad, A. Effect of barley and oat flour types and sourdoughs on dough rheology and bread quality of composite wheat bread. J. Cereal Sci. 2012, 55, 44–52. [Google Scholar] [CrossRef]
- Fang, L.; Wang, W.; Dou, Z.; Chen, J.; Meng, Y.; Cai, L.; Li, Y. Effects of mixed fermentation of different lactic acid bacteria and yeast on phytic acid degradation and flavor compounds in sourdough. LWT 2023, 174, 114438. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, R.; Yuan, W. Type I sourdough steamed bread made by retarded sponge-dough method. Food Chem. 2020, 311, 126029. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Ananthanarayan, L.; Lele, S.S. Dough browning inhibition of multigrain Indian flatbread (chapatti) using a combination of chemical and microwave treatment. J. Food Measur. Charact. 2019, 13, 807–820. [Google Scholar] [CrossRef]
- Habuš, M.; Golubić, P.; Vukušić Pavičić, T.; Čukelj Mustač, N.; Voučko, B.; Herceg, Z.; Ćurić, D.; Novotni, D. Influence of Flour Type, Dough Acidity, Printing Temperature and Bran Pre-processing on Browning and 3D Printing Performance of Snacks. Food Bioproc. Technol. 2021, 14, 2365–2379. [Google Scholar] [CrossRef]
- Quinde-Axtell, Z.; Powers, J.; Baik, B.K. Retardation of discoloration in barley flour gel and dough. Cereal Chem. 2006, 83, 385–390. [Google Scholar] [CrossRef]
- Grgić, T.; Pavišić, Z.; Maltar-Strmečki, N.; Voučko, B.; Čukelj Mustač, N.; Ćurić, D.; Le-Bail, A.; Novotni, D. Ultrasound-assisted Modification of Enzymatic and Antioxidant Activities, Functional and Rheological Properties of Oat and Barley Bran. Food Bioproc. Technol. 2023, 16, 2416–2429. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: Association of Analytical Chemists, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- AACC. Official Methods of Analysis: Approved Methods of the American Association of Cereal Chemists International, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Habuš, M.; Novotni, D.; Gregov, M.; Štifter, S.; Čukelj Mustač, N.; Voučko, B.; Ćurić, D. Influence of particle size reduction and high-intensity ultrasound on polyphenol oxidase, phenolics, and technological properties of wheat bran. J. Food Process. Preserv. 2021, 45, e15204. [Google Scholar] [CrossRef]
- Katina, K.; Salmenkallio-Marttila, M.; Partanen, R.; Forssell, P.; Autio, K. Effects of sourdough and enzymes on staling of high-fibre wheat bread. LWT 2006, 39, 479–491. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. LWT 2002, 35, 407–414. [Google Scholar] [CrossRef]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Numeration of Mesophilic Lactic acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization (ISO): Geneva, Switzerland, 1998.
- ISO 7954:2002; General Guidance for Enumeration of Yeasts and Moulds—Colony Count Technique at 25 degrees C. International Organization for Standardization (ISO): Geneva, Switzerland, 2002.
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van, K.; Riet, T. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Aboshora, W.; Lianfu, Z.; Dahir, M.; Quingran, M.; Musa, A.; Gasmalla, M.A.A.; Omar, K.A. Influence of doum (Hyphaene thebaica L.) flour addition on dough mixing properties, bread quality and antioxidant potential. J. Food Sci. Technol. 2016, 53, 591–600. [Google Scholar] [CrossRef]
- Rawat, M.; Varshney, A.; Rai, M.; Chikara, A.; Pohty, A.L.; Joshi, A.; Binjola, A.; Singh, C.P.; Rawat, K.; Rather, M.A.; et al. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. J. Agric. Food Res. 2023, 12, 100619. [Google Scholar] [CrossRef]
- Mariotti, M.; Garofalo, C.; Aquilanti, L.; Osimani, A.; Fongaro, L.; Tavoletti, S.; Hager, A.S.; Clementi, F. Barley flour exploitation in sourdough bread-making: A technological, nutritional and sensory evaluation. LWT 2014, 59, 973–980. [Google Scholar] [CrossRef]
- Hüttner, E.K.; Dal Bello, F.; Arendt, E.K. Identification of lactic acid bacteria isolated from oat sourdoughs and investigation into their potential for the improvement of oat bread quality. Eur. Food Res. Technol. 2010, 230, 849–857. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Brütsch, L.; Rugiero, S.; Serrano, S.S.; Städeli, C.; Windhab, E.J.; Fischer, P.; Kuster, S. Targeted Inhibition of Enzymatic Browning in Wheat Pastry Dough. J. Agric. Food Chem. 2018, 66, 12353–12360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Cauvain, S.P. Dough retarding and freezing. In Technology of Breadmaking, 2nd ed.; Cauvain, S.P., Young, L.S., Eds.; Springer: New York, NY, USA, 2007; pp. 175–205. [Google Scholar]
- Gill, S.; Vasanthan, T.; Ooraikul, B.; Rossnagal, B. Wheat bread quality as influenced by the substitution of waxy and regular barley flours in their native and cooked forms. J. Cereal Sci. 2002, 36, 239–251. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Relationship of gliadin and glutenin proteins with dough rheology, flour pasting and bread making performance of wheat varieties. LWT 2013, 51, 211–217. [Google Scholar] [CrossRef]
- Grenier, D.; Rondeau-Mouro, C.; Dedey, K.B.; Morel, M.H.; Lucas, T. Gas cell opening in bread dough during baking. Trends Food Sci. Technol. 2021, 109, 482–498. [Google Scholar] [CrossRef]
- Flander, L.; Suortti, T.; Katina, K.; Poutanen, K. Effects of wheat sourdough process on the quality of mixed oat-wheat bread. LWT 2011, 44, 656–664. [Google Scholar] [CrossRef]
- Deasy, A.; Dewi, R.; Tjahjono, A.; Kalirungkut Surabaya, J. The Making of Rice Bran Flour-Based Sourdough Bread. JPA 2022, 10, 28–37. [Google Scholar]
- Liu, W.; Brennan, M.; Serventi, L.; Brennan, C. The Effect of Oat Bran on the Dough Rheology and Quality of Chinese Steamed Bread. GOST 2018, 1, 126–130. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Olaerts, H.; Vandekerckhove, L.; Courtin, C.M. A closer look at the bread making process and the quality of bread as a function of the degree of preharvest sprouting of wheat (Triticum aestivum). J. Cereal Sci. 2018, 80, 188–197. [Google Scholar] [CrossRef]
- Kulp, K. Enzymes as dough improvers. In Advances in Baking Technology; Camel, B.S., Stauffer, C.E., Eds.; Springer: New York, NY, USA, 1993; pp. 152–178. [Google Scholar]
- Yildirim, R.M.; Arici, M. Effect of the fermentation temperature on the degradation of phytic acid in whole-wheat sourdough bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.A.; Chanliaud, E.; Rémésy, C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Miller, R.A.; Hoseney, R.C. Effects of (1 → 3) (1 → 4)-β-D-Glucans of Wheat Flour on Breadmaking. Cereal Chem. 1998, 75, 629–633. [Google Scholar] [CrossRef]
- Gamel, T.H.; Abdel-Aal, E.S.M.; Tosh, S.M. Effect of yeast-fermented and sour-dough making processes on physicochemical characteristics of β-glucan in whole wheat/oat bread. LWT 2015, 60, 78–85. [Google Scholar] [CrossRef]
| Sample Code | Flour and Bran Type | Sourdough Level (g/100 g Dough) | Retardation Time (h) |
|---|---|---|---|
| O-30-0 h | oat | 30 | 0 |
| O-50-0 h | oat | 50 | 0 |
| O-30-24 h | oat | 30 | 24 |
| O-50-24 h | oat | 50 | 24 |
| B-30-0 h | barley | 30 | 0 |
| B-50-0 h | barley | 50 | 0 |
| B-30-24 h | barley | 30 | 24 |
| B-50-24 h | barley | 50 | 24 |
| Parameter | Wheat Flour | Oat Flour | Oat Bran | Barley Flour | Barley Bran |
|---|---|---|---|---|---|
| Total dietary fiber (g/100 g d.w.) | 4.90 ± 0.01 d | 9.29 ± 0.01 c | 20.51 ± 0.03 a | 13.77 ± 0.31 b | 19.77 ± 0.25 a |
| Total minerals as ash (g/100 g d.w.) | 0.70 ± 0.01 e | 1.83 ± 0.00 d | 3.02 ± 0.00 a | 2.33 ± 0.03 c | 2.57 ± 0.03 b |
| Mg (mg/100 g d.w.) | 45.9 ± 2.89 c | 71.30 ± 1.70 b | 120.57 ± 5.46 a | 112.21 ± 2.57 a | 73.58 ± 0.07 b |
| Fe (mg/100 g d.w.) | 1.53 ± 0.08 b | 1.99 ± 0.01 b | 6.99 ± 1.26 a | 2.86 ± 0.25 b | 3.28 ± 0.02 b |
| Zn (mg/100 g d.w.) | 1.25 ± 0.01 b | 2.35 ± 0.05 ab | 3.76 ± 0.81 a | 2.50 ± 0.01 ab | 2.83 ± 0.07 ab |
| Cu (mg/100 g d.w.) | 0.27 ± 0.02 b | 0.44 ± 0.01 ab | 0.56 ± 0.08 a | 0.51 ± 0.01 a | 0.48 ± 0.02 ab |
| TPC (mg FAE/g d.w.) | 0.11 ± 0.00 c | 0.28 ± 0.01 b | * | 0.45 ± 0.01 a | ** |
| Polyphenol oxidase (Δ475/g) | 2.30 ± 0.00 d | 2.52 ± 0.01 d | 6.30 ± 0.01 a | 3.00 ± 0.00 c | 4.50 ± 0.02 b |
| Substrate | pH | TTA (mL 0.1 M NaOH) | µmax (h−1) | A (dpH) | (h) | Ti (h) | p-Value | R2 |
|---|---|---|---|---|---|---|---|---|
| Oat flour | 3.95 ± 0.31 a | 6.50 ± 0.42 d | 0.116 | 1.99 | 1.42 | 7.7 | <0.001 | 0.990 |
| Barley flour | 3.83 ± 0.22 b | 9.33 ± 0.35 b | 0.344 | 1.69 | 8.31 | 10.1 | <0.001 | 0.978 |
| Oat flour and bran | 3.69 ± 0.18 c | 9.95 ± 0.51 a | 0.169 | 2.54 | 1.05 | 6.6 | <0.001 | 0.970 |
| Barley flour and bran | 3.69 ± 0.23 c | 8.35 ± 0.62 c | 0.322 | 2.02 | 6.49 | 8.8 | <0.001 | 0.973 |
| Sourdough | LAB (CFU/g) | Yeast (CFU/g) | pH | TTA (mL 0.1 M NaOH) | Lactic Acid (mg/kg) | Acetic Acid (mg/kg) | FQ |
|---|---|---|---|---|---|---|---|
| Oat flour | 1.85 × 109 | 2.00 × 107 | 4.45 ± 0.05 a | 6.35 ± 0.41 c | 474.88 ± 2.57 b | 24.25 ± 0.65 a | 13.05 c |
| Oat flour and bran | 3.24 × 109 | 2.88 × 107 | 3.89 ± 0.18 c | 7.53 ± 0.22 a | 479.86 ± 2.47 b | 25.19 ± 0.65 a | 12.70 d |
| Barley flour | 2.82 × 109 | 3.06 × 107 | 4.03 ± 0.06 b | 5.86 ± 0.31 d | 582.77 ± 0.41 a | 12.54 ± 1.14 b | 30.98 a |
| Barley flour and bran | 2.26 × 109 | 3.81 × 107 | 3.85 ± 0.10 d | 7.00 ± 0.11 b | 481.50 ± 6.40 b | 12.20 ± 2.28 b | 26.31 b |
| Parameter/Dough | O-30-0 h | O-30-24 h | O-50-0 h | O-50-24 h | B-30-0 h | B-30-24 h | B-50-0 h | B-50-24 h |
|---|---|---|---|---|---|---|---|---|
| pH of dough | 4.72 ± 0.06 ab | 4.40 ± 0.06 b | 4.37 ± 0.06 b | 4.14 ± 0.05 b | 5.01 ± 0.16 a | 4.97 ± 0.24 a | 4.49 ± 0.03 b | 4.42 ± 0.05 b |
| weight loss (%) | NA * | 0.32 ± 0.06 a | NA | 0.36 ± 0.02 a | NA | 0.28 ± 0.04 a | NA | 0.34 ± 0.18 a |
| Parameter/ Bread Type | O-30-0 h | O-30-24 h | O-50-0 h | O-50-24 h | B-30-0 h | B-30-24 h | B-50-0 h | B-50-24 h | |
|---|---|---|---|---|---|---|---|---|---|
| Baking loss (%) | 17.18 ± 0.31 b | 18.76 ± 0.10 ab | 17.92 ± 0.30 b | 17.80 ± 0.84 b | 18.05 ± 0.01 b | 18.70 ± 0.22 ab | 18.85 ± 0.49 a | 20.23 ± 0.44 a | |
| Spread ratio | 4.66 ± 0.08 bc | 5.87 ± 0.07 ab | 4.52 ± 0.03 bc | 6.19 ± 0.44 a | 4.76 ± 0.01 c | 5.71 ± 0.16 a | 5.25 ± 0.43 bc | 6.11 ± 0.04 a | |
| Hardness (N) | 31.30 ± 2.19 bc | 28.58 ± 1.76 c | 34.15 ± 3.10 b | 28.72 ± 0.73 c | 37.35 ± 3.06 ab | 38.33 ± 2.27 a | 38.73 ± 2.09 ab | 33.27 ± 2.79 bc | |
| Cohesiveness | 0.78 ± 0.03 a | 0.70 ± 0.03 b | 0.75 ± 0.02 ab | 0.69 ± 0.02 b | 0.79 ± 0.02 a | 0.74 ± 0.05 ab | 0.77 ± 0.03 a | 0.70 ± 0.02 b | |
| Resilience | 0.75 ± 0.04 ab | 0.66 ± 0.04 c | 0.71 ± 0.02 b | 0.64 ± 0.04 c | 0.78 ± 0.04 a | 0.73 ± 0.04 ab | 0.74 ± 0.02 ab | 0.64 ± 0.01 c | |
| crust | L* | 46.36 ± 0.00 a | 39.12 ± 2.59 b | 43.90 ± 2.30 ab | 39.07 ± 2.60 b | 39.34 ± 0.00 b | 37.96 ± 0.58 b | 37.87 ± 1.70 b | 39.38 ± 1.08 b |
| a* | 11.46 ± 0.00 a | 5.72 ± 0.44 d | 10.29 ± 0.07 ab | 5.63 ± 0.21 d | 10.94 ± 0.00 ab | 6.38 ± 0.20 c | 9.76 ± 0.79 b | 5.42 ± 0.18 d | |
| b* | 28.62 ± 0.00 a | 21.45 ± 0.89 b | 27.31 ± 1.19 a | 21.81 ± 0.56 b | 22.58 ± 0.00 b | 22.02 ± 0.04 bc | 23.43 ± 0.16 b | 17.72 ± 1.03 c | |
| TCD | NA | 11.69 | NA | 8.68 | NA | 4.04 | NA | 7.35 | |
| crumb | L* | 52.52 ± 2.02 a | 49.38 ± 1.23 b | 50.43 ± 3.38 a | 47.64 ± 1.79 b | 41.31 ± 0.23 bc | 41.78 ± 0.04 bc | 40.00 ± 0.64 bc | 36.30 ± 0.57 c |
| a* | 0.36 ± 0.12 d | 0.64 ± 0.07 cd | 0.63 ± 0.14 d | 0.62 ± 0.11 c | 2.00 ± 0.01 b | 2.00 ± 0.08 b | 3.13 ± 0.02 a | 2.85 ± 0.11 a | |
| b* | 14.16 ± 1.20 a | 13.04 ± 0.71 ab | 14.75 ± 1.34 ab | 17.54 ± 4.43 ab | 12.29 ± 0.07 b | 13.87 ± 0.98 ab | 12.62 ± 0.79 b | 11.39 ± 0.56 b | |
| TCD | NA | 3.34 | NA | 3.95 | NA | 2.93 | NA | 3.91 | |
| Dry matter (g/100 g) | 53.67 ± 5.21 a | 53.75 ± 3.19 a | 53.69 ± 4.74 a | 51.04 ± 5.02 a | 55.82 ± 3.56 a | 55.68 ± 4.60 a | 55.56 ± 4.02 a | 57.35 ± 2.72 a | |
| Phytic acid (g/100 g d.w.) | 0.68 ± 0.02 b | 0.42 ± 0.00 c | 0.97 ± 0.02 a | 0.60 ± 0.03 bc | 0.59 ± 0.01 bc | 0.40 ± 0.02 c | 0.67 ± 0.00 b | 0.49 ± 0.00 c | |
| β-glucans (g/100 g d.w.) | 1.07 ± 0.01 b | 0.97 ± 0.00 c | 2.04 ± 0.05 a | 1.96 ± 0.01 a | 0.42 ± 0.01 e | 0.30 ± 0.00 f | 0.96 ± 0.00 c | 0.88 ± 0.01 d | |
| Dependent Variable | Flour Type | Sourdough Level | Retardation Time | Flour Type × Sourdough Level | Flour × Retardation Time | Sourdough Level × Retardation Time | Flour × Sourdough Level × Retardation Time |
|---|---|---|---|---|---|---|---|
| Specific volume | 0.000 * | 0.000 * | 0.000 * | 0.202 * | 0.291 | 0.011 * | 0.003 * |
| Baking loss | 0.001 * | 0.034 * | 0.003 * | 0.016 * | 0.513 | 0.275 | 0.019 * |
| Spread ratio | 0.227 | 0.049 * | 0.000 * | 0.164 | 0.049 * | 0.447 | 0.262 |
| Hardness | 0.000 * | 0.752 | 0.001 * | 0.026 | 0.066 | 0.001 * | 0.063 |
| Cohesiveness | 0.034 * | 0.006 * | <0.001 * | 0.336 | 0.578 | 0.950 | 0.236 |
| Resilience | <0.001 * | <0.001 * | <0.001 * | 0.031 * | 0.608 | 0.370 | 0.067 |
| L* of crust | 0.011 * | 0.161 | 0.027 * | 0.945 | 0.002 * | 0.468 | 0.530 |
| a* of crust | 0.751 | 0.000 * | 0.000 * | 0.039 * | 0.010 * | 0.521 | 0.040 * |
| b* of crust | 0.000 * | 0.045 * | 0.000 * | 0.232 | 0.008 * | 0.101 | 0.006 * |
| L* of crumb | 0.000 * | 0.005 * | 0.000 * | 0.043 * | 0.000 * | 0.024 * | 0.149 |
| a* of crumb | 0.000 * | 0.000 * | 0.002 * | 0.000 * | 0.000 * | 0.332 | 0.002 * |
| b* of crumb | 0.005 * | 0.054 | 0.036 * | 0.637 | 0.031 * | 0.476 | 0.018 * |
| Phytic acid | 0.000 * | 0.000 * | 0.000 * | 0.005 * | 0.008 * | 0.121 | 0.246 |
| β-glucans | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.050 | 0.008 * | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grgić, T.; Drakula, S.; Voučko, B.; Čukelj Mustač, N.; Novotni, D. Sourdough Fermentation of Oat and Barley Flour with Bran and Its Application in Flatbread Made with No-Time and Dough Retardation Methods. Fermentation 2024, 10, 174. https://doi.org/10.3390/fermentation10030174
Grgić T, Drakula S, Voučko B, Čukelj Mustač N, Novotni D. Sourdough Fermentation of Oat and Barley Flour with Bran and Its Application in Flatbread Made with No-Time and Dough Retardation Methods. Fermentation. 2024; 10(3):174. https://doi.org/10.3390/fermentation10030174
Chicago/Turabian StyleGrgić, Tomislava, Saša Drakula, Bojana Voučko, Nikolina Čukelj Mustač, and Dubravka Novotni. 2024. "Sourdough Fermentation of Oat and Barley Flour with Bran and Its Application in Flatbread Made with No-Time and Dough Retardation Methods" Fermentation 10, no. 3: 174. https://doi.org/10.3390/fermentation10030174
APA StyleGrgić, T., Drakula, S., Voučko, B., Čukelj Mustač, N., & Novotni, D. (2024). Sourdough Fermentation of Oat and Barley Flour with Bran and Its Application in Flatbread Made with No-Time and Dough Retardation Methods. Fermentation, 10(3), 174. https://doi.org/10.3390/fermentation10030174







