Unlocking the Potential of Mannosylerythritol Lipids: Properties and Industrial Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Mannosylerythritol Lipids: Structure, Properties, and Production
- (1)
- Using only hydrophobic carbon sources (such as soybean and rapeseed oil), leading to high titres of MELs (up to 150 g/L), but with low purity (ca. 60%) [28]; or
- (2)
- Using only hydrophilic carbon sources (such as glucose), which leads to high purity (~95%), but with low titres (ca. 6 g/L).
4. Mannosylerythritol Applications Described in the Literature
4.1. Biomedical/Pharmaceutical Industries
4.2. Personal Care and Cosmetics
4.3. Agriculture
4.4. Food and Feed Industry
4.5. Environmental Responses
4.6. Others
5. Current and Future Perspectives on MELs in the Market
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid. Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Thavasi, R. (Eds.) Microbial Biosurfactants and Their Environmental and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Precedence Research. Surfactants Market (By Type: Anionic Surfactants, Non-Ionic Surfactants, Cationic Surfactants, Amphoteric Surfactants, Others; By Origin: Synthetic Surfactants, Bio-based Surfactants; By Application: Home Care, Personal Care, Oilfield Chemicals, Food & Beverage, Agrochemicals, Textiles, Plastics, Industrial & Institutional Cleaning)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023–2032. Available online: https://www.precedenceresearch.com/surfactants-market (accessed on 28 February 2024).
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J. Clean. Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Madsen, T.; Boyd, H.B.; Nylén, D.; Pendersen, R.; Petersen, G.; Simonsen, F. Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products. Environ. Proj. 2001, 615, 1–35. [Google Scholar]
- United Nations. Sustainable Development Goals. Available online: https://www.undp.org/sustainable-development-goals (accessed on 28 February 2024).
- European Comission. REACH Regulation. Available online: https://environment.ec.europa.eu/topics/chemicals/reach-regulation_en (accessed on 28 February 2024).
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Krauter, J. Evonik and Unilever Team up for Large-Scale Production of World’s First “Green” Biosurfactant. Evonik. Published December 2019. Available online: https://household-care.evonik.com/en/news/press-releases/evonik-and-unilever-team-up-for-large-scale-production-of-worlds-first-green-biosurfactant-121470.html (accessed on 14 October 2023).
- Wesche, B. BASF Strengthens Its Position in Bio-Surfactants for Personal Care, Home Care and Industrial Formulators with Two Distinct Partnerships. BASF. Published March 2021. Available online: https://www.basf.com/vn/en/media/news-releases/global/2021/03/p-21-148.html (accessed on 14 October 2023).
- Stepan. Stepan Company Completes Acquisition of NatSurFact® Business from Logos Technologies. Published 27 March 2020. Available online: https://www.stepan.com/content/stepan-dot-com/en/news-events/news---events/stepan-company-completes-acquisition-of-natsurfact.html (accessed on 1 March 2024).
- KANEKA Biosurfactant–KANEKA Surfactin. Available online: https://www.kaneka.co.jp/en/business/qualityoflife/nbd_002.html (accessed on 21 April 2024).
- InventionBio. Products. Available online: https://inventionbio.pl/en/products/ (accessed on 21 April 2024).
- Arutchelvi, J.I.; Bhaduri, S.; Uppara, P.V.; Doble, M. Mannosylerythritol lipids: A review. J. Ind. Microbiol. Biotechnol. 2008, 35, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jeon, J.W.; Kim, S.B.; Oh, H.M.; Kwon, T.J.; Yoon, B.D. Surface and physico-chemical properties of a glycolipid biosurfactant, mannosylerythritol lipid, from Candida antarctica. Biotechnol. Lett. 2002, 24, 1637–1641. [Google Scholar] [CrossRef]
- Imura, T.; Ohta, N.; Inoue, K.; Yagi, N.; Negishi, H.; Yanagishita, H.; Kitamoto, D. Naturally engineered glycolipid biosurfactants leading to distinctive self-assembled structures. Chem. A Eur. J. 2006, 12, 2434–2440. [Google Scholar] [CrossRef]
- Fukuoka, T.; Yanagihara, T.; Imura, T.; Morita, T.; Sakai, H.; Abe, M.; Kitamoto, D. Enzymatic synthesis of a novel glycolipid biosurfactant, mannosylerythritol lipid-D and its aqueous phase behavior. Carbohydr. Res. 2011, 346, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Konishi, M.; Fukuoka, T.; Imura, T.; Yamamoto, S.; Kitagawa, M.; Sogabe, A.; Kitamoto, D. Identification of Pseudozyma graminicola CBS 10092 as a Producer of Glycolipid Biosurfactants, Mannosylerythritol Lipids. J. Oleo Sci. 2008, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Morita, T.; Konishi, M.; Imura, T.; Sakai, H.; Kitamoto, D. Structural characterization and surface-active properties of a new glycolipid biosurfactant, mono-acylated mannosylerythritol lipid, produced from glucose by Pseudozyma antarctica. Appl. Microbiol. Biotechnol. 2007, 76, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Hutton, S.; Lajarin-Cuesta, M.; Heaton, R.; Hargreaves, I.; Fracchia, L.; De Rienzo, M.A.D. Production of Mannosylerythritol Lipids (MELs) to be Used as Antimicrobial Agents Against S. aureus ATCC 6538. Curr. Microbiol. 2020, 77, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Guo, J.; Li, Z.; Xu, X.; Wang, J.; Zhai, L.; Liu, J.; Sun, G.; Wang, F.; Xu, Y.; et al. Screening and Research on Skin Barrier Damage Protective Efficacy of Different Mannosylerythritol Lipids. Molecules 2022, 27, 4648. [Google Scholar] [CrossRef]
- Feuser, P.E.; Coelho, A.L.S.; de Melo, M.E.; Scussel, R.; Carciofi, B.A.M.; Machado-de-Ávila, R.A.; de Andrade, C. Apoptosis Induction in Murine Melanoma (B16F10) Cells by Mannosylerythritol Lipids-B; a Glycolipid Biosurfactant with Antitumoral Activities. Appl. Biochem. Biotechnol. 2021, 193, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Glycolipid Biosurfactants, Mannosylerythritol Lipids, Show Antioxidant and Protective Effects against H2O2-Induced Oxidative Stress in Cultured Human Skin Fibroblasts. J. Oleo Sci. 2012, 61, 457–464. [Google Scholar] [CrossRef]
- Bae, I.; Lee, E.S.; Yoo, J.W.; Lee, S.H.; Ko, J.Y.; Kim, Y.J.; Lee, T.R.; Kim, D.; Lee, C.S. Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp. Dermatol. 2019, 28, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.-H.; Lee, S.H.; Oh, S.; Choi, H.; Marinho, P.A.; Yoo, J.W.; Ko, J.Y.; Lee, E.-S.; Lee, T.R.; Lee, C.S.; et al. Mannosylerythritol lipids ameliorate ultraviolet A-induced aquaporin-3 downregulation by suppressing c-Jun N-terminal kinase phosphorylation in cultured human keratinocytes. Korean J. Physiol. Pharmacol. 2019, 23, 113. [Google Scholar] [CrossRef]
- Keković, P.; Borges, M.; Faria, N.T.; Ferreira, F.C. Towards Mannosylerythritol Lipids (MELs) for Bioremediation: Effects of NaCl on M. antarcticus Physiology and Biosurfactant and Lipid Production; Ecotoxicity of MELs. J. Mar. Sci. Eng. 2022, 10, 1773. [Google Scholar] [CrossRef]
- Rau, U.; Nguyen, L.A.; Roeper, H.; Koch, H.; Lang, S. Fed-batch bioreactor production of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl. Microbiol. Biotechnol. 2005, 68, 607–613. [Google Scholar] [CrossRef]
- Faria, N.T.; Nascimento, M.F.; Ferreira, F.A.; Esteves, T.; Santos, M.V.; Ferreira, F.C. Substrates of Opposite Polarities and Downstream Processing for Efficient Production of the Biosurfactant Mannosylerythritol Lipids from Moesziomyces spp. Appl. Biochem. Biotechnol. 2023, 195, 6132–6149. [Google Scholar] [CrossRef]
- Faria, N.T.; Santos, M.V.; Fernandes, P.; Fonseca, L.L.; Fonseca, C.; Ferreira, F.C. Production of glycolipid biosurfactants, mannosylerythritol lipids, from pentoses and d-glucose/d-xylose mixtures by Pseudozyma yeast strains. Process Biochem. 2014, 49, 1790–1799. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Keković, P.; Ribeiro, I.A.C.; Faria, N.T.; Ferreira, F.C. Novel Organic Solvent Nanofiltration Approaches for Microbial Biosurfactants Downstream Processing. Membranes 2023, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Rau, U.; Nguyen, L.A.; Roeper, H.; Koch, H.; Lang, S. Downstream processing of mannosylerythritol lipids produced by Pseudozyma aphidis. Eur. J. Lipid Sci. Technol. 2005, 107, 373–380. [Google Scholar] [CrossRef]
- Kekovic, P.; Nascimento, M.; Faria, N.; Ferreira, F. Device, System and Process for the Enhanced Production of Mannosylerythritol Lipids (mels) Integrating Fermentation and Product Separation from Fermentation Broth by Non-Invasive Methods. WO Patent 2024019627, 19 July 2022. [Google Scholar]
- Kitamoto, D.; Yanagishita, H.; Shinbo, T.; Nakane, T.; Kamisawa, C.; Nakahara, T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Biotechnol. 1993, 29, 91–96. [Google Scholar] [CrossRef]
- Shu, Q.; Niu, Y.; Zhao, W.; Chen, Q. Antibacterial activity and mannosylerythritol lipids against vegetative cells and spores of Bacillus cereus. Food Control 2019, 106, 106711. [Google Scholar] [CrossRef]
- Shu, Q.; Wei, T.; Lu, H.; Niu, Y.; Chen, Q. Mannosylerythritol lipids: Dual inhibitory modes against Staphylococcus aureus through membrane-mediated apoptosis and biofilm disruption. Appl. Microbiol. Biotechnol. 2020, 104, 5053–5064. [Google Scholar] [CrossRef]
- Yamauchi, S.; Furukawa, M.; Kawahara, A.; Sugahara, T.; Yamamoto, S.; Kitabayashi, M.; Sogabe, A.; Shimoda, S.; Hata, E.; Watanabe, K.; et al. Roles of mannosylerythritol lipid-B components in antimicrobial activity against bovine mastitis-causing Staphylococcus aureus. World J. Microbiol. Biotechnol. 2022, 38, 54. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Pang, X.; Wu, Y.; Wu, Y.; Shu, Q.; Chen, Q.; Zhang, X. Synergistic antibacterial effect and mechanism of high hydrostatic pressure and mannosylerythritol Lipid-A on Listeria monocytogenes. Food Control 2022, 135, 108797. [Google Scholar] [CrossRef]
- Liu, X.; Shu, Q.; Chen, Q.; Pang, X.; Wu, Y.; Zhou, W.; Wu, Y.; Niu, J.; Zhang, X. Antibacterial Efficacy and Mechanism of Mannosylerythritol Lipids-A on Listeria monocytogenes. Molecules 2020, 25, 4857. [Google Scholar] [CrossRef]
- Dempster, C.; Marchant, R.; Banat, I.M. Antimicrobial Potential of Biosurfactants as a Novel Combination Therapy against Bacteria That Cause Skin Infections. 2019, 1. Available online: https://www.microbiologyresearch.org/content/journal/acmi/10.1099/acmi.ac2019.po0566 (accessed on 5 May 2024).
- Isoda, H.; Shinmoto, H.; Kitamoto, D.; Matsumura, M.; Nakahara, T. Differentiation of human promyelocytic leukemia cell line HL60 by microbial extracellular glycolipids. Lipids 1997, 32, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Isoda, H.; Kitamoto, D.; Shinmoto, H.; Matsumura, M.; Nakahara, T. Microbial Extracellular Glycolipid Induction of Differentiation and Inhibition of the Protein Kinase C Activity of Human Promyelocytic Leukemia Cell Line HL60. Biosci. Biotechnol. Biochem. 1997, 61, 609–614. [Google Scholar] [CrossRef]
- Isoda, H.; Nakahara, T. Mannosylerythritol lipid induces granulocytic differentiation and inhibits the tyrosine phosphorylation of human myelogenous leukemia cell line K562. Cytotechnology 1997, 25, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wakamatsu, Y.; Shibahara, M.; Nomura, N.; Geltinger, C.; Nakahara, T.; Murata, T.; Yokoyama, K.K. Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999, 59, 482–486. [Google Scholar]
- Morita, Y.; Tadokoro, S.; Sasai, M.; Kitamoto, D.; Hirashima, N. Biosurfactant mannosyl-erythritol lipid inhibits secretion of inflammatory mediators from RBL-2H3 cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Isoda, H.; Shinmoto, H.; Matsumura, M.; Nakahara, T. The Neurite-Initiating Effect of Microbial Extracellular Glycolipids in PC12 Cells. Cytotechnology 1999, 31, 165–172. [Google Scholar] [CrossRef]
- Shibahara, M.; Zhao, X.; Wakamatsu, Y.; Nomura, N.; Nakahara, T.; Jin, C.; Nagaso, H.; Murata, T.; Yokoyama, K.K. Mannosylerythritol Lipid Increases Levels of Galactoceramide in and Neurite Outgrowth from PC12 Pheochromocytoma Cells. Cytotechnology 2000, 33, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, S.; Hattori, Y.; Maitani, Y. Biosurfactant MEL-A enhances cellular association and gene transfection by cationic liposome. J. Control. Release 2006, 112, 362–368. [Google Scholar] [CrossRef]
- Ueno, Y.; Hirashima, N.; Inoh, Y.; Furuno, T.; Nakanishi, M. Characterization of Biosurfactant-Containing Liposomes and Their Efficiency for Gene Transfection. Biol. Pharm. Bull. 2007, 30, 169–172. [Google Scholar] [CrossRef]
- Inoh, Y.; Kitamoto, D.; Hirashima, N.; Nakanishi, M. Biosurfactants of MEL-A increase gene transfection mediated by cationic liposomes. Biochem. Biophys. Res. Commun. 2001, 289, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Inoh, Y.; Furuno, T.; Hirashima, N.; Kitamoto, D.; Nakanishi, M. Rapid delivery of small interfering RNA by biosurfactant MEL-A-containing liposomes. Biochem. Biophys. Res. Commun. 2011, 414, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Bakur, A.; Elshaarani, T.; Niu, Y.; Chen, Q. Comparative study of antidiabetic, bactericidal, and antitumor activities of MEL@AgNPs, MEL@ZnONPs, and Ag-ZnO/MEL/GA nanocomposites prepared by using MEL and gum arabic. RSC Adv. 2019, 9, 9745–9754. [Google Scholar] [CrossRef] [PubMed]
- Bakur, A.; Niu, Y.; Kuang, H.; Chen, Q. Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. AMB Express 2019, 9, 62. [Google Scholar] [CrossRef]
- Bakur, A.; Hongyun, L.; Elshaarani, T.; Albashir, D.; Mohammed, A.; Chen, Q. Antioxidant and Anticancer Properties of Biosynthesized GA/Ag-Fe3O4@ Nanocomposites. J. Clust. Sci. 2022, 33, 903–911. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, J.; Cheng, X.; Yang, Y.; Yu, Y.; Wang, L.; Dong, Q.; Chi, Z.; Liu, C. Cosmetic-Derived Mannosylerythritol Lipid-B-Phospholipid Nanoliposome: An Acid-Stabilized Carrier for Efficient Gastromucosal Delivery of Amoxicillin for In Vivo Treatment of Helicobacter pylori. ACS Omega 2022, 7, 29086–29099. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, J.; Cheng, X.; Yang, Y.; Yu, Y.; Wang, L.; Dong, Q.; Chi, Z.; Liu, C. Berberine-loaded mannosylerythritol lipid-B nanomicelles as drug delivery carriers for the treatment of Helicobacter pylori biofilms in vivo. Eur. J. Pharm. Biopharm. 2023, 193, 105–118. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Zhang, C.; Chi, Z.; Chi, Z.; Liu, G. Efficient production of mannosylerythritol lipids by a marine yeast Moesziomyces aphidis XM01 and their application as self-assembly nanomicelles. Mar. Life Sci. Technol. 2022, 4, 373–383. [Google Scholar] [CrossRef]
- Im, J.H.; Nakane, T.; Yanagishita, H.; Ikegami, T.; Kitamoto, D. Mannosylerythritol lipid, a yeast extracellular glycolipid, shows high binding affinity towards human immunoglobulin G. BMC Biotechnol. 2001, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Imura, T.; Fukuoka, T.; Morita, T.; Sakai, H.; Abe, M.; Kitamoto, D. Kinetic studies on the interactions between glycolipid biosurfactant assembled monolayers and various classes of immunoglobulins using surface plasmon resonance. Colloids Surf. B Biointerfaces 2007, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeong, E.S.; Kim, K.N.; Park, S.H.; Kim, J.W. Nanoemulsification of pseudo-ceramide by molecular association with mannosylerythritol lipid. Colloids Surf. B Biointerfaces 2014, 116, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, A.W.; Kanemaru, M.Y.S.; de Souza, F.G.; Duarte, M.C.T.; de Andrade, C.J.; Pastore, G.M. Enhanced antimicrobial and antioxidant capacity of Thymus vulgaris, Lippia sidoides, and Cymbopogon citratus emulsions when combined with mannosylerythritol a lipid biosurfactant. Food Res. Int. 2023, 163, 112213. [Google Scholar] [CrossRef]
- Kitagawa, M.; Nishimoto, K.; Tanaka, T. Cosmetic Pigments, Their Production Method, and Cosmetics Containing the Cosmetic Pigments. U.S. Patent 9,181,436, 10 November 2015. [Google Scholar]
- Morita, T.; Kitagawa, M.; Yamamoto, S.; Suzuki, M.; Sogabe, A.; Imura, T.; Fukuoka, T.; Kitamoto, D. Activation of Fibroblast and Papilla Cells by Glycolipid Biosurfactants, Mannosylerythritol Lipids. J. Oleo Sci. 2010, 59, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kitagawa, M.; Yamamoto, S.; Sogabe, A.; Imura, T.; Fukuoka, T.; Kitamoto, D. Glycolipid Biosurfactants, Mannosylerythritol Lipids, Repair the Damaged Hair. J. Oleo Sci. 2010, 59, 267–272. [Google Scholar] [CrossRef]
- Morita, T.; Kitagawa, M.; Suzuki, M.; Yamamoto, S.; Sogabe, A.; Yanagidani, S.; Imura, T.; Fukuoka, T.; Kitamoto, D. A Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, Shows Potential Moisturizing Activity toward Cultured Human Skin Cells: The Recovery Effect of MEL-A on the SDS-damaged Human Skin Cells. J. Oleo Sci. 2009, 58, 639–642. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morita, T.; Fukuoka, T.; Imura, T.; Yanagidani, S.; Sogabe, A.; Kitamoto, D.; Kitagawa, M. The Moisturizing Effects of Glycolipid Biosurfactants, Mannosylerythritol Lipids, on Human Skin. J. Oleo Sci. 2012, 61, 407–412. [Google Scholar] [CrossRef]
- Kondo, T.; Yasui, C.; Banno, T.; Asakura, K.; Fukuoka, T.; Ushimaru, K.; Koga, M.; Minamikawa, H.; Saika, A.; Morita, T.; et al. Self-Assembling Properties and Recovery Effects on Damaged Skin Cells of Chemically Synthesized Mannosylerythritol Lipids. ChemBioChem 2022, 23, e202100631. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, Y.; Tsukiji, H. Mannosylerythritol Lipid B Enhances the Skin Permeability of the Water-Soluble Compound Calcein via OH Stretching Vibration Changes. Colloids Interfaces 2020, 4, 10. [Google Scholar] [CrossRef]
- Mawani, J.S.; Mali, S.N.; Pratap, A.P. Formulation and evaluation of antidandruff shampoo using mannosylerythritol lipid (MEL) as a bio-surfactant. Tenside Surfactants Deterg. 2023, 60, 44–53. [Google Scholar] [CrossRef]
- Hua, Z.; Chen, Y.; Du, G.; Chen, J. Effects of biosurfactants produced by Candida antarctica on the biodegradation of petroleum compounds. World J. Microbiol. Biotechnol. 2004, 20, 25–29. [Google Scholar] [CrossRef]
- Kitamoto, D.; Ikegami, T.; Suzuki, G.T.; Sasaki, A.; Takeyama, Y.-I.; Idemoto, Y.; Koura, N.; Yanagishita, H. Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida antarctica). Biotechnol. Lett. 2001, 23, 1709–1714. [Google Scholar] [CrossRef]
- Farooq, U.; Szczybelski, A.; Ferreira, F.C.; Faria, N.T.; Netzer, R. A Novel Biosurfactant-Based Oil Spill Response Dispersant for Efficient Application under Temperate and Arctic Conditions. ACS Omega 2024, 9, 9503–9515. [Google Scholar] [CrossRef] [PubMed]
- Barreau, S.; Packet, D.; Couleon, P.; Fouquet, M. Petroleum Demulsifier. U.S. Patent 10,889,76, 12 January 2021. [Google Scholar]
- Fukuoka, T.; Shinozaki, Y.; Tsuchiya, W.; Suzuki, K.; Watanabe, T.; Yamazaki, T.; Kitamoto, D.; Kitamoto, H. Control of enzymatic degradation of biodegradable polymers by treatment with biosurfactants, mannosylerythritol lipids, derived from Pseudozyma spp. yeast strains. Appl. Microbiol. Biotechnol. 2016, 100, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Wang, Y.; Liu, X.L.; Zhou, J.Z.; Li, Y.H. Anthocyanin Nutrition Carrier and Preparation Method Thereof. China Patent 110934300, 31 March 2020. [Google Scholar]
- Fan, L.; Chen, Q.; Mairiyangu, Y.; Wang, Y.; Liu, X. Stable vesicle self-assembled from phospholipid and mannosylerythritol lipid and its application in encapsulating anthocyanins. Food Chem. 2021, 344, 128649. [Google Scholar] [CrossRef]
- Fan, L.; Xie, P.; Wang, Y.; Liu, X.; Li, Y.; Zhou, J. Influences of mannosylerythritol lipid-A on the self-assembling structure formation and functional properties of heat-induced β-lactoglobulin aggregates. Food Hydrocoll. 2019, 96, 310–321. [Google Scholar] [CrossRef]
- Shu, Q.; Wei, T.; Liu, X.; Liu, S.; Chen, Q. The dough-strengthening and spore-sterilizing effects of mannosylerythritol lipid-A in frozen dough and its application in bread making. Food Chem. 2022, 369, 131011. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gu, S.; Shi, Y.; Chen, Q. Alleviative effects of mannosylerythritol lipid-A on the deterioration of internal structure and quality in frozen dough and corresponding steamed bread. Food Chem. 2024, 431, 137122. [Google Scholar] [CrossRef]
- Fukuoka, T.; Shinozaki, Y.; Tsuchiya, W.; Suzuki, K.; Watanabe, T.; Yamazaki, T.; Kitamoto, D.; Kitamoto, H. Application of Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, as Agrospreaders. J. Oleo Sci. 2015, 64, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ga’al, H.; Yang, G.; Fouad, H.; Guo, M.; Mo, J. Mannosylerythritol Lipids Mediated Biosynthesis of Silver Nanoparticles: An Eco-friendly and Operative Approach against Chikungunya Vector Aedes albopictus. J. Clust. Sci. 2021, 32, 17–25. [Google Scholar] [CrossRef]
- Yoshida, S.; Koitabashi, M.; Nakamura, J.; Fukuoka, T.; Sakai, H.; Abe, M.; Kitamoto, D.; Kitamoto, H. Effects of biosurfactants, mannosylerythritol lipids, on the hydrophobicity of solid surfaces and infection behaviours of plant pathogenic fungi. J. Appl. Microbiol. 2015, 119, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.; Zorner, P.S.; Alibek, K.; Milovanovic, M.; Mazumder, S.; Dixon, T.; Fotsch, A. Materials and Methods for the control of Nematodes. WO Patent WO2018094075A1, 28 November 2019. [Google Scholar]
- Matosinhos, R.D.; Cesca, K.; Carciofi, B.A.M.; de Oliveira, D.; de Andrade, C.J. The Biosurfactants Mannosylerythritol Lipids (MELs) as Stimulant on the Germination of Lactuca sativa L. Agriculture 2023, 13, 1646. [Google Scholar] [CrossRef]
- Madihalli, C.; Sudhakar, H.; Doble, M. Mannosylerythritol Lipid-A as a Pour Point Depressant for Enhancing the Low-Temperature Fluidity of Biodiesel and Hydrocarbon Fuels. Energy Fuels 2016, 30, 4118–4125. [Google Scholar] [CrossRef]
- Ferreira, F.; Faria, N.; Fonseca, C. Production of Fuels from Microbial Glycolipids with Lipid Chains Comprising 6 to 14 Carbons. WO2015174870A1, 19 November 2015. [Google Scholar]
- José de Andrade, C.; Maria Pastore, G. Comparative study on microbial enhanced oil recovery using mannosylerithritol lipids and surfactin. Int. J. Sci. World 2016, 4, 69. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Jayamurthy, H.; Reddy, K.K.; Kanjilal, S.; Prasad, R.B.; Pandey, A. Studies on biosurfactants from Pseudozyma sp. NII 08165 and their potential application as laundry detergent additives. Biochem. Eng. J. 2013, 78, 85–92. [Google Scholar] [CrossRef]
- Hellmuth, H.; Bode, N.; Dreja, M.; Buhl, A. Detergent with Mannosylerythritol Lipid. Published Online 28 April 2016. Available online: https://patents.google.com/patent/DE102014221889A1/en (accessed on 26 October 2023).
- Kitamoto, D.; Yanagishita, H.; Endo, A.; Nakaiwa, M.; Nakane, T.; Akiya, T. Remarkable antiagglomeration effect of a yeast biosurfactant, diacylmannosylerythritol, on ice-water slurry for cold thermal storage. Biotechnol. Prog. 2001, 17, 362–365. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and Skin Function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Hwang, Y.K.; Sung-Ah, B.I.N.; Kim, Y.J.; Lee, J.H. Skin Whitening Composition Containing Mannosylerythritol Lipid. WO2018048127A1, 17 February 2021. [Google Scholar]
- Ito, S.; Suzuki, M.; Suzuki, K.; Kobayashi, Y. Feed Additive and Feed. U.S. Patent 20100249058, 30 September 2010. [Google Scholar]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Pandey, A. Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour. Technol. 2015, 191, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Fai, A.E.; Resende Simiqueli, A.P.; de Andrade, C.J.; Ghiselli, G.; Pastore, G.M. Optimized production of biosurfactant from Pseudozyma tsukubaensis using cassava wastewater and consecutive production of galactooligosaccharides: An integrated process. Biocatal. Agric. Biotechnol. 2015, 4, 535–542. [Google Scholar] [CrossRef]
- Mannosylerythritol Lipids Market, by End-Use Industries (Household Detergent (Laundry, Dish Wash, Personal Care & Cosmetics (Skincare, Hair Care), Pharmaceuticals, Food, Others)), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East & Africa)—Size, Share, Outlook, and Opportunity Analysis, 2023–2030. Coherent Market Insights. Published June 2023. Available online: https://www.coherentmarketinsights.com/market-insight/mannosylerythritol-lipids-market-3692 (accessed on 14 October 2023).
- Wu, L.-M.; Lai, L.; Lu, Q.; Mei, P.; Wang, Y.-Q.; Cheng, L.; Liu, Y. Comparative studies on the surface/interface properties and aggregation behavior of mono-rhamnolipid and di-rhamnolipid. Colloids Surf. B Biointerfaces 2019, 181, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.E.; Tian, F.; Malm, S.W.; Olivares, C.; Pacheco, R.P.; Simonich, M.T.; Hunjan, A.S.; Tanguay, R.L.; Klimecki, W.T.; Polt, R.; et al. Biodegradability and toxicity of monorhamnolipid biosurfactant diastereomers. J. Hazard. Mater. 2019, 364, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.; Nitschke, M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control 2013, 29, 138–142. [Google Scholar] [CrossRef]
- Dhar, P.; Havskjold, H.; Thornhill, M.; Roelants, S.; Soetaert, W.; Kota, H.R.; Chernyshova, I. Toward green flotation: Interaction of a sophorolipid biosurfactant with a copper sulfide. J. Colloid. Interface Sci. 2021, 585, 386–399. [Google Scholar] [CrossRef]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids Production by Candida bombicola ATCC 22214 and its Potential Application in Microbial Enhanced Oil Recovery. Front. Microbiol. 2015, 6, 1324. [Google Scholar] [CrossRef] [PubMed]
- Irata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Pala, M.; Castelein, M.G.; Dewaele, C.; Roelants, S.L.K.W.; Soetaert, W.K.; Stevens, C.V. Tuning the antimicrobial activity of microbial glycolipid biosurfactants through chemical modification. Front. Bioeng. Biotechnol. 2024, 12, 7185. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, Y.; Osman, M.; Nakahara, H.; Sano, Y.; Ishiguro, R.; Matsumoto, M. Significance of β-sheet formation for micellization and surface adsorption of surfactin. Colloids Surf. B Biointerfaces 1995, 4, 341–348. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.H. Characterization of Surfactin Produced by Bacillus subtilis Isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.V.; Silveira, E.; Pereira, B.B. Toxicity and applications of surfactin for health and environmental biotechnology. J. Toxicol. Environ. Health Part B 2018, 21, 382–399. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.W.F.; Cara, A.B.; Lechuga-Villena, M.; García-Román, M.; Melo, V.M.M.; Gonçalves, L.R.B.; Vaz, D.A. Aquatic toxicity and biodegradability of a surfactant produced by Bacillus subtilis ICA56. J. Environ. Sci. Health Part A 2017, 52, 174–181. [Google Scholar] [CrossRef]
- Mohd Isa, M.H.; Shamsudin, N.H.; Al-Shorgani, N.K.N.; Alsharjabi, F.A.; Kalil, M.S. Evaluation of antibacterial potential of biosurfactant produced by surfactin-producing Bacillus isolated from selected Malaysian fermented foods. Food Biotechnol. 2020, 34, 1–24. [Google Scholar] [CrossRef]
- DataPhysics Instruments GmbH. Determination of Critical Micelle Concentration with DataPhysics DCAT Series; DataPhysicsr: Filderstadt, Germany.
- Sigma-Aldrich. Safety Data Sheet Triton X-100; Sigma-Aldrich: St. Louis, MO, USA, 2024. [Google Scholar]
- Kitamoto, D.; Fuzishiro, T.; Yanagishita, H.; Nakane, T.; Nakahara, T. Production of mannosylerythritol lipids as biosurfactants by resting cells of Candida antarctica. Biotechnol. Lett. 1992, 14, 305–310. [Google Scholar] [CrossRef]
- Jarvis, F.G.; Johnson, M.J. A Glyco-lipide Produced by Pseudomonas Aeruginosa. J. Am. Chem. Soc. 1949, 71, 4124–4126. [Google Scholar] [CrossRef]
- Gorin, P.A.J.; Spencer, J.F.T.; Tulloch, A.P. Hydroxy Fatty Acid Glycosided of Sophorose from Torulopsis Magnoliae. Can. J. Chem. 1961, 39, 846–855. [Google Scholar] [CrossRef]
- Joshi, T. A Short History and Preamble of Surfactants. Int. J. Appl. Chem. 2017, 13, 283–292. [Google Scholar]
- Miao, Y.; To, M.H.; Siddiqui, M.A.; Wang, H.; Lodens, S.; Chopra, S.S.; Kaur, G.; Roelants, S.L.; Lin, C.S.K. Sustainable biosurfactant production from secondary feedstock—Recent advances, process optimization and perspectives. Front Chem. 2024, 12, 1327113. [Google Scholar] [CrossRef]
- Ashby, R.D.; McAloon, A.J.; Solaiman, D.K.Y.; Yee, W.C.; Reed, M. A process model for approximating the production costs of the fermentative synthesis of sophorolipids. J. Surfactants Deterg. 2013, 16, 683–691. [Google Scholar] [CrossRef]
- Lang, S.; Wullbrandt, D. Rhamnose lipids—Biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shen, L.; Yu, F.; Zhao, M.; Jin, M.; Deng, S.; Long, X. Enhanced fermentation of biosurfactant mannosylerythritol lipids on the pilot scale under efficient foam control with addition of soybean oil. Food Bioprod. Process. 2023, 138, 60–69. [Google Scholar] [CrossRef]
- Morita, T.; Konishi, M.; Fukuoka, T.; Imura, T.; Kitamoto, D. Microbial conversion of glycerol into glycolipid biosurfactants, mannosylerythritol lipids, by a basidiomycete yeast, Pseudozyma antarctica JCM 10317T. J. Biosci. Bioeng. 2007, 104, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.T.; Santos, M.; Ferreira, C.; Marques, S.; Ferreira, F.C.; Fonseca, C. Conversion of cellulosic materials into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma spp. under SHF and SSF processes. Microb. Cell Fact. 2014, 13, 155. [Google Scholar] [CrossRef]
- Mawani, J.; Jadhav, J.; Pratap, A. Fermentative Production of Mannosylerythritol Lipids using Sweetwater as Waste Substrate by Pseudozyma antarctica (MTCC 2706). Tenside Surfactants Deterg. 2021, 58, 246–258. [Google Scholar] [CrossRef]
- Andrade, C.J.; de Andrade, L.M.; de Rocco, S.A.; Sforça, M.L.; Pastore, G.M.; Jauregi, P. A novel approach for the production and purification of mannosylerythritol lipids (MEL) by Pseudozyma tsukubaensis using cassava wastewater as substrate. Sep. Purif. Technol. 2017, 180, 157–167. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Barreiros, R.; Oliveira, A.C.; Ferreira, F.C.; Faria, N.T. Moesziomyces spp. cultivation using cheese whey: New yeast extract-free media, β-galactosidase biosynthesis and mannosylerythritol lipids production. Biomass Convers. Biorefin. 2022, 14, 6783–6796. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wu, J.; Wang, W.; Chen, Q. Production and characterization of a new glycolipid, mannosylerythritol lipid, from waste cooking oil biotransformation by Pseudozyma aphidis ZJUDM34. Food Sci. Nutr. 2019, 7, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.F.; Coelho, T.; Reis, A.; Gouveia, L.; Faria, N.T.; Ferreira, F.C. Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach. Microorganisms 2022, 10, 2390. [Google Scholar] [CrossRef]
- Beck, A.; Werner, N.; Zibek, S. Mannosylerythritol Lipids: Biosynthesis, Genetics, and Production Strategies. In Biobased Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–167. [Google Scholar] [CrossRef]
- Roelants, S.; Solaiman, D.K.Y.; Ashby, R.D.; Lodens, S.; Van Renterghem, L.; Soetaert, W. Production and Applications of Sophorolipids. In Biobased Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–119. [Google Scholar] [CrossRef]
- Saika, A.; Koike, H.; Fukuoka, T.; Morita, T. Tailor-made mannosylerythritol lipids: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6877–6884. [Google Scholar] [CrossRef]
- Suh, S.J.; Invally, K.; Ju, L.K. Rhamnolipids: Pathways, Productivities, and Potential. In Biobased Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–203. [Google Scholar] [CrossRef]
- Shephard, J.J.; Callear, S.K.; Imberti, S.; Evans, J.S.O.; Salzmann, C.G. Microstructures of negative and positive azeotropes. Phys. Chem. Chem. Phys. 2016, 18, 19227–19235. [Google Scholar] [CrossRef] [PubMed]
- Gunther, M. Mikrobielle Synthese, Aufarbeitung, Modifizierung und Tensideigenschaften von Mannosylerythritollipinden und Cellobioselipiden; Berichte aus Forschung und Entwicklung IGB; Fraunhofer IGB: Stuttgart, Germany, 2015; Volume 66. [Google Scholar]
- Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M. Isolation and Analysis of Low Molecular Weight Microbial Glycolipids. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3705–3723. [Google Scholar] [CrossRef]
- Global Market Insights. Natural Cosmetics Market Size—By Product Type (Skin Care and Sun Care, Hair Care, Body Care, Men’s Grooming, Makeup, Fragrance), by Packaging Type, by Price Range, by Consumer Group, by Distribution Channel, & Global Forecast 2024–2032. Published December 2023. Available online: https://www.gminsights.com/industry-analysis/natural-cosmetics-market (accessed on 12 March 2024).
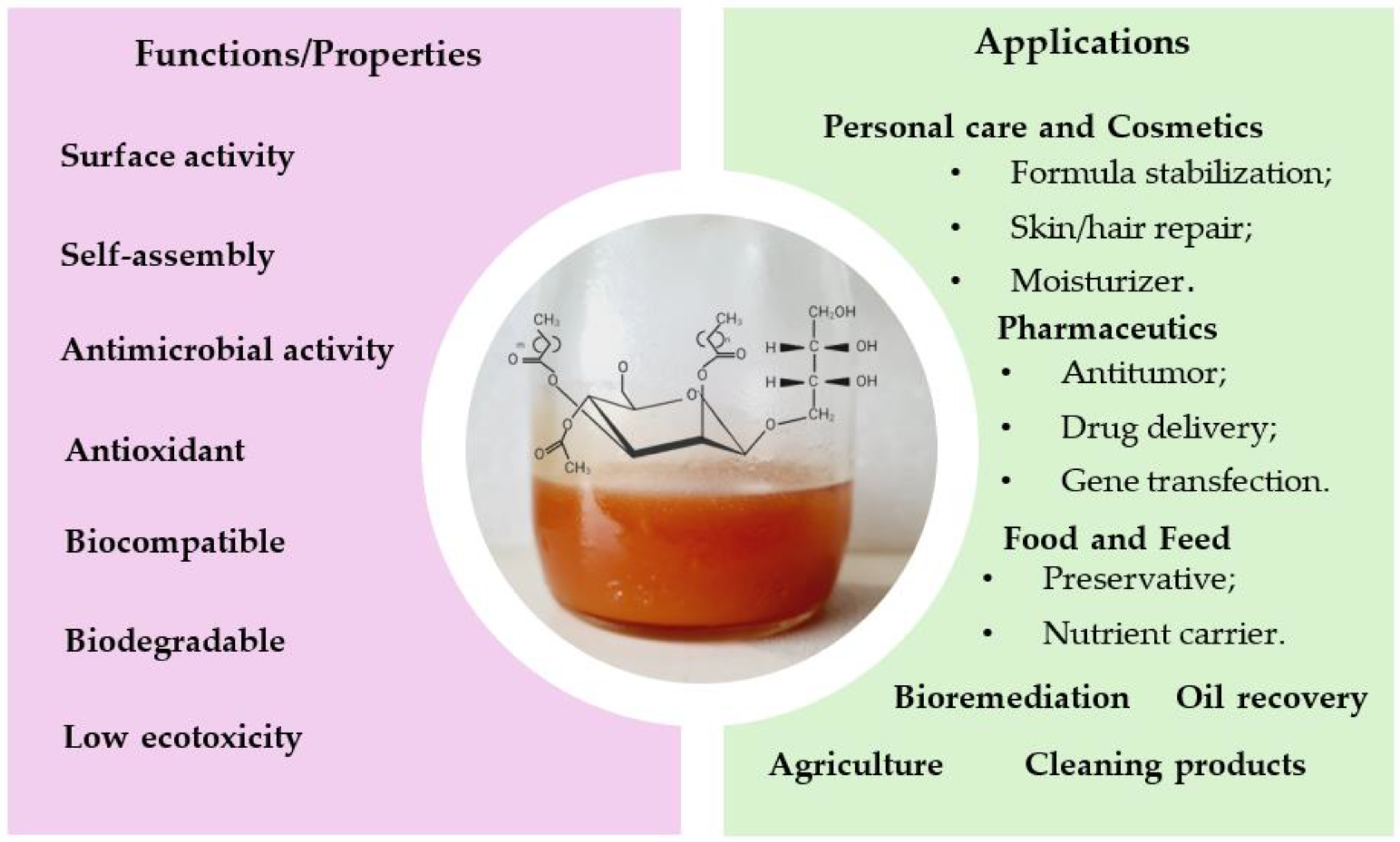
| Application Area | Specification | Brief Description of the Results | MELs Used | References | |||
|---|---|---|---|---|---|---|---|
| Biomedical/Pharmaceutics | Anti-microbial activity | ▪ Both MELs were strongly active against Gram-positive bacteria (Bacillus subtilis, Micrococcus luteus, Mycobacterium rhodoochrous, Staphylococcus aureus). | MIC (μg/mL) | MEL-A 99% and MEL-B 99% | [34] | ||
| MEL-A | MEL-B | ||||||
| B. subtilis | 6.2 | 2.5 | |||||
| M. luteus | 3.1 | 12.5 | |||||
| M. rhodoochrous | 25 | 25 | |||||
| S. aureus | 12.5 | 25 | |||||
| ▪ MELs had antimicrobial activity against S. aureus and biofilm disruption activity. | 500 | MEL-A, -B, -C and -D mixture | [21] | ||||
| ▪ MEL-A inhibited the germination of Bacillus cereus spores. | 1250 | MEL-A 80% | [35] | ||||
| ▪ MEL-A inhibited planktonic cells and biofilm of S. aureus. | 625 | MEL-A 80% | [36] | ||||
| ▪ MEL-B inhibited the growth of bovine mastitis causative S. aureus. | 10 | MEL-B | [37] | ||||
| ▪ MEL-A inhibited Listeria monocytogenes by damaging its cell membrane and morphology. Its combination with hydrostatic pressure led to a higher bactericidal effect than the hydrostatic pressure alone. | 32 | MEL-A 80% | [38,39] | ||||
| ▪ MELs inhibited the growth of E. coli and P. aeruginosa. The combination of MELs and antibiotics potentiated antibiotics’ efficiency. | E. coli | 300 | NA | [40] | |||
| P. aeruginosa | 75 | ||||||
| Antitumor | ▪ MELs induced the differentiation of human promyelocytic leukemia cells HL60 and inhibited protein kinase C activity. | MEL-A and -B | [41,42] | ||||
| ▪ MELs inhibited tyrosine kinase activity, inhibiting proliferation and inducing the differentiation of human myelogenous leukemia cells K562. | MEL mixture | [43] | |||||
| ▪ MEL-B reduced cell viability and induced death by apoptosis of B16F10 mouse melanoma cells. | MEL-B 95% Toyobo | [23] | |||||
| Biomedical/Pharmaceutics (continuation) | ▪ MELs stimulated tyrosinase activity and melanin production, leading to apoptosis and cell-differentiation of B16 mouse melanoma cells. | NA | [44] | ||||
| Anti- inflammatory | ▪ MELs inhibited the secretion of inflammatory mediators by rat basophilic leukemia RBL-2H3 cells (a mast cell line). | MEL-A and MEL-B | [45] | ||||
| Neural repair | ▪ MELs induced the outgrowth of neurites from and enhanced the activity of acetylcholinesterase in PC12 pheochromocytoma cells. | MEL-A | [46,47] | ||||
| Genetic material transfection or drug- carrying | ▪ MEL-A increased the efficiency of gene transfection by cationic liposomes with a cholesterol derivative or DC-Chol. | MEL-A | [48,49,50] | ||||
| ▪ MEL-A-containing cationic liposome was able to deliver siRNA rapidly and directly. | MEL-A | [51] | |||||
| ▪ MELs were used as stabilizing agents for silver and zinc oxide nanocomposites, gold nanoparticles and synthesis of silver and magnetic iron oxide nanocomposites, to be used in human liver cancer cell inhibition (HepG2). | NA | [52,53,54] | |||||
| ▪ Nanoliposomes made of soybean lecithin and cholesterol, when incorporated with MEL-B, had enhanced stability at pH 3–7 and delivered amoxicillin for Helicobacter pylori infection treatment in vivo. | MEL-B, Toyobo | [55] | |||||
| ▪ MEL-B nanomicelles successfully carried berberine for H. pylori biofilm disintegration and infection eradication. | MEL-B, Toyobo | [56] | |||||
| Drug delivery | ▪ Preparation of MEL nanomiceles for drug delivery (clarithromycin). It was shown that, by varying the pH, it is possible to control clarithromycin delivery (in 2 h, at pH 1.2 37.1% of the drug was delivered, while, at pH 7.4, only 9.7% was released). | MEL-A | [57] | ||||
| Immunoglobulin purification | ▪ MEL-A showed high binding affinity towards HIgG, HIgA and HIgM. | MEL-A | [58,59] | ||||
| Cosmetics and personal care | Formulation stabilization | ▪ Nanoemulsification of pseudo-ceramide was stabilized by molecular association with MELs. | Damy Chemicals | [60] | |||
| MELs stabilized the foaming, emulsification, and wetting properties of sodium lauryl sulphate. | MEL-B | [61] | |||||
| ▪ Coating cosmetic pigments (lip primer, foundation and sunscreen) with MELs enhanced their skin adhesion. | NA | [62] | |||||
| Skin whitening | ▪ MELs inhibited melanogenesis via suppressing ERK–CREB–MiTF–tyrosinase signalling in human melanocytes and a three-dimensional human skin equivalent. | MEL-B 85%, DK BIO | [25] | ||||
| Hair growth promotion | ▪ MEL-A produced from soybean oil increased cultured fibroblast cells and 3D human skin model cell viability and activated human papilla cells. | MEL-A 80.1% | [63] | ||||
| Cosmetics and personal care (continuation) | Damaged hair repair | ▪ MEL-A and MEL-B showed similar activity to ceramides for hair damage repair, and increased hair flexibility. | MEL-A 99% MEL-B 90% | [64] | |||
| Skin repair and moisturization | ▪ MELs ameliorated UVA-induced aquaporin-3 downregulation by suppressing c-Jun N-terminal kinase phosphorylation in cultured human keratinocytes. | MELs from DKBIO | [26] | ||||
| ▪ MEL-A had a recovery effect on SDS-damaged skin cells | MEL-A | [65] | |||||
| ▪ MEL-A and MEL-B produced with olive oil showed activities similar to natural ceramides on the cell viability of cultured human skin cells and repaired SDS-induced damage; MEL-B increased the water content in the stratum corneum and reduced water loss through perspiration. | MEL-A 100% MEL-B 100% | [66] | |||||
| ▪ MELs with carbon chains with 10 or more carbons exhibited better cell damage repair than a natural C18 ceramide, particularly MEL-D C10 (MELs purified by acetylation level and carbon chain size; see original paper) | Purified MELs | [67] | |||||
| ▪ MEL-B protected both HaCaT and 3D skin cell models from UVB- and SDS-induced damage by upregulating the expression of the key skin barrier damage-associated mRNA genes and proteins LOR, FLG, and TGM1. | Purified MEL-A, -B and -C | [22] | |||||
| ▪ MEL-B liposomes increased skin permeability to water-soluble compounds (calcein) in mice. | MEL-B, Toyobo | [68] | |||||
| Antioxidant | ▪ MEL-C had antioxidant activity through DPPH radical and superoxide anion scavenging and protection of cultured human fibroblast cells against H2O2-induced oxidative stress | MEL-C 80.7–92.5% | [24] | ||||
| Anti microbial | ▪ MELs had antimicrobial activity against Malassezia furfur, the yeast that causes dandruff. A shampoo formulated with MELs and SLS had increased anti-dandruff activity | NA | [69] | ||||
| Bioremediation/Environmental responses | Oil spills | ▪ MELs increased the bioavailability and biodegradation rate of n-alkanes, diesel, kerosene and crude oil (MEL mixture: 68% MEL-A, 28% -B and -C and 4% -D). | NA MEL mixture | [70,71,72] | |||
| ▪ Patent using MELs as petroleum demulsifier agents | NA | [73] | |||||
| Biodegradtion control | ▪ Biodegradation of an agricultural biodegradable plastic composed of poly(butylene succinate-co-adipate) by cutinase-like esterases and microorganisms was inhibited by MELs. | MEL-A, -B, and -C | [74] | ||||
| Food | Nutrient carriers | ▪ MEL-A was used in the formulation of a stable anthocyanin nutrient carrier. Compared with free anthocyanins, the encapsulated anthocyanins had higher retention rates when exposed to storage and simulated gastrointestinal fluid conditions; their antioxidant capacity after simulated intestinal digestion was enhanced. | MEL-A >95% | [75,76] | |||
| Food functionality | MEL-A reduced aggregation from β-lactoglobulin aggregates, creating microscale MEL-A-β-lg complexes. The foaming stability and emulsion properties were enhanced in the presence of MEL-A, improving food texture. | MEL-A | [77] | ||||
| Food (continuation) | Food preservation | ▪ MEL-A enhanced the rheological properties and water holding capacity of frozen dough, minimizing the freezable water content, while killing B. cereus cells and spores. | MIC (μg/mL) | MEL-A 80% | [35,78,79] | ||
| 1250 | |||||||
| ▪ Emulsification of essential oils (EO) (Thymus vulgaris, Lippia sidoides and Cymbopogon citratus) with MEL-B led to an enhancement of essential oils’ antioxidant activity and preservation of antimicrobial activity. | B. subtilis | Only MEL 500 | MEL + EO 120 | MEL-B | [61] | ||
| Penicillium sp. | 250 | 62.23 | |||||
| Agriculture | Agro-spreader | ▪ MELs were used as agrochemical spreader for biopesticides for hydrophobic plant surfaces (MEL mixture: 58% MEL-A, 25% MEL-B and 10% MEL-D). | MEL mixture | [80] | |||
| Wetting agent | ▪ MEL solutions showed good wetting ability on poorly wettable Gramineae plant surfaces. | MEL-A, -B, and -C | [80] | ||||
| Biocide | ▪ MEL-Ag nanoparticles displayed activity against mosquito larvae and pupae | MEL mixture | [81] | ||||
| Powdery mildew was suppressed on MEL-treated leaves. | MEL-A | [82] | |||||
| ▪ MELs, combined with other ingredients, were used for nematodes control. | NA | [83] | |||||
| ▪ MEL-B had a biostimulant and phytotoxic effect on lettuce plant germination and growth for given concentrations. | MEL-B 95% Toyobo | [84] | |||||
| Fuel additive | ▪ MEL-A enhanced the fluidity of fuels at low temperatures. | MEL-A | [85] | ||||
| Others | Jet biofuel | ▪ MELs were used as precursors for fuel with lipid chains comprising 6 to 14 carbons production. | NA | [86] | |||
| Enhanced oil recovery | ▪ MEL-B could create emulsions with heavy oils. | MEL-B | [87] | ||||
| Detergent | ▪ MELs had stability over wide pH and temperature ranges and improved detergent efficiency in removing stains from fabric in a proportion of 1:1 (w detergent/w MELs) | Crude MEL-A, -B and -C mixture | [88,89] | ||||
| Ice prevention | Suppression of agglomeration and growth of ice particles | MEL-A | [90] | ||||
| CMC/CAC (mM) | Surface Activity (mN/m) | Stability | Environmental Impact | Antimicrobial Activity | |||||
|---|---|---|---|---|---|---|---|---|---|
| pH | T (°C) | Salt (NaCl) | IC50 1 (mg/L) | Biodegradability | MIC 4 (μg/mL) | ||||
| MELs | -A | 0.004 [17] | 28.4 [17] | 4–10 [16] | Up to 90 [16] | Up to 100 mM (~0.6%) [16] | 999.95 [27] | Readily biodegradable [16] | 32 [39] |
| -B | 0.0045 [17] | 28.2 [17] | NA | ||||||
| Rhamnolipids | Mono- | 0.4 [97] | 27.4 [97] | 4–10 [97] | Up to 120 [97] | 50–1000 mM (~0.3–5.5%) [97] | 545.65 [27] | Readily biodegradable [98] | 78.1–2500 [99] |
| Di- | 0.46 [97] | 31.3 [97] | |||||||
| Sophorolipids | 0.04–0.4 [100] | 38.5–40 [100] | 2–12 [101] | Up to 100 [101] | Up to 10–13% [101] | 722.90 [27] | Readily biodegradable [102] | 470 [103] | |
| Surfactin | 0.0094 [104] | 30 [104] | 5–13 [105] | Up to 100 [105] | Up to 6% [105] | >500 2 [106] | Readily biodegradable [107] | 10 [108] | |
| Triton x-100 | 0.4 [109] | 30.6 [109] | NA | NA | NA | 26 3 [106] | Not readily biodegradable [110] | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, J.D.; Nascimento, M.F.; Keković, P.; Ferreira, F.C.; Faria, N.T. Unlocking the Potential of Mannosylerythritol Lipids: Properties and Industrial Applications. Fermentation 2024, 10, 246. https://doi.org/10.3390/fermentation10050246
de Almeida JD, Nascimento MF, Keković P, Ferreira FC, Faria NT. Unlocking the Potential of Mannosylerythritol Lipids: Properties and Industrial Applications. Fermentation. 2024; 10(5):246. https://doi.org/10.3390/fermentation10050246
Chicago/Turabian Stylede Almeida, Joana Dias, Miguel Figueiredo Nascimento, Petar Keković, Frederico Castelo Ferreira, and Nuno Torres Faria. 2024. "Unlocking the Potential of Mannosylerythritol Lipids: Properties and Industrial Applications" Fermentation 10, no. 5: 246. https://doi.org/10.3390/fermentation10050246
APA Stylede Almeida, J. D., Nascimento, M. F., Keković, P., Ferreira, F. C., & Faria, N. T. (2024). Unlocking the Potential of Mannosylerythritol Lipids: Properties and Industrial Applications. Fermentation, 10(5), 246. https://doi.org/10.3390/fermentation10050246






