Abstract
Due to its unique physicochemical properties, Pullulan is an exopolysaccharide with many applications in the food, biomedical, and pharmaceutical industries. Aiming to reduce its production cost, an interesting alternative is to consider other possibilities of raw materials, including the production of this biopolymer in a lignocellulosic biorefinery concept. Xylose is the main sugar of hemicellulosic hydrolysates obtained from different biomasses, and it is a sugar still not extensively exploited regarding its potential for pullulan production. This study aimed to evaluate the production of pullulan from sugarcane bagasse hemicellulosic hydrolysate by cultivating Aureobasidium pullulans ATCC 42023 in a bubble column reactor. The hemicellulosic hydrolysate was obtained through dilute acid treatment carried out in a stirred tank reactor before being detoxified to remove microbial growth inhibitors. The maximum concentration of 28.62 ± 1.43 g/L of pullulan was obtained after 120 h of fermentation in a bubble column reactor in batch mode. Analysis of spectroscopic properties through FTIR of the obtained pullulan revealed α-(1→6)-linked maltosyl units, similar to those of commercial samples of the biopolymer. XRD analysis showed that the prepared pullulan is amorphous, and a homogeneous morphology with a smooth surface of the pullulan was observed in SEM analysis. This study showed the potential of the production of pullulan from sugarcane bagasse hemicellulosic hydrolysate in a bubble column bioreactor, an alternative strategy for the industrial production of this biopolymer.
1. Introduction
The research focused on topics such as the reduction in the emissions of greenhouse gases and environmentally friendly technologies is fundamental to overcome current world challenges. In this context, linked to sustainable development, one important approach lies in deploying biorefineries to produce green products such as biofuels and biopolymers. Among biopolymers, pullulan has been mainly considered for its specific physicochemical and biological properties. It is a water-soluble homopolysaccharide that can be produced by fungi from renewable sources [1,2]. It is a non-hygroscopic, biodegradable, and biocompatible polymer composed of maltotriose units (three glucose units linked by α-(1→4) glycosidic bonds) linked by α-(1→6) glycosidic bonds [2,3,4].
Pullulan is of economic importance considering its highlighted properties: its non-mutagenicity, non-toxicity, resistance to oils, and edible and odorless nature. It is mainly used in the pharmaceutical (stabilizers, emulsifiers, gelling agents, water-binding agents, controlled release of drugs, and 3D/4Dprinting), chemical (coating, packaging, as sizing agent), and agri-food (starch substitute) industrial sectors [5,6]. In addition, pullulan has been recognized as a material of interest in tissue engineering [2]. This biopolymer is produced industrially under specific conditions through starch fermentation using wild strains of Aureobasidium pullulans, which are non-pathogenic and non-toxigenic microorganisms [7].
Despite its commercial importance and significant applications in various fields, the large-scale use of pullulan is limited [8]. The main bottleneck for commercial use is its high cost (USD 25–100/kg) compared to other biopolymers such as xanthan gum and dextran [9]. Various approaches have been adopted to reduce the cost of production of this biopolymer. Commercial-grade glucose, xylose, sucrose, mannose, galactose, and fructose have been mainly used as carbon sources for pullulan production at a relatively high cost [10]. Therefore, using alternative carbon sources that are inexpensive, renewable, and available in large quantities, such as agro-industrial waste and by-products, could be an exciting approach for the industrial production of pullulan.
Previous studies have successfully reported work carried out using by-products such as rice-hull-residue hydrolysate [11], potato starch hydrolysate [10,12], sugarcane bagasse enzymatic hydrolysate [3], and sesame seed oil cake [9] as carbon sources for pullulan production.
Sugarcane cultivation is one of the main agricultural activities in Brazil. Its annual production was estimated at 652.9 million tons in 2023 [13]. Sugarcane bagasse, an agro-industrial by-product of the sucro-alcohol sector, has been used as a source of energy and biofuel production, but also as a potential raw material for different value-added bioproducts, due to its composition, which is rich in cellulose, hemicellulose, and lignin. However, to date, the use of this resource for the production of pullulan has not been widely explored.
The production of pullulan from sugarcane bagasse can be an interesting alternative to improve the economic viability of biorefineries, mainly considering the use of a hemicellulosic fraction of the raw material. Sugarcane bagasse hemicellulosic hydrolysate has been scarcely evaluated for pullulan production, with a previous work in the literature having reported the use of a UV mutant of A. pullulans cultured in a xylose-rich hydrolysate, reaching the production of 12.65 g/L of pullulan in a bench scale stirred tank biorector, in 7 days of the process [14].
Besides the raw material, the optimization of operational parameters and the selection of the kind of bioreactor are important for the economic production of biopolymers. Bench studies in bioreactors are fundamental to evaluate new options of the process with potential for industrial-scale production. Among the options, bubble column bioreactors are simple and cheap systems scarcely evaluated for pullulan production. In a previous work, Hilares et al. (2019) [3] evaluated a bubble column reactor to produce pullulan from an enzymatic hydrolysate of sugarcane bagasse (from all carbohydrate fractions, cellulose, and hemicellulose), resulting in the production of 25.19 g/L of pullulan in 96 h of the process.
The current investigation aimed to explore the potential of the hemicellulosic fraction of sugarcane bagasse to produce pullulan through the cultivation of Aureobasidium pullulans ATCC 42023. The experiments were initially established in Erlenmeyer flasks and then applied to a bubble column reactor operating in batch. This was the first successful application of Aureobasidium pullulans ATCC 42023 in the production of pullulan from sugarcane bagasse hemicellulosic hydrolysate in a bubble column reactor.
2. Materials and Methods
2.1. Raw Material
Sugarcane bagasse was kindly donated by the company Ipiranga Agroindustrial LTDA (Descalvado, São Paulo, Brazil). It was first dried under sunlight until it reached water content lower than 10% to be stored away from light and humidity at room temperature. Its composition, analyzed as per Mesquita et al. (2016) [15], corresponded to 40.35 ± 3.45% cellulose (glucan content), 27.94 ± 2.68% hemicellulose (content of xylan + arabinosyl + acetyl), 26.26 ± 0.62% lignin, and 5.45 ± 0.16% extractives.
2.2. Hemicellulosic Hydrolysate Preparation
The preparation of hemicellulosic hydrolysate was carried out in a stainless-steel stirred tank reactor with a capacity of 80 L. A quantity of 3 kg of previously dried sugarcane bagasse was brought into contact with 30 L of sulfuric acid solution 1% in the reactor for 20 min at 120 °C with stirring at 80 min−1. The relationship between the duration of pretreatment and the temperature in the reactor, also called severity factor (SF) (Equation (1)), was determined through logarithmic extrapolation of the ordinate of the reaction, as reported by Tengborg et al. [16] (Equation (2)). The severity concept equation can be transformed to obtain the combined severity factor (CSF) by including pH [17] (Equation (3)).
Here, R = severity, T = reaction temperature (°C), T0 = reference temperature (100 °C), and t = retention time (min).
The suspension thus obtained was then concentrated under vacuum at 75 °C in order to obtain an approximate hemicellulosic hydrolysate concentration of 142 g/L of xylose. The concentrated hydrolysate obtained was detoxified to eliminate toxic compounds according to Mussatto and Roberto [18]. In this way, the hydrolysate was over-titrated with a sodium hydroxide solution up to pH 10, then the pH was reduced to 7 by adding phosphoric acid. In each case, the precipitate was removed via centrifugation and vacuum-filtrated. Next, an adsorption process was carried out by mixing 300 mL of hydrolysate in a 500 mL Erlenmeyer flask with activated carbon in a 1/40 (w/v) ratio. The mixture obtained was stirred in a shaker at a speed of 200 min−1 for a contact time of one hour at 40 °C. The mixture obtained at equilibrium was centrifuged before being filtered. The liquid obtained was autoclaved before being stored in the refrigerator at 4 °C for future use.
2.3. Microorganism and Inoculum Preparation
The strain of A. pullulans ATCC 42023 was stored at 4 °C in Potato Dextrose Agar in the Laboratory of Biopolymers, Bioreactors and Simulation of Processes of the Engineering School of Lorena, University of Sao Paulo.
The inoculum was prepared in 125 mL Erlenmeyer flasks containing 50 mL of medium composed of 35 g/L of xylose, 0.6 g/L of (NH4)2SO4, 1 g/L of NaCl, 0.1 g/L MgSO4, 1 g/L K2HPO4, and 2g/L of yeast extract. The medium components were previously autoclaved for 15 min at a temperature of 120 °C. Then, a loopful of a slant storage culture was transferred into the Erlenmeyer flasks, which were incubated with continuous stirring at a speed of 200 min−1 at a temperature of 28 °C for 48 h. After, the cells obtained were centrifuged at 2000× g for 10 min at a temperature of 25 °C. The suspension was washed twice with distilled water to obtain an initial concentration of 1 g/L in the fermentation medium. In this way, a sample of the cell suspension was taken, and cell concentration was determined through optical density measurements at 600 nm, related with dry biomass through a previously prepared curve.
Figure 1 shows the morphology of the fungus A. pullulans after two days of growth, observed under an optical microscope.
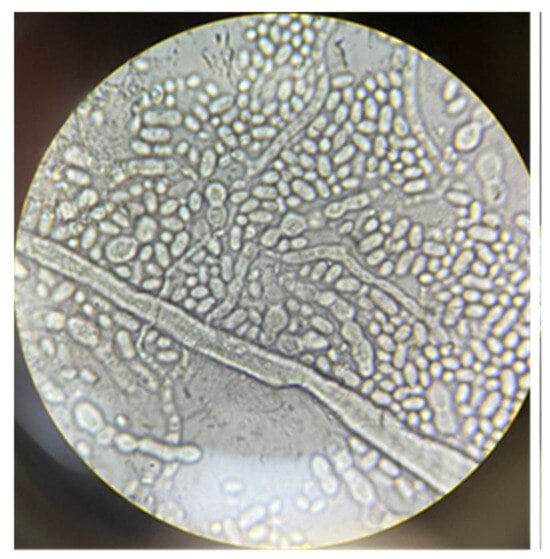
Figure 1.
Morphology of the A. pullulans after two days of growth (400× magnification) in medium and fermentation conditions.
Fermentations were performed using a medium based on commercial purified monomeric sugars (xylose and glucose) or on hemicellulosic hydrolysate as a carbon source. For the medium prepared with hydrolysate, the composition was as follows: 107 g/L of Xylose, 8.5 g/L of glucose, 3 g/L of NaNO3, 2 g/L of K2HPO4, 0.2 g/L of NaCl, and 0.1 g/L of MgSO4. The medium prepared with commercial sugars was prepared to reproduce this composition.
The source carbons and all other reagents were autoclaved separately at 120 °C for 15 min before being mixed in the aseptic room. The initial pH of the fermentation medium was 5.5.
2.3.1. Production of Pullulan in Erlenmeyer Flasks
Batch fermentations were performed in 125 mL Erlenmeyer flasks containing 50 mL of fermentation medium. Approximately 400 µL of the cell suspension was transferred to inoculate the 50 mL fermentation medium. This was placed under continuous stirring on a shaker operating at a speed of 200 min−1 for 120 h at a temperature of 26 °C. Each test was triplicated.
2.3.2. Production of Pullulan in Bubble Column Reactor in Batch Mode
Fermentations were carried out in a jacketed bubble column reactor with a useful volume of 120 mL and an internal diameter of 3.80 cm. Aeration rate of 0.4 vvm was used. Foam control was ensured daily by adding an anti-foam agent. The temperature of the medium was controlled at 26 °C using a thermostatic bath for 120 h of fermentation. Samples were periodically collected for the concentration of pullulan, sugars, and cell determination.
2.4. Isolation and Purification of the Product
Samples were periodically removed after the first samples were collected on day 0 (blanks). Approximately 5 mL was collected at each sampling, then centrifuged at 2000× g for 15 min at 4 °C. The obtained biomass was washed twice with distilled water via centrifugation. The solid was dried for 24 h in the oven at 105 °C to determine dry weight, which was converted in biomass concentration in g/L [3]. A quantity of 4 mL of the supernatant obtained after centrifugation was precipitated with 8 mL of cold ethanol for 24 h at 4 °C in a refrigerator, then centrifuged at 2000× g for 15 min before being washed twice with ethanol. The solid fraction (raw pullulan) recovered was subsequently dialyzed with deionized water for 24 h to remove salts before being precipitated by adding ethanol. Estimation of pullulan concentration in g/L was calculated by drying the precipitate at 105 °C to a constant weight [14].
2.5. Analytical Methods
The composition of the hydrolysate in sugars and acetic acid before and after fermentation was analyzed by using a High-Performance Liquid Chromatography device, which is equipped with a Refractive Index Detector RID-6A and an HPX-87H column (300 7.8 mm (Bio-Rad, Agilent, Santa Clara, CA, USA) containing 0.001 N H2SO4 as mobile phase and 0.6 mL/min as flow rate, with column temperature of 45 °C. Samples for HPLC analysis were previously filtered through a Sep Pak C18 filter (Waters Corporation 34 Maple Street Milford, MA, USA). The volume of 20 μL was the injection value in agreement with the work reported by Ahmed et al. [19]. Furans and phenolic compounds were also evaluated via HPLC, using a C18 column at 25 °C with a UV–VIS detector, and eluted with a solution of acetonitrile:water (1:8) and 1% acetic acid at a flow rate of 0.8 mL/min.
Fourier-transform infrared spectroscopy (FTIR) (Perkin Elmer-spectrum TWO ATR 2015, PerkinElmer, Waltham, MA, USA) was used to characterize the pullulan produced, and results were compared with a sample of commercial pullulan (food grade) kindly supplied by Dinaco, Brazil. The analysis wavenumber was between 4000 and 600 cm−1 at a resolution of 4 cm−1. A quantity of 1.5 mg of samples previously heated in the oven was mixed with 250 mg of KBr crystals and kept in a desiccator at 30 °C for 24 h under reduced pressure [20].
The diffraction pattern of the prepared pullulan was analyzed on an Empyrean powder diffractometer from the company PANalytical, Almelo, The Netherlands, consisting of a copper anode for the X-ray source (λ = 1.540598 A° and a detector coupled to a monochromator).
The analysis of morphology of the prepared pullulan was carried out using a scanning electron microscope with an EDX-EBSD system (Quanta 200F, FEI, Eindhoven, The Netherlands).
3. Results and Discussion
3.1. Hydrolysate Detoxification
The acid hydrolysis process can generate phenolic compounds and furan derivatives that are known as toxic substances for microorganisms [21]. For this reason, the hemicellulose hydrolysate was subjected to detoxification procedures combining activated carbon as a support material with a pH variation, aimed at reducing these compound concentrations, improving the fermentability of the microorganisms. The toxic compounds present in the non-detoxified hydrolysate are often related to slow kinetics with limited productivity yield in fermentation.
The concentrations of toxic compounds, including sugars and acetic acid, before and after the detoxification procedure in sugarcane bagasse hydrolysate are presented in Table 1.

Table 1.
Concentrations of toxic compounds and sugars before and after detoxification by activated carbon in sugarcane bagasse hemicellulosic hydrolysate.
With a severity factor of 3.8, the pretreatment temperature had a very strong effect on the hydrolysis of hemicelluloses in the hydrolysate, with a concentration of xylose obtained of 20.15 g/L, corresponding to a xylan hydrolysis yield of about 71%. After concentration, xylose reached 135.72 g/L. This result was in agreement with previously reported work [22].
The results shown in Table 1 also reveal that the procedure including activated carbon is effective in completely or partially eliminating toxic compounds from hydrolysate. As can be seen, the concentrations of sugars (glucose and xylose) in the hydrolysate after detoxification decreased considerably. This reduction can be explained by some adsorption of sugars by precipitates formed in the pH change process or during the adsorption process of inhibitors by activated carbon [23]. In addition to the sugar concentrations, Table 1 shows that furans and phenolic compounds were adsorbed during the detoxification of the hydrolysate. Table 1 also verifies a slight increase in the concentration of acetic acid after the detoxification process, which can be related to some volume reduction observed after the pH increase step.
In general, activated carbon was very effective in the removal of furfural, hydroxymethylfurfural, and phenolics. This behavior was similar to those obtained by Daza-Serna et al. [24] in the detoxification of hemicellulosic hydrolysate of wheat straw.
3.2. Pullulan Production by Aureobasidium Pullulans in Erlenmeyer Flasks
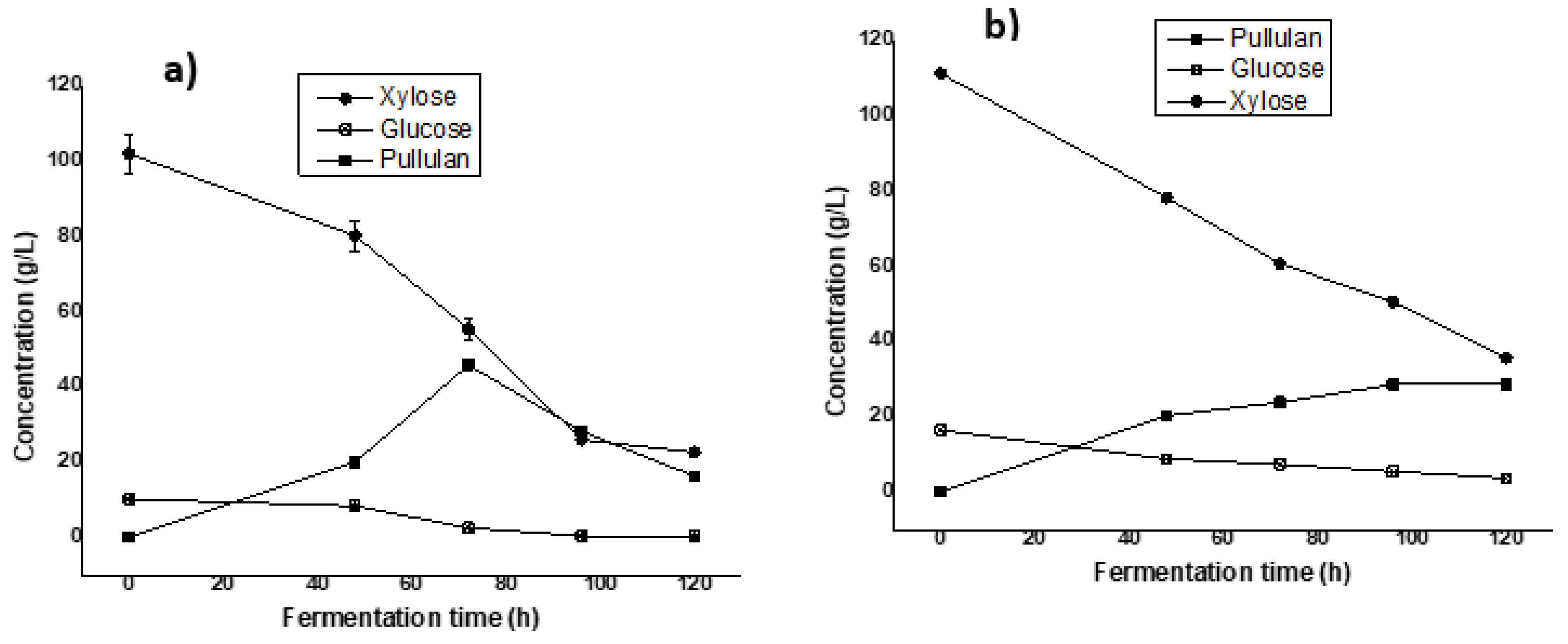
A glucose/xylose (1/9 mass ratio) simulated medium was used as a carbon source with the aim of evaluating the capacity of its pullulan production by Aureobasidium pullulans ATCC 42023 in Erlenmeyer flasks. The kinetic profiles of fermentation in Erlenmeyer flasks using both commercial sugars and hydrolysate-based medium are shown in Figure 2.
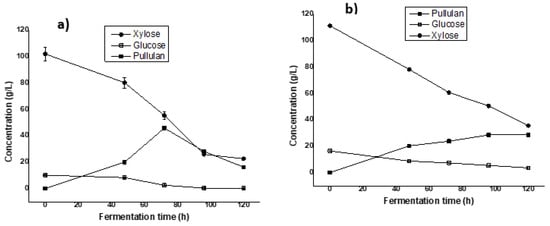
Figure 2.
Fermentation kinetic profiles of Aureobasidium pullulans ATCC 42023 during pullulan production in Erlenmeyer flasks from commercial sugars (a) and (b) hemicellulosic hydrolysate-based mediums.
The initial concentration of cells in the fermentation medium was chosen based on the literature. Indeed, different reported cultivations with different culture mediums and different strains of A. pullulans have used low initial cell concentrations, around 1 g/L [25,26,27]. However, future work should consider the influence of this variable in the process.
The results shown in Figure 2 indicate a maximum production of 45.85 ± 1.08 g/L of pullulan after 5 days of fermentation (Figure 2a). This result is higher than the 17.63 g/L of pullulan obtained in a commercial xylose-based medium reported by Chen et al. [14] with A. pullulans AY82. Furthermore, a study reported by Liu et al. [28] showed a maximum concentration of 58.9 g/L of pullulan using the mixture of sugars using a glucose/xylose ratio of 75%:25% as a carbon source.
On the other hand, the evaluation of the potential for pullulan production with the hemicellulosic hydrolysate of sugarcane bagasse as the sole carbon source was tested firstly in the Erlenmeyer flasks (Figure 2b) following the same conditions. The initial sugar concentrations in the fermentation medium were 107 g/L of xylose, 8.5 g/L of glucose, and 13.85 g/L of arabinose. A maximum production of 33.17 ± 1.60 g/L of pullulan was obtained in 120 h of fermentation in the Erlenmeyer flasks. The maximum concentration thus obtained was lower than that using commercial sugars as a carbon source. This difference can be explained by the fact that hemicellulose hydrolysate, even after detoxification, contains toxic compounds that inhibit microbial growth and consequently reduce pullulan production [14]. Furthermore, it was reported by Mussatto and Roberto [29] that acetic acid, which derives from the acetyl groups present in hemicellulose during acid treatment, has an inhibitory power that can negatively influence the production yield of biomolecules. Indeed, the inhibition of enzymes such as D-xylose reductase and xylitol dehydrogenase, which are involved in the metabolism of xylose by the presence of glucose, leads during fermentation to the consumption of glucose before that of xylose [30,31]. These results demonstrate that acetic acid and phenolic derivatives could be responsible for the reduction in pullulan production when using a sugarcane bagasse hemicellulose hydrolysate and indicate future works for better understanding the effect of each of the inhibitors present in hydrolysate on pullulan production.
The other interesting point is regarding the repression of carbon catabolites, a cellular regulation mechanism in which microorganisms preferentially select certain carbon sources from those available in the culture medium, which appears to be illustrated in Figure 2. Despite this, xylose was consumed from the beginning of the process, possibly because the inoculum was prepared with xylose as the carbon source, thereby adapting the microorganism to this carbohydrate. But, as shown in Figure 2, xylose was not fully assimilated by the microorganism. This could be due to the presence of glucose, which might repress xylose utilization, leading to reduced pentose consumption and product yield. Future studies will investigate whether, with equal amounts of glucose and xylose as carbon sources, the microorganism can utilize these sugars simultaneously. According to Chen et al. [14], a yield of 17.63 g/l of pullulan was obtained from xylose using A. pullulans AY82, indicating that it can produce pullulan from both xylose and glucose.
The preference for glucose could also be related to the mechanism of transport of this sugar across the cell membrane. As A. pullulans exhibits great nutritional diversity due to the different ecological preferences of its diverse strains, there are numerous sugar transporters in its membrane. For xylose, for example, Gostinčar et al. [32] reported sugar transporters within the major facilitator superfamily, which includes H+ symporters, one of which transports various monosaccharides but has a high affinity for glucose. Therefore, glucose and xylose can compete for some transporters. However, this topic has not been well reported in the literature and requires further and deeper studies.
Anyway, the literature reports pullulan production using xylose as the sole carbon source [14,33]. However, studies deeply investigating the use of a mix of glucose and xylose are still scarce. The biosynthesis of this biopolymer from xylose is a complex metabolic process that can be traced through the pentose phosphate pathway (PPP), as proposed by Guo et al. [34] and discussed in the recent review by Cruz-Santos et al. [35].
3.3. Kinetics of Pullulan Production from Sugarcane Bagasse Hemicellulose Hydrolysate in a Bubble Column Reactor
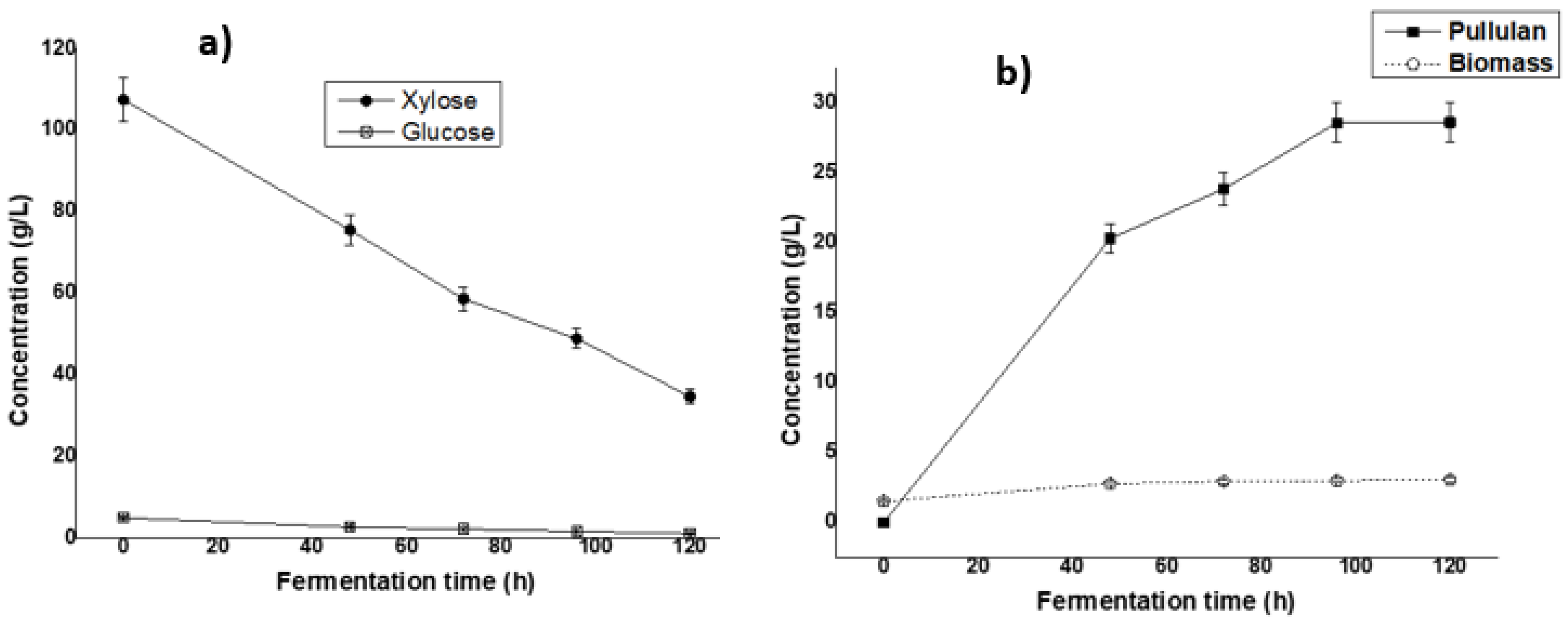
Many Aureobasidium strains have been reported for the production of pullulan from glucose, but very few studies have used xylose as the carbon source [36]. Xylose, the main sugar of the hemicellulosic hydrolysate, has already been used as a carbon source for pullulan production in Erlenmeyer flasks but has never been reported in a bubble column reactor. After confirming the potential production in the Erlenmeyer flask, a study of the batch fermentation kinetics of pullulan production by cultivation of Aureobasidium pullulans ATCC 42023 in the bubble column reactor using the detoxificated sugarcane bagasse hemicellulosic hydrolysate, with an initial pH of 5.5, was performed, and results are shown in Figure 3.
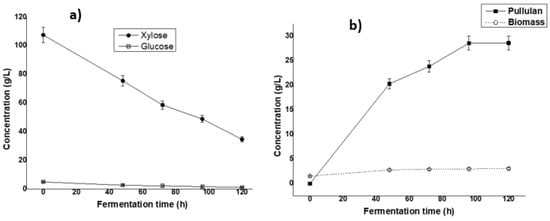
Figure 3.
Profile of sugar consumption (a) and biomass/pullulan production (b) during the fermentation of sugarcane bagasse hemicellulose by Aureobasidium pullulans ATCC 42023 in a bubble column bioreactor. Data are shown as means ± SDs (n = 3).
High pullulan production was observed on the second day and increased slightly from 3 to 5 days. The maximum concentration of pullulan was observed on the fifth day of fermentation with a value of 28.62 ± 1.43 g/L. During the same period, the biomass slightly increased throughout the experimental duration, indicating that pullulan production can be independent or partially dependent on cell growth.
Using the bubble column reactor, the production was comparable to that obtained in the Erlenmeyer flask. The slight difference could be explained by the failure to control the optimum fermentation factors, particularly aeration, which is a main factor in controlling fermentation using the bubble column reactor. Therefore, further study is recommended to verify the performance of the bubble column reactor through an optimization process for the production of pullulan using hemicellulosic hydrolysate.
The obtained pullulan concentration was comparable to that in other works but lower than that reported by Chen et al. [14] on pullulan production by sugarcane bagasse hemicellulose hydrolysate in a 5 L fermenter. Sucrose, which remains the main substrate for the industrial production of pullulan, is controversial due to its high cost (USD 900 per ton of sucrose), followed by glucose, which, despite its lower cost than sucrose, has a relatively low efficiency in producing pullulan [36]. Xylose is promising and can improve the economic viability of pullulan production [37].
Since sugarcane hemicellulose hydrolysate has excellent potential for pullulan production, further studies should focus on the hydrolysate detoxification technique and improvement of fermentation conditions in order to optimize pullulan production with hemicellulosic hydrolysate.
The concentrations of pullulan obtained in this study are of great interest and even considerable compared to similar work dedicated to the production of pullulan from various food wastes by wild and mutant strains of Aureobasidium pullulans (Table 2).

Table 2.
Comparison of pullulan production from sugarcane bagasse hemicellulosic hydrolysate carried out in this study and other previously reported performances.
3.4. Characterization of Exopolysaccharide Prepared with Sugarcane Bagasse Hemicellulose Hydrolysate
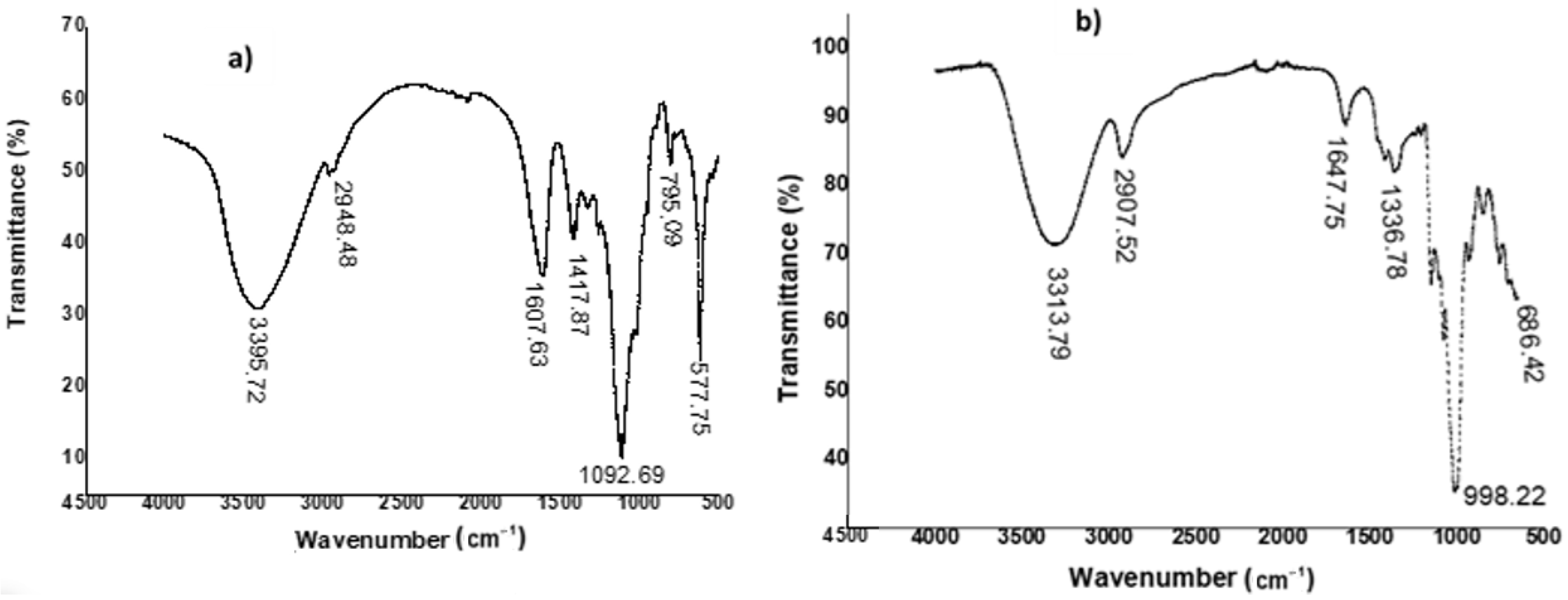
The FTIR spectra of pullulan produced from sugarcane bagasse hemicellulose hydrolysate and commercial pullulan used as a reference are shown in Figure 4.
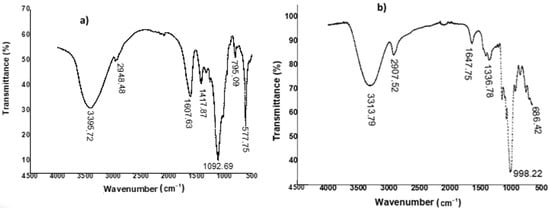
Figure 4.
FTIR spectra of pullulan recovered from sugarcane bagasse hemicellulose hydrolysate (a) and commercial pullulan (b).
As can be observed, a broad absorption band is observed at 3395.72 cm−1, which can be attributed to the stretching vibration of hydroxyl groups (–OH) mainly observed in polysaccharide structures. We also noted a weak peak that appeared almost at 2948.48 cm−1, which could be attributed to the sp3 bonds of the methyl groups [38,39]. Several other high-intensity peaks were also detected at 1607.63, 1417.87, and 1092.69 cm−1, attributed respectively to the C–O, C–OH, and C–O–C elongation bands of esters [40]. Peaks that could be attributed to α-1,6-glucopyranoside and α-1,4-glucopyranoside bonds were detected at 795.09 and 577.75 cm−1, respectively [3,41]. In addition, Table 3 compares the spectral data of the exopolysaccharide produced to those of commercial pullulan.

Table 3.
Comparative infrared spectroscopy data from sugarcane bagasse hemicellulose hydrolysate and commercial pullulan.
It appears that the spectral data of commercial pullulan, as well as those produced from sugarcane bagasse hemicellulose hydrolysate, presented similar characteristics, thus confirming the pullulan structure of this exopolysaccharide and possibly having applications for the development of medical devices, as a food additive, or as an adhesive [7].
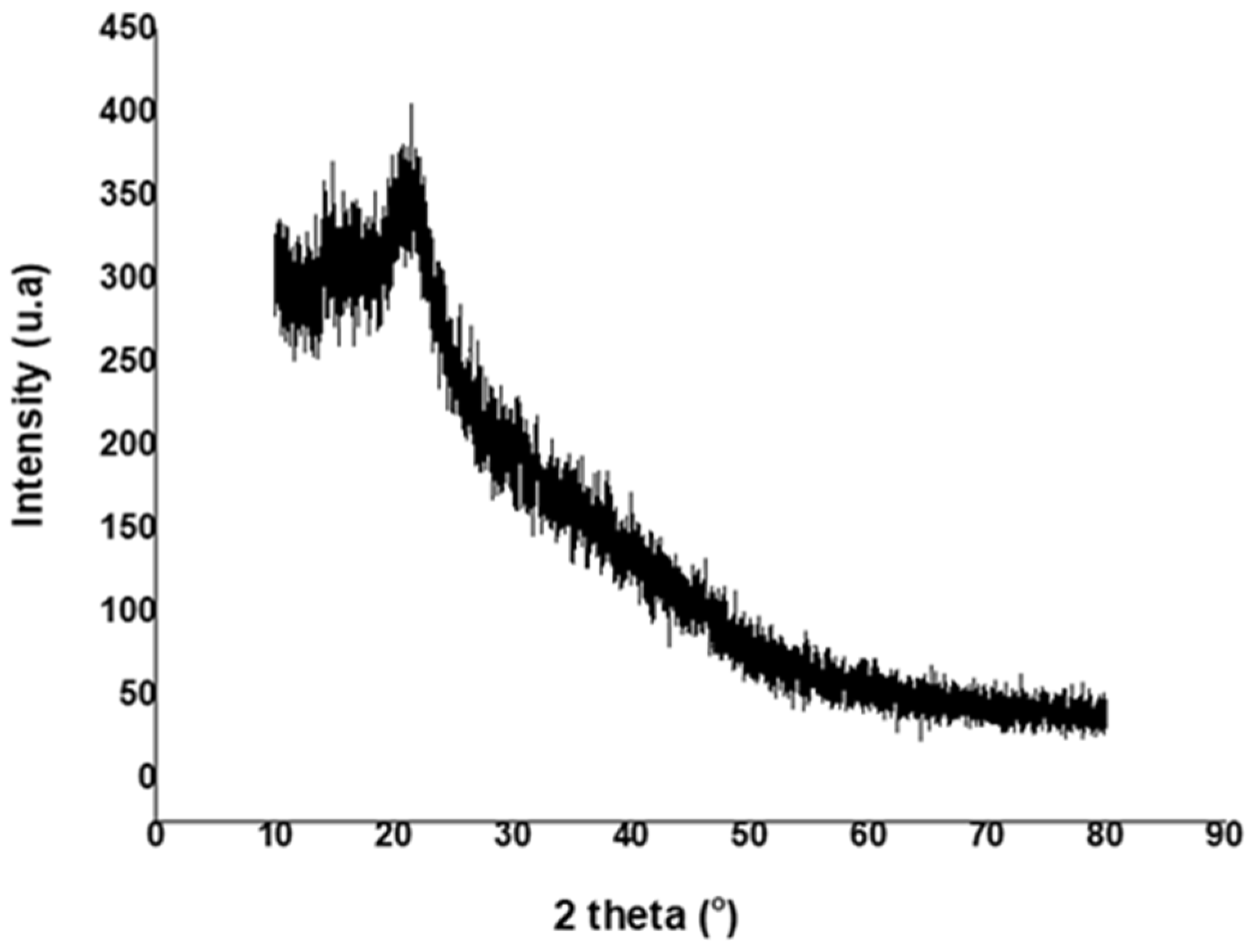
The X-ray diffractogram of synthesized pullulan is shown in Figure 5. A weak peak was revealed at around 2θ = 20°, indicating the amorphous nature of pullulan [4,41]. This observation was consistent with other XRD analysis results of pullulan reported by several other researchers [42,43].
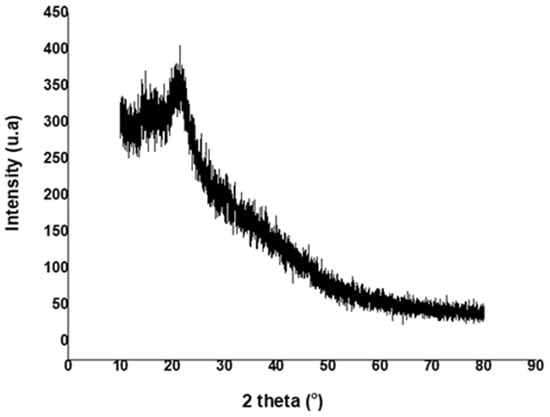
Figure 5.
The XRD pattern of the pullulan obtained from sugarcane bagasse hemicellulose hydrolysate.
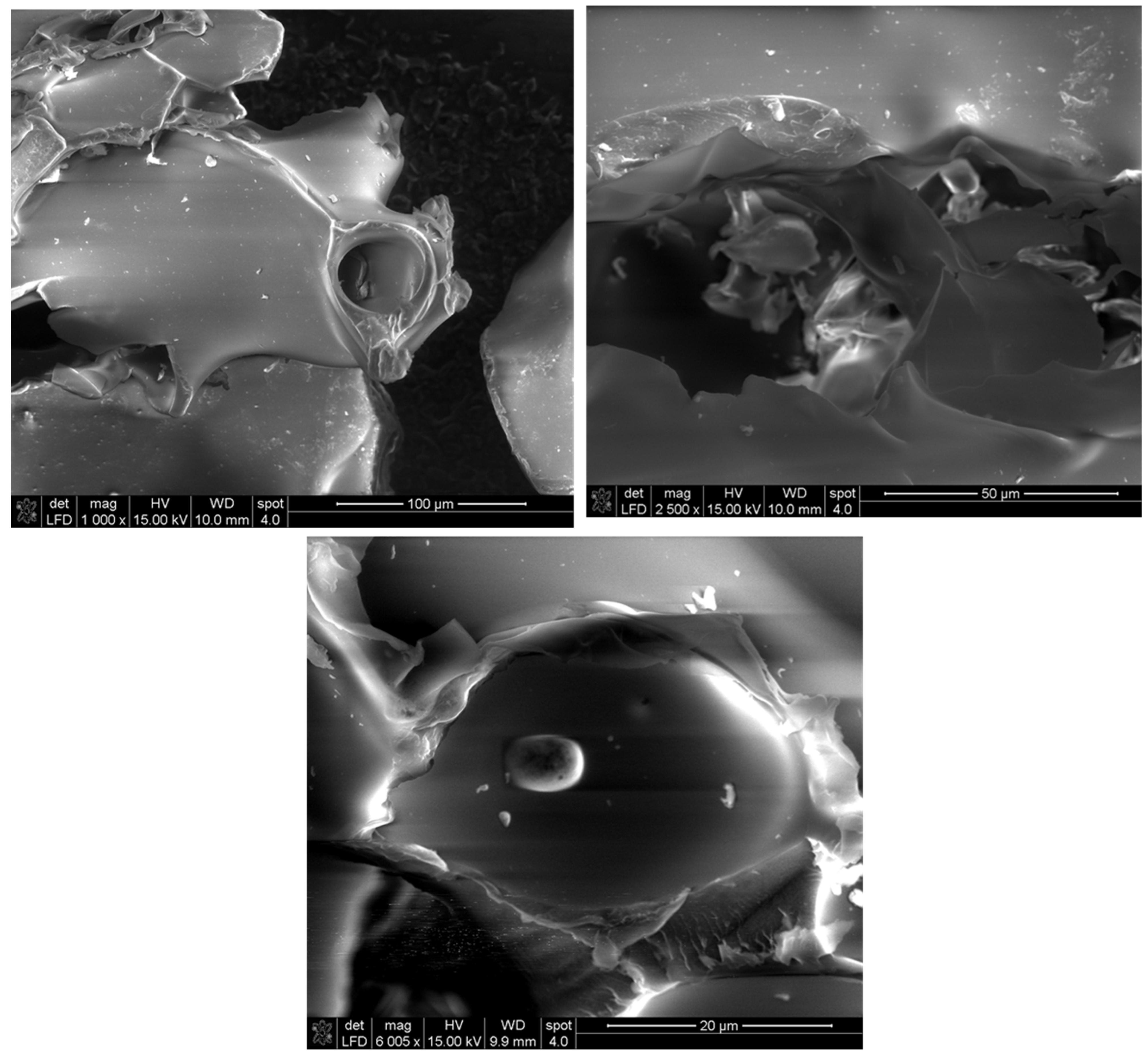
The morphology of the prepared biopolymer was studied at different magnifications (1000×; 2500×, and 6000×) using SEM. The results presented in Figure 6 show the smooth surfaces of the synthesized pullulan photomicrographs regardless of the magnification of the equipment. These results corroborate those reported by [44,45].
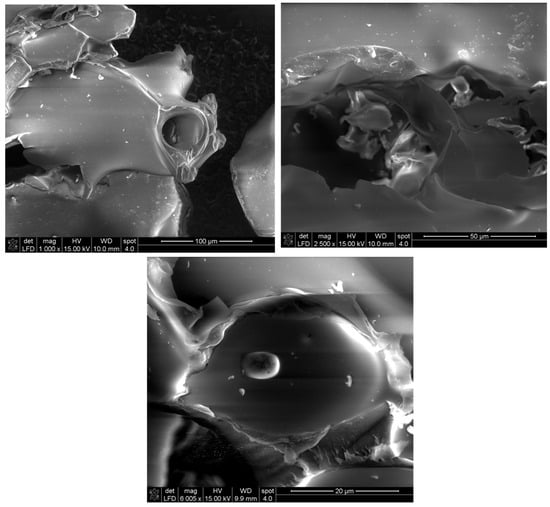
Figure 6.
SEM micrographs of produced pullulan at different magnifications.
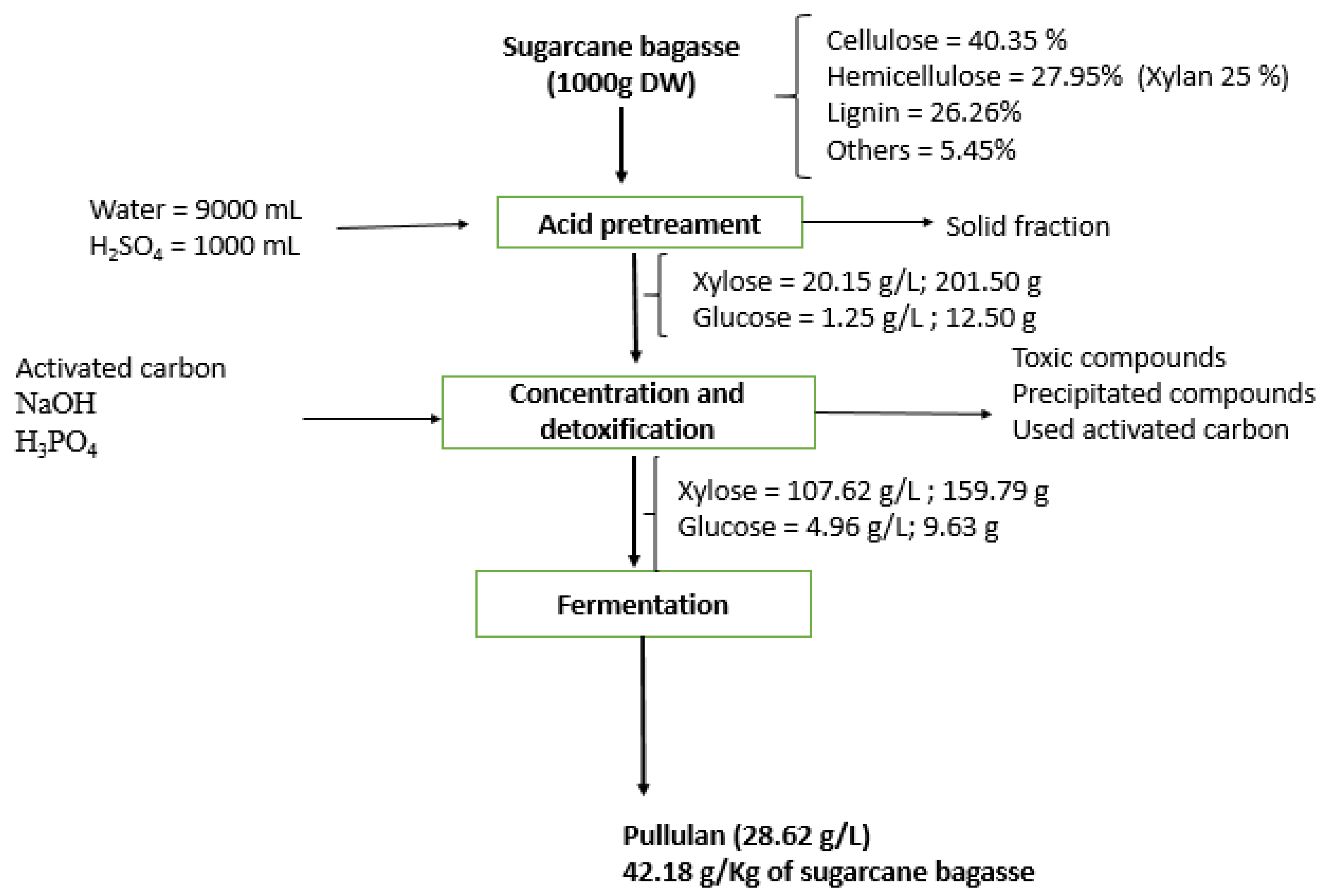
The material balance for the main phases of pullulan synthesis from sugarcane bagasse is presented in Figure 7.
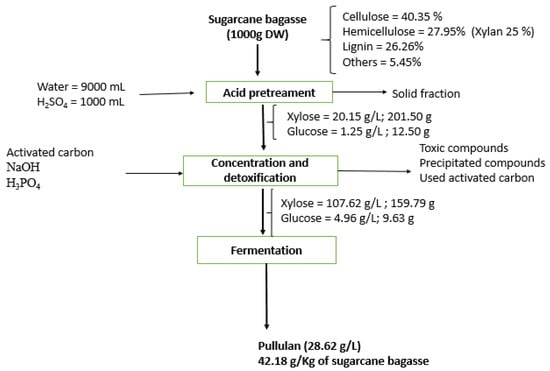
Figure 7.
Material balance in main phases for pullulan production from sugarcane bagasse hemicellulosic hydrolysate.
4. Conclusions
This study aimed to evaluate the hemicellulose hydrolysate of sugarcane bagasse in pullulan production using the strain Aureobasidium pullulans ATCC 42023 in a bubble column reactor. The results indicate a maximum concentration of 28.62 ± 1.43 g/L of pullulan, obtained after 120 h of fermentation. This investigation showed that xylose from sugarcane waste hydrolysate can be used as a carbon substitute to produce pullulan. A comprehensive techno-economic process assessment is underway to monitor all significant factors that may further influence the process’s expansion toward full-scale conversion.
Author Contributions
Conceptualization, R.F.T.T. and J.C.S.; methodology, R.F.T.T., M.M.C.-S. and S.B.M.; software, R.F.T.T.; validation, R.F.T.T., J.C.S. and M.M.C.-S.; formal analysis, V.P.S., S.B.L.N., J.A.A.K. and S.S.d.S.; investigation, J.C.S.; resources, J.C.S. and S.S.d.S.; data curation, R.F.T.T.; writing—original draft preparation, R.F.T.T., V.P.S. and F.A.F.A.; writing—review and editing, J.C.S.; visualization, F.A.F.A.; supervision, J.C.S.; project administration, R.F.T.T.; funding acquisition, J.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil, processes number #150097/2022-0, # 304166/2022-7, #305416/2021-9), INCT Yeasts: “Biodiversity, preservation and biotechnological innovation", funded by CNPq, Brasília, Brazil, grant #406564/2022, and Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation—FAPESP, grant #2020/12059-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barcelos, M.C.; Vespermann, K.A.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2019, 60, 1475–1495. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, H.; Gao, J.; Song, H.; Bai, W. High-level production of pullulan and its biosynthesis regulation in Aureobasidium pullulans BL06. Front. bioeng. Biotechnol. 2023, 11, 1131875. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; Resende, J.; Orsi, C.A.; Ahmed, M.A.; Lacerda, T.M.; Da Silva, S.S.; Santos, J.C. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int. J. Biol. Macromol. 2019, 127, 169–177. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan production in stirred tank reactor by a colour-variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. Technol. Appl. 2021, 2, 100086. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, C.; Zhang, G.; Wang, C.; Wei1, G. Enhanced β-glucan and pullulan production by Aureobasidium pullulans with zinc sulfate supplementation. Appl. Microbiol. Biot. 2020, 104, 1751–1760. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, S.; Ahmad, A.; Chen, L.; Bai, W. Ultra-highmolecular weight pullulan-based material with high deformability and shape-memory properties. Carbohydr. Polym. 2022, 295, 119836. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Hosseini, S.S. Production, optimization and characterization of pullulan from sesame seed oil cake as a new substrate by Aureobasidium pullulans. Carbohydr. Polym. Technol. Appl. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Chen, J.; Fang, Y.; Wu, L.; Xu, Y.; Wang, S. Production of pullulan from raw potato starch hydrolysates by a new strain of Auerobasidium pullulans. Int. J. Biol. Macromol. 2016, 82, 740–743. [Google Scholar] [CrossRef]
- Wang, D.; Ju, X.; Zhou, D.; Wei, G. Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresour. Technol. 2014, 164, 12–19. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Ma, S.J.; Chang, F.; Xue, W.J. Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz. J. Microbiol. 2017, 48, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, A.W.; Brown, F.; Farha, M.N.; Rosan, T.M.; Folberth, G.A.; Hayes, F.; Sitch, S. Impacts of ground-level ozone on sugarcane production. Sci. Total Environ. 2023, 904, 166817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Li, F.; Liu, M.; Zhang, X.; Guo, X.; Xiao, D. Production of pullulan from xylose and hemicellulose hydrolysate by Aureobasidium pullulans AY82 with pH control and DL-dithiothreitol addition. Biotechnol. Bioprocess Eng. 2014, 19, 282–288. [Google Scholar] [CrossRef]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess Biosyst. Eng. 2016, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Tengborg, C.; Stenberg, K.; Galbe, M.; Zacchi, G.; Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B. Comparison of SO2 and H2SO4 impregnation of softwood prior to steam pretreatment on ethanol production. Appl. Biochem. Biotechnol. 1998, 70, 3–15. [Google Scholar] [CrossRef]
- Chen, S.F.; Du, B.; Chambliss, K.C.; van Walsum, G.P. Pseudo reaction kinetics of organic degradation products in diluteacid-catalyzed corn stover pretreatment hydrolysates. Biotechnol. Bioeng. 2007, 98, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.E.; Roberto, I.E.C. Hydrolysate detoxification with activated charcoal for xylitol production by Candida guilliermondii. Biotechnol. Lett. 2001, 23, 1681–1684. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Rehman, M.S.U.; Terán-Hilares, R.; Khalid, S.; Han, J.I. Optimization of twin gear-based pretreatment of rice straw for bioethanol production. Energy Conv. Manag. 2017, 141, 120–125. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Gowthami, E.; Swathi, B.; Elakkiya, S.; Srivastava, S.N.; Ravikumar, R.; Gowdhaman, D.; Ponnusami, V. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydr. Polym. 2013, 92, 697–703. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Steyer, J.P.; Carrere, H. Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour. Technol. 2012, 104, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crops Prod. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Fonseca, B.G.; Puentes, J.G.; Mateo, S.; Sanchez, S.; Moya, A.J.; Roberto, I.C. Detoxification of rice straw and olive tree pruning hemicellulosic hydrolysates employing Saccharomyces cerevisiae and its effect on the ethanol production by Pichia stipitis. J. Agric. Food Chem. 2013, 61, 9658–9665. [Google Scholar] [CrossRef] [PubMed]
- Daza-Serna, L.; Masi, A.; Serna-Loaiza, S.; Pfnier, J.; Stark, G.; Mach, R.L.; Friedl, A. Detoxification strategy of wheat straw hemicellulosic hydrolysate for cultivating Trichoderma reesei: A contribution towards the wheat straw biorefinery. Biomass Conv. Bioref. 2023, 13, 16495–16509. [Google Scholar] [CrossRef]
- Youssef, F.; Roukas, T.; Biliaderis, C.G. Pullulan production by a non-pigmented strain of Aureobasidium pullulans using batch and fed-batch culture. Process Biochem. 1999, 34, 355–366. [Google Scholar] [CrossRef]
- Hilares, R.T.; Orsi, C.A.; Ahmed, M.A.; Marcelino, P.F.; Menegatti, C.R.; da Silva, S.S.; Dos Santos, J.C. Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour. Technol. 2017, 230, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz Oktay, B.; Bozdemir, M.T.; Özbaş, Z.Y. Evaluation of some agro-industrial wastes as fermentation medium for pullulan production by Aureobasidium pullulans AZ-6. Curr. Microbiol. 2022, 79, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, X.; Chen, C.; Chi, Z.; Zhang, Y.; Cui, Q.; Liu, Y.J. Robust production of pigment-free pullulan from lignocellulosic hydrolysate by a new fungus co-utilizing glucose and xylose. Carbohydr. Polym. 2020, 241, 116400. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolysates for use in fermentative process: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Santos, S.C.; Dionísio, S.R.; De Andrade, A.L.D.; Roque, L.R.; Da Costa, A.C.; Ienczak, J.L. Fermentation of xylose and glucose mixture in intensified reactors by Scheffersomyces stipitis to produce ethanol. IJBB 2015, 9, 539–544. [Google Scholar]
- Olivares-Marin, I.K.; Madrigal-Perez, L.A.; Canizal-Garcia, M.; García-Almendárez, B.E.; González-Hernández, J.C.; Regalado-Gonzalez, C. Interactions between carbon and nitrogen sources depend on RIM15 and determine fermentative or respiratory growth in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2018, 102, 4535–4548. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014, 15, 1–29. [Google Scholar] [CrossRef]
- Kennedy, I.I.D.E.; West, T.P. Effect of yeast extract addition to a mineral salts medium containing hydrolyzed plant xylan on fungal pullulan production. Z. für Naturforschung C 2018, 73, 319–323. [Google Scholar] [CrossRef]
- Guo, J.; Huang, S.; Chen, Y.; Guo, X.; Xiao, D. Heterologous expression of Spathaspora passalidarum xylose reductase and xylitol dehydrogenase genes improved xylose fermentation ability of Aureobasidium pullulans. Microbial Cell Factories 2018, 17, 64. [Google Scholar] [CrossRef]
- Cruz-Santos, M.M.; Antunes, F.A.; de Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.; Santos, J.C. Production and applications of Pullulan from Lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Ponnusami, V. Review on production, downstream processing and characterization of microbial pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Zamare, D.; Manikanta, A. Selection and utilization of agro-industrial waste for biosynthesis and hyper-production of pullulan: A review. Biosynthetic Technol. Environ. Chall. 2018, 89–103. [Google Scholar]
- Hamidi, M.; Kennedy, J.F.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Production optimization, characterization and gene expression of pullulan from a new strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2019, 138, 725–735. [Google Scholar] [CrossRef]
- Van den Eynde, K.; Boon, V.; Gaspar, R.C.; Fardim, P. Biofabrication of Functional Pullulan by Aureobasidium pullulans under the Effect of Varying Mineral Salts and Sugar Stress Conditions. Molecules 2023, 28, 2478. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.W.; Qin, Z.Y.; Xu, J.X.; Kong, B.H.; Liu, Q.; Wang, H. Preparation and characterization of pea protein isolate-pullulan blend electrospun nanofiber films. Int. J. Biol. Macromol. 2020, 157, 641–647. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Y.; Huang, J.; Lai, H.; Yuan, D.; Tang, X.; Yang, L. Self-organized thermo-responsive poly (lactic-co-glycolic acid)-graft-pullulan nanoparticles for synergistic thermo-chemotherapy of tumor. Carbohydr. Polym. 2020, 237, 116104. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.R.; Kumari, N.; Bhunia, B.K.; Sarkar, B.; Mandal, B.B.; Ghosh, A. Synthesis and characterization of a non-cytotoxic and biocompatible acrylamide grafted pullulan—Application in pH responsive controlled drug delivery. Int. J. Biol. Macromol. 2018, 120, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Pandey, A.; Kennedy, J.F. Hyper-production of pullulan from de-oiled rice bran by Aureobasidium pullulans in a stirred tank reactor and its characterization. Bioresour. Technol. Rep. 2020, 11, 100494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).