Anaerobic Conversion of Proteinogenic Amino Acids When Methanogenesis Is Inhibited: Carboxylic Acid Production from Single Amino Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Microbial Community Analyses
3. Results and Discussion
3.1. Characterization of Inoculum Sludge
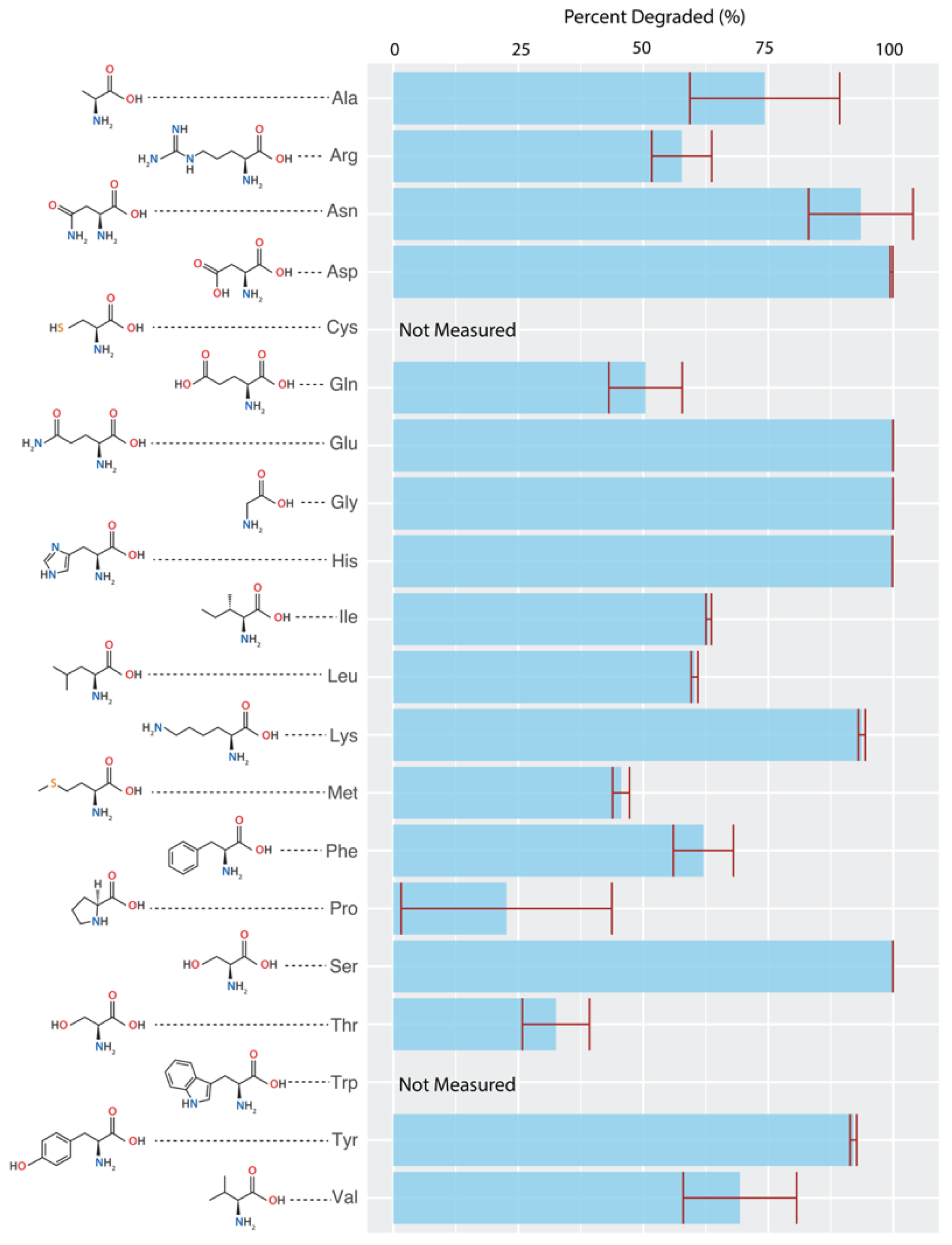
3.2. Amino Acid Degradation
3.3. Production of Volatile Fatty Acids
3.4. Microbial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Scarborough, M.J.; Lawson, C.E.; DeCola, A.C.; Gois, I.M. Microbiomes for sustainable biomanufacturing. Curr. Opin. Microbiol. 2022, 65, 8–14. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, B.; Dai, X.; Wang, H.; Li, N.; Yang, D. Effects of thermal hydrolysis on the metabolism of amino acids in sewage sludge in anaerobic digestion. Waste Manag. 2019, 88, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Ye, J.; Xu, H.; Zhu, Z.; Xu, T. Effects of different amino acids and their configurations on methane yield and biotransformation of intermediate metabolites during anaerobic digestion. J. Environ. Manag. 2021, 296, 113152. [Google Scholar] [CrossRef] [PubMed]
- Miholits, E.M.; Malina, J.F. Effects of Amino Acids on Anaerobic Digestion. J. (Water Pollut. Control Fed.) 1968, 40, R42–R53. [Google Scholar]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.; Plugge, C.M.; Strik, D.P.; Grootscholten, T.I.; Buisman, C.J.; Hamelers, H.V. Chain Elongation with Reactor Microbiomes: Open-Culture Biotechnology to Produce Biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Martos, J.-L.; Greses, S.; Magdalena, J.-A.; Iribarren, D.; Tomás-Pejó, E.; González-Fernández, C. Life cycle assessment of volatile fatty acids production from protein-and carbohydrate-rich organic wastes. Bioresour. Technol. 2021, 321, 124528. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Wang, S.; Ping, Q.; Li, Y. Comprehensively understanding metabolic pathways of protein during the anaerobic digestion of waste activated sludge. Chemosphere 2022, 297, 134117. [Google Scholar] [CrossRef]
- Sangavai, C.; Bharathi, M.; Ganesh, S.P.; Chellapandi, P. Kinetic modeling of Stickland reactions-coupled methanogenesis for a methanogenic culture. AMB Express 2019, 9, 82. [Google Scholar] [CrossRef]
- Barker, H. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 1981, 50, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Nisman, B. The stickland reaction. Bacteriol. Rev. 1954, 18, 16–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Zhang, X.; Huang, H.; Qin, Z.; Liu, C.; Chen, Y. Amino Acid Configuration Affects Volatile Fatty Acid Production during Proteinaceous Waste Valorization: Chemotaxis, Quorum Sensing, and Metabolism. Environ. Sci. Technol. 2022, 56, 8702–8711. [Google Scholar] [CrossRef]
- Regueira, A.; Lema, J.M.; Carballa, M.; Mauricio-Iglesias, M. Metabolic modeling for predicting VFA production from protein-rich substrates by mixed-culture fermentation. Biotechnol. Bioeng. 2020, 117, 73–84. [Google Scholar] [CrossRef]
- Salvador, A.F.; Cavaleiro, A.J.; Paulo, A.M.S.; Silva, S.A.; Guedes, A.P.; Pereira, M.A.; Stams, A.J.M.; Sousa, D.Z.; Alves, M.M. Inhibition Studies with 2-Bromoethanesulfonate Reveal a Novel Syntrophic Relationship in Anaerobic Oleate Degradation. Appl. Environ. Microbiol. 2019, 85, e01733-18. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Dobrowolski, J.C.; Rode, J.E.; Sadlej, J. Cysteine conformations revisited. Comput. Theor. Chem. 2007, 810, 129–134. [Google Scholar] [CrossRef]
- La Cour, R.; Jørgensen, H.; Schjoerring, J.K. Improvement of tryptophan analysis by liquid chromatography-single quadrupole mass spectrometry through the evaluation of multiple parameters. Front. Chem. 2019, 7, 797. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Barter, R.L.; Yu, B. Superheat: An R package for creating beautiful and extendable heatmaps for visualizing complex data. J. Comput. Graph. Stat. 2018, 27, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.R.; Pullammanappallil, P.C. Protein degradation during anaerobic wastewater treatment: Derivation of stoichiometry. Biodegradation 2001, 12, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Zepeda, A.; Durán, S.; Du Pont, G.; Calderón, J. Asparagine degradation in Rhizobium etli. Microbiology 1996, 142, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Barker, H.A. Two pathways of glutamate fermentation by anaerobic bacteria. J. Bacteriol. 1974, 117, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Andreesen, J.R.; Bahl, H.; Gottschalk, G. Introduction to the physiology and biochemistry of the genus Clostridium. In Clostridia; Springer: Boston, MA, USA, 1989; pp. 27–62. [Google Scholar]
- Scarborough, M.J.; Lynch, G.; Dickson, M.; McGee, M.; Donohue, T.J.; Noguera, D.R. Increasing the economic value of lignocellulosic stillage through medium-chain fatty acid production. Biotechnol. Biofuels 2018, 11, 200. [Google Scholar] [CrossRef]
- Mohiuddin, S.S.; Khattar, D. Biochemistry, Ammonia; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Stadtman, T.C.; White Jr, F. Tracer studies on ornithine, lysine, and formate metabolism in an amino acid fermenting Clostridium. J. Bacteriol. 1954, 67, 651–657. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef]
- Levine, U.Y.; Looft, T.; Allen, H.K.; Stanton, T.B. Butyrate-producing bacteria, including mucin degraders, from the swine intestinal tract. Appl. Environ. Microbiol. 2013, 79, 3879–3881. [Google Scholar] [CrossRef]
- Alföldi, L.; Raskó, I.; Kerekes, E. L-serine deaminase of Escherichia coli. J. Bacteriol. 1968, 96, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Borek, B.A.; Waelsch, H. The enzymatic degradation of histidine. J. Biol. Chem. 1953, 205, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Thauer, R.K. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Brindle, P.A.; Baker, F.C.; Tsai, L.W.; Reuter, C.C.; Schooley, D.A. Sources of Propionate for the Biogenesis of Ethyl-Branched Insect Juvenile Hormones: Role of Isoleucine and Valine. Proc. Natl. Acad. Sci. USA 1987, 84, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Menahan, L.; Schultz, L. Metabolism of leucine and valine within the rumen. J. Dairy. Sci. 1964, 47, 1080–1085. [Google Scholar] [CrossRef]
- Gagliano, M.; Braguglia, C.; Petruccioli, M.; Rossetti, S. Ecology and biotechnological potential of the thermophilic fermentative Coprothermobacter spp. FEMS Microbiol. Ecol. 2015, 91, fiv018. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Bize, A.; Guillot, A.; Monnet, V.; Madigou, C.; Chapleur, O.; Mazéas, L.; He, P.; Bouchez, T. Metaproteomics of cellulose methanisation under thermophilic conditions reveals a surprisingly high proteolytic activity. ISME J. 2014, 8, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Javier-Lopez, R.; Mandolini, E.; Dzhuraeva, M.; Bobodzhanova, K.; Birkeland, N.-K. Fervidobacterium pennivorans subsp. keratinolyticus subsp. nov., a Novel Feather-Degrading Anaerobic Thermophile. Microorganisms 2022, 11, 22. [Google Scholar] [CrossRef]
- Wu, B.; Wang, X.; Deng, Y.-Y.; He, X.-L.; Li, Z.-W.; Li, Q.; Qin, H.; Chen, J.-T.; He, M.-X.; Zhang, M. Adaption of microbial community during the start-up stage of a thermophilic anaerobic digester treating food waste. Biosci. Biotechnol. Biochem. 2016, 80, 2025–2032. [Google Scholar] [CrossRef]
- Ueki, A.; Goto, K.; Ohtaki, Y.; Kaku, N.; Ueki, K. Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int. J. Syst. Evol. Microbiol. 2017, 67, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Parera Olm, I.; Sousa, D.Z. Upgrading dilute ethanol to odd-chain carboxylic acids by a synthetic co-culture of Anaerotignum neopropionicum and Clostridium kluyveri. Biotechnol. Biofuels Bioprod. 2023, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Baena, S.; Fardeau, M.L.; Labat, M.; Ollivier, B.; Garcia, J.L.; Patel, B.K. Desulfovibrio aminophilus sp. nov., a novel amino acid degrading and sulfate reducing bacterium from an anaerobic dairy wastewater lagoon. Syst. Appl. Microbiol. 1998, 21, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Shetty, S.A.; Lagkouvardos, I.; Ritari, J.; Chamlagain, B.; Douillard, F.P.; Paulin, L.; Piironen, V.; Clavel, T.; Plugge, C.M.; et al. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ. Microbiol. Rep. 2016, 8, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Troise, A.D.; Nijsse, B.; Roviello, G.N.; Fogliano, V.; de Vos, W.M. Intestinimonas-like bacteria are important butyrate producers that utilize Nε-fructosyllysine and lysine in formula-fed infants and adults. J. Funct. Foods 2020, 70, 103974. [Google Scholar] [CrossRef]
- Wei, Z.; Ma, S.; Chen, R.; Wu, W.; Fan, H.; Dai, L.; Deng, Y. Aminipila luticellarii sp. nov., an anaerobic bacterium isolated from the pit mud of strong aromatic Chinese liquor, and emended description of the genus Aminipila. Int. J. Syst. Evol. Microbiol. 2021, 71, 004710. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas Jan, D. Ecology of Bacillaceae. Microbiol. Spectr. 2015, 3, TBS-0017-2013. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef]
- Iino, T.; Mori, K.; Tanaka, K.; Suzuki, K.I.; Harayama, S. Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese corbicula clam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1840–1845. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Badger, S.; Lai, C.-H.; Listgarten, M.A.; Visconti, R.A.; Socransky, S.S. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and Description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from Humans with Periodontal Disease. Int. J. Syst. Evol. Microbiol. 1981, 31, 432–445. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Kar Sajib, S. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Sirirote, P.; Watts, D.; Chulalaksananukul, S. Heavy metal resistant anaerobic bacterial strains from brewery digester sludge. Int. J. Glob. Warm. 2013, 5, 127–134. [Google Scholar] [CrossRef]




| Amino Acid | Chemical Name (As Added) | Concentration Added (g L−1) | Media pH |
|---|---|---|---|
| Alanine | L-alanine | 9.34 | 6.69 |
| Arginine | L-arginine | 9.90 | 9.68 |
| Asparagine | L-asparagine monohydrate | 15.63 | 7.10 |
| Aspartate | L-aspartic acid potassium salt | 17.83 | 6.88 |
| Cysteine | L-cysteine | 15.14 | 6.00 |
| Glutamine | L-glutamine | 10.15 | 6.78 |
| Glutamate | Sodium L-glutamate monohydrate | 12.98 | 6.98 |
| Glycine | Glycine | 15.64 | 6.20 |
| Histidine | L-histidine | 9.70 | 7.36 |
| Isoleucine | L-isoleucine | 5.46 | 6.28 |
| Leucine | L-leucine | 5.47 | 6.34 |
| Lysine | L-lysine monohydrochloride | 8.15 | 6.73 |
| Methionine | L-methionine | 8.48 | 7.07 |
| Phenylalanine | L-phenylalanine | 5.15 | 6.96 |
| Proline | L-proline | 6.54 | 7.34 |
| Serine | L-serine | 13.14 | 6.88 |
| Threonine | L-threonine | 9.31 | 6.90 |
| Tryptophan | L-tryptophan | 5.67 | 5.67 |
| Tyrosine | L-tyrosine | 5.96 | 5.96 |
| Valine | L-valine | 6.10 | 6.39 |
| TS (%) | VS (% of TS) | Soluble COD (mg L−1) | Soluble NH3-N (mg L−1) | pH | |
|---|---|---|---|---|---|
| Sludge 1 | 2.7 | 61 | 972 | 254 | 7.56 |
| Sludge 2 | 1.6 | 97 | 755 | 573 | 7.22 |
| Sludge 3 | 1.9 | 70 | 534 | 361 | 7.64 |
| Sludge 4 | 3.2 | 61 | 755 | 447 | 7.51 |
| Sludge 5 | 2.1 | 62 | 755 | 567 | 6.95 |
| Sludge 6 | 2.1 | 64 | 1080 | 505 | 7.25 |
| Average | 2.3 | 69 | 809 | 451 | 7.36 |
| Stdev | 0.6 | 14 | 192 | 125 | 0.26 |
| Amino Acid | Final Amino Acid Concentration (mg COD L−1) | Percent Conversion (%) 1 | |||
|---|---|---|---|---|---|
| Propionic Acid | Butyric Acid | Iso-Butyric Acid | Iso-Valeric Acid | ||
| Alanine | 2280 ± 1230 | 7.2 ± 0.83 | 1.9 ±0.40 | 0.08 ± 0.05 | 0.02 ± 0.01 |
| Arginine | 3900 ± 822 | 0.10 ± 0.13 | 3.7 ± 0.10 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| Asparagine | 571 ± 920 | 1.1 ± 0.17 | 1.9 ± 0.53 | 0.00 ± 0.00 | 0.01 ± 0.00 |
| Aspartate | 30.7 ± 19.4 | 0.06 ± 0.02 | 0.21 ± 0.11 | 0.01 ± 0.00 | 0.02 ± 0.01 |
| Cysteine | Not Measured | 0.03 ± 0.03 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Glutamine | 4660 ± 545 | 0.23 ± 0.11 | 3.3 ± 0.94 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| Glutamate | 1.46 ± 2.53 | 0.27 ± 0.17 | 9.5 ± 2.2 | 0.06 ± 0.07 | 0.06 ± 0.03 |
| Glycine | 3.93 ± 0.83 | 0.02 ± 0.02 | BDL 2 | 0.01 ± 0.01 | 0.03 ± 0.01 |
| Histidine | 14.0 ± 1.67 | 0.13 ± 0.12 | 5.3 ± 2.1 | 0.04 ± 0.03 | 0.03 ± 0.02 |
| Isoleucine | 3340 ± 97.5 | 0.04 ± 0.02 | 0.11 ± 0.05 | BDL 2 | BDL 2 |
| Leucine | 3570 ± 97.2 | 0.55 ± 0.18 | 0.09 ± 0.02 | 0.16 ± 0.08 | 6.1 ± 0.97 |
| Lysine | 559 ± 67.6 | 0.07 ± 0.04 | 16 ± 3.2 | 0.02 ± 0.01 | 0.05 ± 0.04 |
| Methionine | 4870 ± 63.3 | 2.4 ± 0.45 | 2.5 ± 0.32 | 0.00 ± 0.00 | 0.01 ± 0.01 |
| Phenylalanine | 3340 ± 492 | 0.70 ± 0.10 | 0.04 ± 0.07 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Proline | 6954 ± 1800 | 0.10 ± 0.07 | 0.17 ± 0.21 | 0.01 ± 0.01 | 0.03 ± 0.02 |
| Serine | 5.18 ± 1.53 | 0.79 ± 0.51 | 14 ± 1.3 | 0.02 ± 0.03 | 0.01 ± 0.01 |
| Threonine | 5980 ± 598 | 3.5 ± 1.6 | 1.8 ± 0.47 | 0.01 ± 0.00 | 0.04 ± 0.02 |
| Tryptophan | Not Measured | 0.03 ± 0.00 | 0.0 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Tyrosine | 744 ± 91.5 | 0.08 ± 0.03 | 0.67 ± 0.14 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Valine | 2740 ± 2370 | 0.48 ± 0.31 | 0.10 ± 0.09 | 1.9 ± 0.21 | 0.03 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conrado, L.; McCoy, J.; Rabinovich, L.; Davoudimehr, M.; Stamatopoulou, P.; Scarborough, M. Anaerobic Conversion of Proteinogenic Amino Acids When Methanogenesis Is Inhibited: Carboxylic Acid Production from Single Amino Acids. Fermentation 2024, 10, 237. https://doi.org/10.3390/fermentation10050237
Conrado L, McCoy J, Rabinovich L, Davoudimehr M, Stamatopoulou P, Scarborough M. Anaerobic Conversion of Proteinogenic Amino Acids When Methanogenesis Is Inhibited: Carboxylic Acid Production from Single Amino Acids. Fermentation. 2024; 10(5):237. https://doi.org/10.3390/fermentation10050237
Chicago/Turabian StyleConrado, Leandro, Jacob McCoy, Leo Rabinovich, Mona Davoudimehr, Panagiota Stamatopoulou, and Matthew Scarborough. 2024. "Anaerobic Conversion of Proteinogenic Amino Acids When Methanogenesis Is Inhibited: Carboxylic Acid Production from Single Amino Acids" Fermentation 10, no. 5: 237. https://doi.org/10.3390/fermentation10050237
APA StyleConrado, L., McCoy, J., Rabinovich, L., Davoudimehr, M., Stamatopoulou, P., & Scarborough, M. (2024). Anaerobic Conversion of Proteinogenic Amino Acids When Methanogenesis Is Inhibited: Carboxylic Acid Production from Single Amino Acids. Fermentation, 10(5), 237. https://doi.org/10.3390/fermentation10050237








