Electron Beam on Fermentation Medium as an Alternative Disinfection Method for Ethanol Distilleries: A Comprehensive Review
Abstract
1. Introduction
2. Contaminating Microorganisms
Effects of Contaminants on Wort
- Yeast cream with a lower yeast cell concentration;
- A larger volume of wine undesirably directed to acid treatment;
- A higher buffering capacity of the yeast cream due to dilution in a higher proportion of wine;
- A higher dose of acid required for acid treatment of the yeast cream;
- Reduced yeast cell viability during acid treatment;
- Increased cost of performing this acid treatment process.
3. Current Control Methods
4. Electron Beam
4.1. Electron Beam Application
- The possibility of applying a high dose rate, which allows a larger volume of wort to be treated per unit of time [54];
- Investment equivalent with costs to achieve the same treatment capacity as other techniques [81];
- Greater ease of licensing and acceptance by the community since radiation emission stops as soon as the electrical power supply is interrupted [15];
- Ease of operation and control of the electron beam [65];
- In the case of ethanol production in Brazil, the high energy demand can be met by the cogeneration.
4.2. Effects of Electron Beam on Wort Treatment
5. A Practical Example of Wort Irradiation
5.1. Materials and Irradiation
5.2. Main Findings
Contamination Control
6. Opportunities and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renewable Fuel Association World Fuel Ethanol Production by Region. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 4 November 2023).
- MME Scenarios for Ethanol Supply and Otto Cycle Demand—2018–2030. Available online: http://www.epe.gov.br/sites-pt/publicacoes-dados-abertos/publicacoes/PublicacoesArquivos/publicacao-255/topico-392/EPE-DPG-SGB-Bios-NT-01-2017-r0_Cenarios_de_Oferta_de_Etanol.pdf (accessed on 4 November 2023).
- Unica Cane’s Bioelectricity in Numbers-January 2017. Available online: http://www.siamig.com.br/uploads/a309a426211622d6594e960fa9202c12.pdf (accessed on 4 November 2023).
- Melo, M. Políticas Públicas Brasileiras de Biocombustíveis: Estudo Comparativo Entre os Programas de Incentivos à Produção, com Ênfase em Etanol e Biodiesel. Ph.D. Thesis, Universidade Federal de Uberlândia, Uberlândia, Brazil, 2018. [Google Scholar]
- EPA United States Environmental Protection Agency. Available online: https://www.epa.gov/renewable-fuel-standard-program/renewable-identification-numbers-rins-under-renewable-fuel-standard (accessed on 4 November 2023).
- MAPA Ministério da Agricultura, Pecuária e Abastecimento. Projeções do Agronegócio—Brasil 2020/21 a 2030/31, Projeções de Longo Prazo. Secretaria de Política Agrícola. SIRE/Embrapa. Available online: https://www.gov.br/agricultura/pt-br/assuntos/politica-agricola/todas-publicacoes-de-politica-agricola/projecoes-do-agronegocio/projecoes-do-agronegocio-2020-2021-a-2030-2031.pdf/view (accessed on 4 November 2023).
- IMEA Instituto Mato-Grossense de Economia Agropecuária. 2024. Available online: https://www.imea.com.br/imea-site/ (accessed on 23 January 2024).
- CONAB National Supply Company. Available online: https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras (accessed on 20 January 2024).
- Khullar, E.; Kent, A.D.; Leathers, T.D.; Bischoff, K.M.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Contamination Issues in a Continuous Ethanol Production Corn Wet Milling Facility. World J. Microbiol. Biotechnol. 2013, 29, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Beckner, M.; Ivey, M.L.; Phister, T.G. Microbial Contamination of Fuel Ethanol Fermentations: Bioethanol Contamination. Lett. Appl. Microbiol. 2011, 53, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.M.D.; Sica, P.; Pires, L.D.A.N.; Spironello, L.; Mota, L.A.; Peixoto, G.T.; Calegari, R.P.; Basso, T.O.; Tonso, A.; Gomes, M.P.; et al. Integration of Corn and Cane for Ethanol Production: Effects of Lactobacilli Contamination on Fermentative Parameters and Use of Ionizing Radiation Treatment for Disinfection. Fermentation 2023, 9, 89. [Google Scholar] [CrossRef]
- Day, W.H.; Serjak, W.C.; Stratton, J.R.; Stone, L. Contamination Inhibition, Antibiotics as Contamination-Control Agents in Grain Alcohol Fermentations. J. Agric. Food Chem. 1954, 2, 252–258. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hoff, R.B.; Molognoni, L.; Deolindo, C.T.P.; De Oliveira, T.; Mattos, J.L.S.; Oliveira, L.V.A.D.; Daguer, H. Residues of Antibiotics in Yeasts from Ethanol Production: A Possible Contamination Route for Feedingstuffs. J. Environ. Sci. Health Part B 2021, 56, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Douradinho, R.; Sica, P.; Tonoli, F.; Mattos, E.; Oliveira, M.; Pinto, A.; Mota, L.; Faria, T.; Costa, V.F.; Leite, G.; et al. Osmotic Stress Alleviation in Saccharomyces Cerevisiae for High Ethanol Fermentations with Different Wort Substrates. Stresses 2023, 3, 813–826. [Google Scholar] [CrossRef]
- Calegari, R.P.; Silva, E.A.D.; Silva, A.P.M.D.; Gomes, M.P.; Mota, L.A.; Arthur, V.; Baptista, A.S. Wort Disinfection Treatment with Electron Beam for Bioethanol Production. Sci. Agric. 2023, 80, e20210260. [Google Scholar] [CrossRef]
- Ceccato-Antonini, S.R. Conventional and Nonconventional Strategies for Controlling Bacterial Contamination in Fuel Ethanol Fermentations. World J. Microbiol. Biotechnol. 2018, 34, 80. [Google Scholar] [CrossRef]
- Stupiello, J.P.; Horii, J. Considerações Sobre Tratamentos de Caldo de Cana Para Fermentação Alcoólica. Álcool E Açúcar 1981, 3, 40–46. [Google Scholar]
- Lopes, J.J.; Pyle, L.D. Bioprocess Modelling, Simulation and Control for Ethanol Continuous Production in Brazil. Rev. Microbiol. 2005, 1, 14–15. [Google Scholar]
- Amorim, H.V.; Oliveira, A.J.; Campos, H. Infecção, Problema Sério Na Produção de Álcool. Congr. Nac. Soc. Téc. Açucar. Bras. 1981, 2, 158–168. [Google Scholar]
- Alterthum, F.; Cruz, M.R.M.; Vairo, M.L.R.; Gambassi, D.M. Efeito dos Microrganismos Contaminantes da Fermentação Alcoólica nas Microdestilarias. Açúcar Álcool E Subproduto 1984, 3, 42–49. [Google Scholar]
- Sica, P.; Prado, L.M.L.M.; Granja, P.; Carvalho, E.M.D.; Mattos, E.D.C.; Calegari, R.P.; Silverio, M.; Martins, B.C.; Baptista, A.S. Effects of Energy Cane (Saccharum spp.) Juice on Corn Ethanol (Zea mays) Fermentation Efficiency: Integration towards a More Sustainable Production. Fermentation 2021, 7, 30. [Google Scholar] [CrossRef]
- Skinner, K.A.; Leathers, T.D. Bacterial Contaminants of Fuel Ethanol Production. J. Ind. Microbiol. Biotechnol. 2004, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S. Post-Harvest Deterioration of Sugarcane. Sugar Tech 2009, 11, 109–123. [Google Scholar] [CrossRef]
- Lopes, M.L.; Paulillo, S.C.D.L.; Godoy, A.; Cherubin, R.A.; Lorenzi, M.S.; Giometti, F.H.C.; Bernardino, C.D.; Amorim Neto, H.B.D.; Amorim, H.V.D. Ethanol Production in Brazil: A Bridge between Science and Industry. Braz. J. Microbiol. 2016, 47, 64–76. [Google Scholar] [CrossRef]
- Bonatelli, M.L.; Ienczak, J.L.; Labate, C.A. Sugarcane Must Fed-Batch Fermentation by Saccharomyces Cerevisiae: Impact of Sterilized and Non-Sterilized Sugarcane Must. Antonie Van Leeuwenhoek 2019, 112, 1177–1187. [Google Scholar] [CrossRef]
- Frederick, M.K.B. Estudo Genético, Fisiológico e Molecular de Lactobacillus Fermentum Envolvidos na Floculação de Leveduras. Master’s Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 1994. [Google Scholar]
- Oliva-Neto, P.; Yokoya, F. Effects of Nutritional Factors on Growth of Lactobacillus Fermentum Mixed with Saccharomyces Cerevisiae in Alcoholic Fermentation. Rev. Microbiol. 1997, 28, 25–31. [Google Scholar]
- Amorim, H.V.; Lopes, M.L.; de Castro Oliveira, J.V.; Buckeridge, M.S.; Goldman, G.H. Scientific Challenges of Bioethanol Production in Brazil. Appl. Microbiol. Biotechnol. 2011, 91, 1267–1275. [Google Scholar] [CrossRef]
- Rose, A.H.; Harrison, J.S. The Yeasts, 2nd ed.; Academic Press: London, UK, 1993; ISBN 978-0-12-596415-9. [Google Scholar]
- Basso, L.C.; Basso, T.O.; Rocha, S.N. Ethanol Production in Brazil: The Industrial Process and Its Impact on Yeast Fermentation. In Biofuel Production-Recent Developments and Prospects; Dos Santos Bernardes, M.A., Ed.; InTech: Vienna, Austria, 2011; ISBN 978-953-307-478-8. [Google Scholar]
- Douradinho, R.; Sica, P.; Oliveira, M.; Uchoa Pinto, A.; Mota, L.; Mattos, E.; Perecin, D.; Garcilasso, V.; de Almeida, J.M.A.R.; Piedade, S.; et al. Assessing Ionizing Radiation and Chlorine Dioxide (ClO2) as Potential Aseptization Treatments for Yeast Recycling on Mixed Wort of Corn and Sugarcane in Brazil. Stresses 2024, 4, 155–171. [Google Scholar] [CrossRef]
- Neitzel, T.; Lima, C.S.; Biazi, L.E.; Collograi, K.C.; Carvalho Da Costa, A.; Vieira Dos Santos, L.; Ienczak, J.L. Impact of the Melle-Boinot Process on the Enhancement of Second-Generation Ethanol Production by Spathaspora Passalidarum. Renew. Energy 2020, 160, 1206–1216. [Google Scholar] [CrossRef]
- Nolasco Junior, J. Desenvolvimento de Processo Termico Otimizado Para Mosto de Caldo de Cana na Fermentação Alcoolica. Master’s Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2005. [Google Scholar]
- Brazzach, M.L.; Aquarone, E. Emprego de Desinfetantes Quimicos em Fermentacao de Mosto de Melaco de Cana. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1970. [Google Scholar]
- Basso, L.C.; De Amorim, H.V.; De Oliveira, A.J.; Lopes, M.L. Yeast Selection for Fuel Ethanol Production in Brazil. FEMS Yeast Res. 2008, 8, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Neto, J.M.; Covre, E.A.; Rosa, B.C.; Ceccato-Antonini, S.R. Can Ethanol Partially or Fully Replace Sulfuric Acid in the Acid Wash Step of Bioethanol Production to Fight Contamination by Lactobacillus Fermentum? Braz. J. Chem. Eng. 2020, 37, 323–332. [Google Scholar] [CrossRef]
- Caetano, A.C.G.; Madaleno, L.L. Controle de Contaminantes Bacterianos Na Fermentação Alcoólica Com a Aplicação de Biocidas Naturais. Ciência Tecnol. 2011, 2, 27–37. [Google Scholar]
- Brown, N.A.; de Castro, P.A.; de Castro Pimentel Figueiredo, B.; Savoldi, M.; Buckeridge, M.S.; Lopes, M.L.; de Lima Paullilo, S.C.; Borges, E.P.; Amorim, H.V.; Goldman, M.H.S.; et al. Transcriptional Profiling of Brazilian Saccharomyces cerevisiae Strains Selected for Semi-Continuous Fermentation of Sugarcane Must. FEMS Yeast Res. 2013, 13, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Lino, F.S.D.O.; Bajic, D.; Vila, J.C.C.; Sánchez, A.; Sommer, M.O.A. Complex Yeast–Bacteria Interactions Affect the Yield of Industrial Ethanol Fermentation. Nat. Commun. 2021, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.S.; Cruz, I.A.; Américo-Pinheiro, J.H.P.; Soriano, R.N.; de Souza, R.L.; Bilal, M.; Iqbal, H.M.N.; Bharagava, R.N.; Romanholo Ferreira, L.F. Interaction between Saccharomyces Cerevisiae and Lactobacillus Fermentum during Co-Culture Fermentation. Biocatal. Agric. Biotechnol. 2020, 29, 101756. [Google Scholar] [CrossRef]
- Dellias, M.D.T.F.; Borges, C.D.; Lopes, M.L.; da Cruz, S.H.; de Amorim, H.V.; Tsai, S.M. Biofilm Formation and Antimicrobial Sensitivity of Lactobacilli Contaminants from Sugarcane-Based Fuel Ethanol Fermentation. Antonie Van Leeuwenhoek 2018, 111, 1631–1644. [Google Scholar] [CrossRef]
- Saunders, L.P.; Bischoff, K.M.; Bowman, M.J.; Leathers, T.D. Inhibition of Lactobacillus Biofilm Growth in Fuel Ethanol Fermentations by Bacillus. Bioresour. Technol. 2019, 272, 156–161. [Google Scholar] [CrossRef]
- Muthaiyan, A.; Limayem, A.; Ricke, S.C. Antimicrobial Strategies for Limiting Bacterial Contaminants in Fuel Bioethanol Fermentations. Prog. Energy Combust. Sci. 2011, 37, 351–370. [Google Scholar] [CrossRef]
- Bischoff, K.M.; Zhang, Y.; Rich, J.O. Fate of Virginiamycin through the Fuel Ethanol Production Process. World J. Microbiol. Biotechnol. 2016, 32, 76. [Google Scholar] [CrossRef] [PubMed]
- Attri, S.; Goel, G. Sterilization in Bioprocesses. In Basic Biotechniques for Bioprocess and Bioentrepreneurship; Elsevier: Amsterdam, The Netherlands, 2023; pp. 329–339. ISBN 978-0-12-816109-8. [Google Scholar]
- Alcarde, A.R.; Walder, J.M.M.; Horii, J. Comparison Between Gamma Radiation and Kamoran HJ in the Decontamination of Sugarcane Must. J. Food Process. Preserv. 2001, 25, 137–147. [Google Scholar] [CrossRef]
- Roach, D.R.; Khatibi, P.A.; Bischoff, K.M.; Hughes, S.R.; Donovan, D.M. Bacteriophage-Encoded Lytic Enzymes Control Growth of Contaminating Lactobacillus Found in Fuel Ethanol Fermentations. Biotechnol. Biofuels 2013, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Brexó, R.P.; Sant’Ana, A.S. Impact and Significance of Microbial Contamination during Fermentation for Bioethanol Production. Renew. Sustain. Energy Rev. 2017, 73, 423–434. [Google Scholar] [CrossRef]
- Lagunas-Solar, M.C.; Matthews, S.M. Radionuclide and Electric Accelerator Sources for Food Irradiation. Radiat. Phys. Chem. 1977 1985, 25, 691–702. [Google Scholar] [CrossRef]
- Del Mastro, N.L. Development of Food Irradiation in Brazil. Prog. Nucl. Energy 1999, 35, 229–248. [Google Scholar] [CrossRef]
- Alcarde, A.R.; Walder, J.M.M.; Horii, J. Fermentation of Irradiated Sugarcane Must. Sci. Agric. 2003, 60, 677–681. [Google Scholar] [CrossRef]
- Acosta, S.; Lodos, J. The Action of Gamma Radiation on Sugar Cane as a New Sterilization Method. Sugar Azucar 1982, 77, 55–59. [Google Scholar]
- Podadera, P. Estudo das Propriedades do Açúcar Líquido Invertido Processado com Radiação Gama e Feixe de Elétrons. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Nobre, T.D.P.; Horii, J.; Alcarde, A.R. Viabilidade Celular de Saccharomyces Cerevisiae Cultivada Em Associação Com Bactérias Contaminantes Da Fermentação Alcoólica. Ciênc. E Tecnol. Aliment. 2007, 27, 20–25. [Google Scholar] [CrossRef]
- Douradinho, R.S. Aplicação de Feixe de Elétrons para Esterilização de Mosto Obtido a Partir da Hidrólise de Milho em Caldo de Cana Energia, Visando à Produção de Etanol. Ph.D. Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2023. [Google Scholar]
- Okuno, E. Efeitos Biológicos Das Radiações Ionizantes: Acidente Radiológico de Goiânia. Estud. Av. 2013, 27, 185–200. [Google Scholar] [CrossRef]
- Tauhata, L.; Salati, I.P.A.; Di Prinzio, R.; Di Prinzio, A. Radioproteção e Dosimetria: Fundamentos; Comissão Nacional de Energia Nuclear: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- CNEN. Glossário Do Setor Nuclear e Radiológico Brasileiro. Ph.D. Thesis, Comissão Nacional de Energia Nuclear, Rio de Janeiro, Brazil, 2021.
- Fellows, P.J. Tecnologia do Processamento de Alimentos: Princípios e Prática; Artmed: São Paulo, Brazil, 2018. [Google Scholar]
- Molins, R.A. (Ed.) Food Irradiation: Principles and Applications; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-35634-9. [Google Scholar]
- Nouailhetas, Y.; Almeida, C.E.B.; Pestana, S. Radiações Ionizantes e a Vida; National Comission of Nuclear Energy: Rio de Janeiro, Brazil, 2021.
- Soares, J.R. Desenvolvimento de Sistema Para Seguimento de Produto e Aquisição de Dados do Processo de Irradiação em Irradiadores de Grande Porte. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Capodaglio, A.G. Critical Perspective on Advanced Treatment Processes for Water and Wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Rela, P.R.; Sampa, M.H.O.; Duarte, C.L.; Costa, F.E.; Sciani, V. Development of an Up-Flow Irradiation Device for Electron Beam Wastewater Treatment. Radiat. Phys. Chem. 2000, 57, 657–660. [Google Scholar] [CrossRef]
- Jacovone, R.M.S. Estudo do comportamento eletroquímico do óxido de grafeno reduzido/Ni sintetizado por radiação ionizante. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- Miller, R.B. Electronic Irradiation of Foods: An Introduction to the Technology; Food engineering series; Springer: New York, NY, USA, 2005; ISBN 978-0-387-23784-8. [Google Scholar]
- Phillips, G.O. Radiation Chemistry of Carbohydrates. In Advances in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1962; Volume 16, pp. 13–58. ISBN 978-0-12-007216-3. [Google Scholar]
- Lung, H.-M.; Cheng, Y.-C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial Decontamination of Food by Electron Beam Irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- García Gómez-Tejedor, G.; Fuss, M.C. (Eds.) Radiation Damage in Biomolecular Systems; Biological and Medical Physics, Biomedical Engineering; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-2563-8. [Google Scholar]
- Van Gerwen, S.J.C.; Rombouts, F.M.; Riet, K.V.; Zwietering, M.H. A Data Analysis of the Irradiation Parameter D10 for Bacteria and Spores under Various Conditions. J. Food Prot. 1999, 62, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J. Irradiation for Better Foods. Trends Food Sci. Technol. 2006, 17, 148–152. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Wholesomeness of Irradiated Food: Report of a Joint FAO/IAEA/WHO Expert Committee [on the Wholesomeness of Irradiated Food; Geneva, 27 October–3 November 1980]; Technical report series/World Health Organization; World Health Organization: Geneva, Switzerland; World Food and Agricultural Organization: Rome, Italy; World International Atomic Energy Agency: Vienna, Austria, 1981; ISBN 978-92-4-120659-4.
- Crawford, L.M.; Ruff, E.H. A Review of the Safety of Cold Pasteurization through Irradiation. Food Control 1996, 7, 87–97. [Google Scholar] [CrossRef]
- IAEA The Radiation Chemistry of Polysaccharides; IAEA: Vienna, Austria, 2016; ISBN 978-92-0-101516-7.
- Scherz, H. Formation of Deoxycompounds and Malondialdehyde in Irradiated Aqueous Solutions of Carbohydrates and Related Compounds. Radiat. Res. 1970, 43, 12. [Google Scholar] [CrossRef]
- Diehl, J.F. Safety of Irradiated Foods; CRC Press: Boca Raton, FL, USA, 1995; ISBN 978-0-429-08184-2. [Google Scholar]
- Brazilian Sugarcane and Bioenergy Industry Association. Available online: https://unicadata.com.br/historico-de-producao-e-moagem (accessed on 4 November 2023).
- Yang, G.; Yin, Y.; Wang, J. Microbial Community Diversity during Fermentative Hydrogen Production Inoculating Various Pretreated Cultures. Int. J. Hydrogen Energy 2019, 44, 13147–13156. [Google Scholar] [CrossRef]
- IIA Electron Beam Technology. International Irradiation Association. Available online: www.iiaglobal.com (accessed on 4 November 2023).
- Lainetti, F.d.F. Desenvolvimento do Projeto Arquitetônico de uma Unidade Móvel de Irradiação do IPEN-CNEN/SP para o Tratamento de Efluentes Industriais. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2019. [Google Scholar]
- Lima, R.B.; de Aguiar, C.L.; Galaverna, R.; Baptista, A.S.; Eberlin, M.N.; Arthur, V. Sucrose and Color Profiles in Sugarcane (Saccharum sp.) Juice Analyzed by UFLC-ELSD and Synapt High-Definition Mass Spectrometry during Radiation Treatment. Radiat. Phys. Chem. 2016, 121, 99–105. [Google Scholar] [CrossRef]
- Chi, Z.; Zhao, X.; Daugaard, T.; Dalluge, D.; Rover, M.; Johnston, P.; Salazar, A.M.; Santoscoy, M.C.; Smith, R.; Brown, R.C.; et al. Comparison of Product Distribution, Content and Fermentability of Biomass in a Hybrid Thermochemical/Biological Processing Platform. Biomass Bioenergy 2019, 120, 107–116. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of Degradation Compounds from Lignocellulosic Biomass in the Biorefinery: Sugar Reaction Mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H.; Shibabe, S.; Ito, H. Gamma Irradiation on Fermentation Mashes Consisting Mainly of Cane Molasses. Agric. Biol. Chem. 1969, 33, 473–479. [Google Scholar] [CrossRef]
- Sica, P. Sugarcane Breeding for Enhanced Fiber and Its Impacts on Industrial Processes. In Sugarcane—Biotechnology for Biofuels; Sarwar Khan, M., Ed.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-935-2. [Google Scholar]
- Duncan, C.L.; Colmer, A.R. Coliforms Associated with Sugarcane Plants and Juices. Appl. Microbiol. 1964, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Souto, B.M.; Tupinambá, D.D.; Bergmann, J.C.; Kyaw, C.M.; Kruger, R.H.; Barreto, C.C.; Quirino, B.F. Microbial Diversity in Sugarcane Ethanol Production in a Brazilian Distillery Using a Culture-Independent Method. J. Ind. Microbiol. Biotechnol. 2015, 42, 73–84. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, W.L. (Ed.) Dosimetry for Radiation Processing; Taylor & Francis: London, UK; New York, NY, USA, 1989; ISBN 978-0-85066-740-0. [Google Scholar]
- Oliveira, A.J.; Gallo, C.R.; Alcarde, V.E.; Godoy, A.; Amorim, H.V. Métodos Para o Controle Microbiológico Na Produção de Álcool e Açúcar; University of São Paulo: Piracicaba, Brazil, 1996. [Google Scholar]
- Wang, M.; Han, J.; Dunn, J.B.; Cai, H.; Elgowainy, A. Well-to-Wheels Energy Use and Greenhouse Gas Emissions of Ethanol from Corn, Sugarcane and Cellulosic Biomass for US Use. Environ. Res. Lett. 2012, 7, 045905. [Google Scholar] [CrossRef]
- Khan, A.R.; Hoq, M.M. Lactic Acid Bacteria as Contaminant in Alcohol Fermentation. Bangladesh J. Microbiol. 1990, 7, 119–121. [Google Scholar]
- Borrely, S.I. Utilização da Radiação Ionizante na Esterilização de Produtos: Radioesterilização; Instituto de Pesquisas Energéticas e Nucleares—IPEN/CNEN/USP: São Paulo, Brazil, 2019. [Google Scholar]
- Jacques, K.A.; Lyons, T.P.; Kelsall, D.R. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries, 4th ed.; Nottingham University Press: Nottingham, UK, 2003; ISBN 978-1-897676-13-4. [Google Scholar]
- Oliva-Neto, P.; Yokoya, F. Evaluation of Bacterial Contamination in a Fed-Batch Alcoholic Fermentation Process. World J. Microbiol. Biotechnol. 1994, 10, 697–699. [Google Scholar] [CrossRef]
- Zeller, A.L.V.; Oliveira, A.J.; Zago, E.A. Conservação de xarope de cana-de-açúcar pelo emprego da radiação gama. STAB—Açúcar Álcool E Subprodutos Bras. 1984, 2, 29–36. [Google Scholar]
- Watanabe, H.; Sato, T. Changes in Content and Composition of Sugar in Molasses Caused by Gamma-Irradiation. J. Ferment. Technol. Jpn. 1980, 58, 363–366. [Google Scholar]
- Beyers, M., Brodrick, H.T., Van Niekerk, W.C.A., Eds.; National symposium on food irradiation. In Proceedings of the National Symposium on Food Irradiation, Pretoria, South Africa, 4–5 October 1979; Atomic Energy Board: Pretoria, South Africa, 1979. ISBN 978-0-86960-711-4. [Google Scholar]
- Oh, S.-H.; Lee, Y.-S.; Kim, J.-H.; Kim, J.-H.; Lee, J.-W.; Kim, M.R.; Yook, H.-S.; Byun, M.-W. Effect of pH on Non-Enzymatic Browning Reaction during γ-Irradiation Processing Using Sugar and Sugar–Glycine Solutions. Food Chem. 2006, 94, 420–427. [Google Scholar] [CrossRef]
- Division of Scientific and Technical Information, International Atomic Energy Agency. Training Manual on Food Irradiation Technology and Techniques, 2nd ed.; IAEA, Joint FAO IAEA Division of Atomic Energy in Food and Agriculture, Ed.; Documentation STI/DOC/10: Technical Reports Series; Internat. Atomic Energy Agency: Vienna, Austria, 1982; ISBN 978-92-0-115082-0. [Google Scholar]
- Castillo, G. Budget: Electron Accelerator. Application: Agroindustry. Available online: https://www.rosatom.ru/en/index.html (accessed on 20 October 2022).
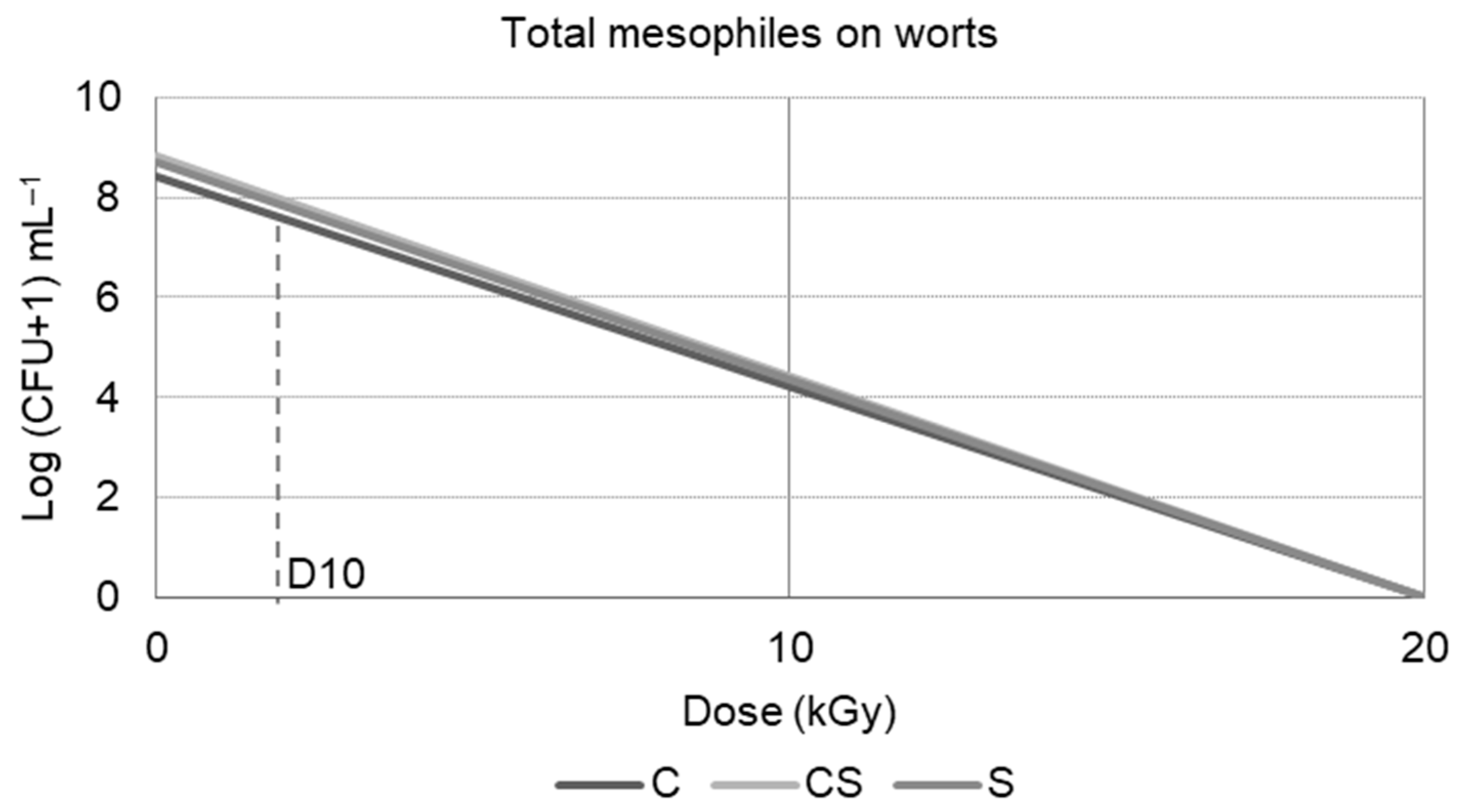
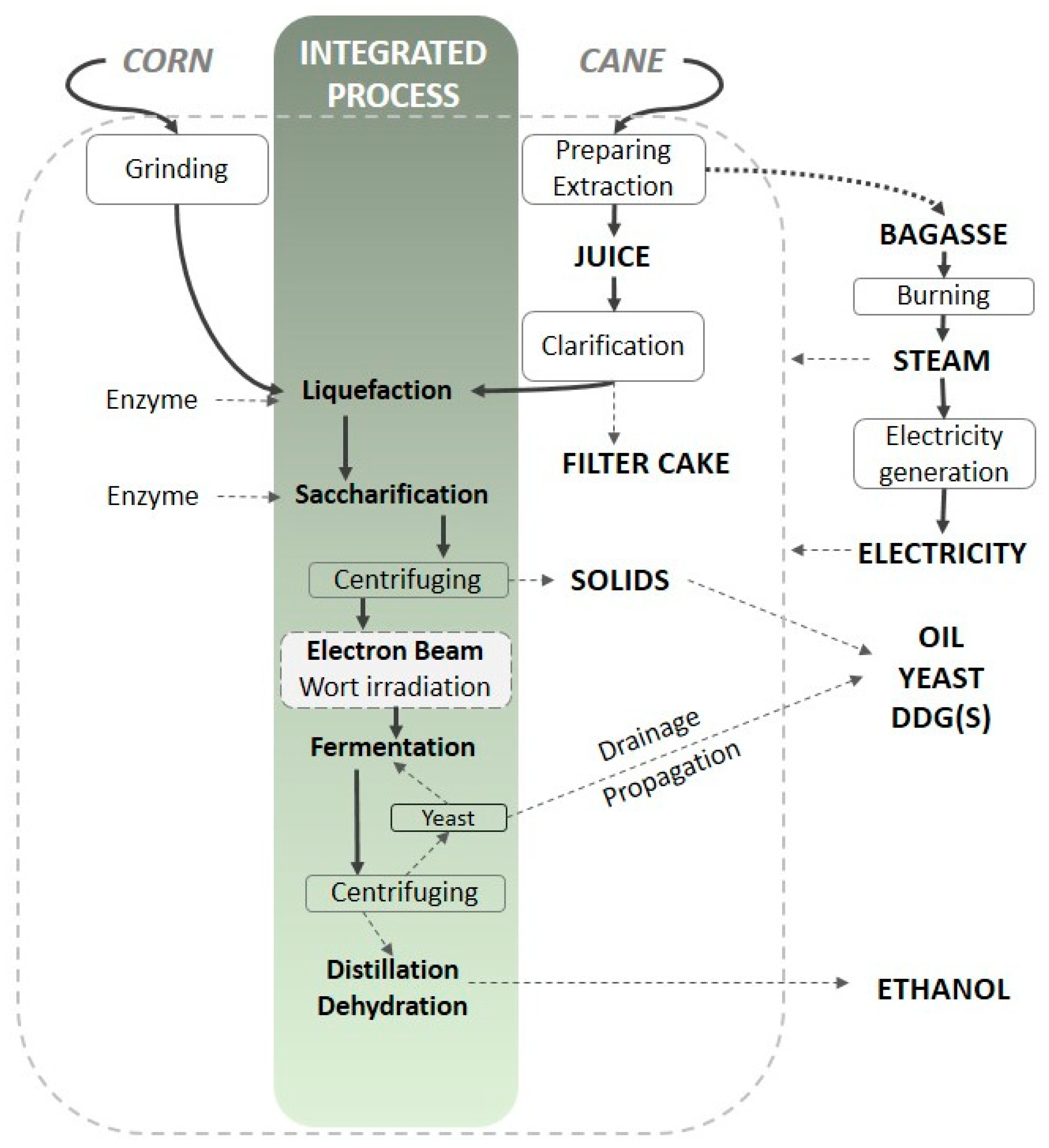
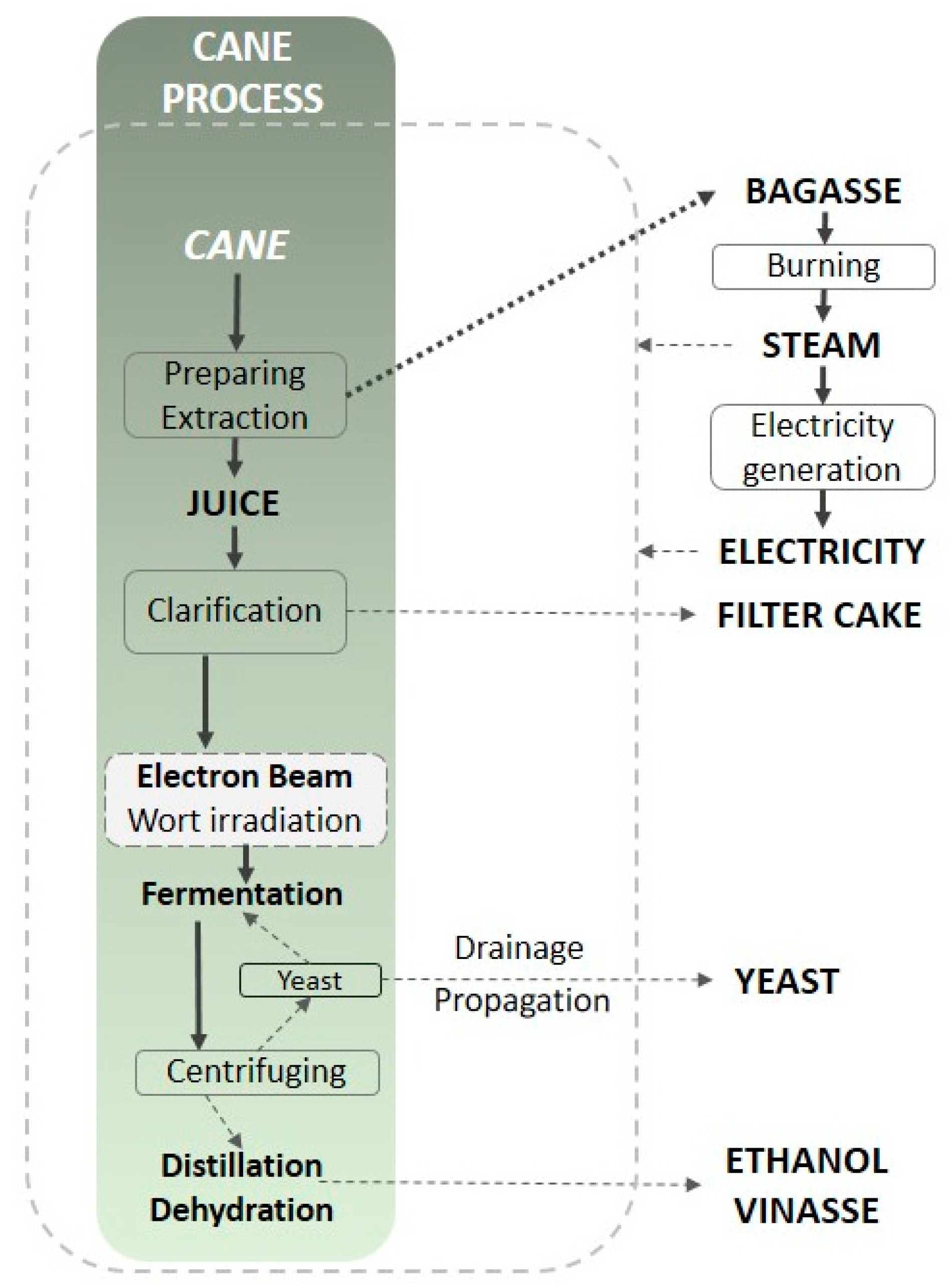

| Abbreviation | C0 | C20 | CS0 | CS20 | S0 | S20 |
|---|---|---|---|---|---|---|
| Description | Corn hydrolyzed in water with no radiation | Corn hydrolyzed in water irradiated with 20 kGy | Corn hydrolyzed in cane juice with no radiation | Corn hydrolyzed in cane juice irradiated with 20 kGy | Sugarcane juice with no radiation | Sugarcane juice irradiated with 20 kGy |
| Corn (g) | 639 | 639 | 0 | |||
| Water (mL) | 1000 | 0 | 0 | |||
| Juice (mL) | 0 | 1000 | 1000 | |||
| Fermentable sugars from juice (g L−1) | 0 | 58 | 304 | |||
| Fermentable sugars in wort (g L−1) | 302 | 342 | 304 | |||
| Treatment | Bacteria | Mesophilic | Fermentable Sugars | |
|---|---|---|---|---|
| Log (UFC+1) mL−1 | g L−1 | |||
| T1 | C0 | 8.5 ± 0.10 a | 8.4 ± 0.13 a | 298.7 ± 1.56 b |
| T2 | C20 | 0.0 ± 0.00 b | 0.0 ± 0.00 b | 298.8 ± 7.53 b |
| T3 | CS0 | 8.8 ± 0.09 a | 8.8 ± 0.05 a | 335.6 ± 1.04 a |
| T4 | CS20 | 0.0 ± 0.00 b | 0.0 ± 0.00 b | 335.3 ± 9.10 a |
| T5 | S0 | 8.7 ± 0.06 a | 8.7 ± 0.07 a | 298.1 ± 0.49 b |
| T6 | S20 | 0.0 ± 0.00 b | 0.0 ± 0.00 b | 298.3 ± 0.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douradinho, R.; Sica, P.; Perecin, D.; Oliveira, M.; Pinto, A.U.; Mota, L.; Mattos, E.; Almeida, J.M.D.; Piedade, S.; Arthur, V.; et al. Electron Beam on Fermentation Medium as an Alternative Disinfection Method for Ethanol Distilleries: A Comprehensive Review. Fermentation 2024, 10, 193. https://doi.org/10.3390/fermentation10040193
Douradinho R, Sica P, Perecin D, Oliveira M, Pinto AU, Mota L, Mattos E, Almeida JMD, Piedade S, Arthur V, et al. Electron Beam on Fermentation Medium as an Alternative Disinfection Method for Ethanol Distilleries: A Comprehensive Review. Fermentation. 2024; 10(4):193. https://doi.org/10.3390/fermentation10040193
Chicago/Turabian StyleDouradinho, Rafael, Pietro Sica, Danilo Perecin, Matheus Oliveira, Alana Uchoa Pinto, Layna Mota, Eduardo Mattos, João Monnerat De Almeida, Sonia Piedade, Valter Arthur, and et al. 2024. "Electron Beam on Fermentation Medium as an Alternative Disinfection Method for Ethanol Distilleries: A Comprehensive Review" Fermentation 10, no. 4: 193. https://doi.org/10.3390/fermentation10040193
APA StyleDouradinho, R., Sica, P., Perecin, D., Oliveira, M., Pinto, A. U., Mota, L., Mattos, E., Almeida, J. M. D., Piedade, S., Arthur, V., Horii, J., Coelho, S., & Baptista, A. (2024). Electron Beam on Fermentation Medium as an Alternative Disinfection Method for Ethanol Distilleries: A Comprehensive Review. Fermentation, 10(4), 193. https://doi.org/10.3390/fermentation10040193






