Pullulan Production from Sugarcane Bagasse Hemicellulosic Hydrolysate by Aureobasidium pullulans ATCC 42023 inBubble Column Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
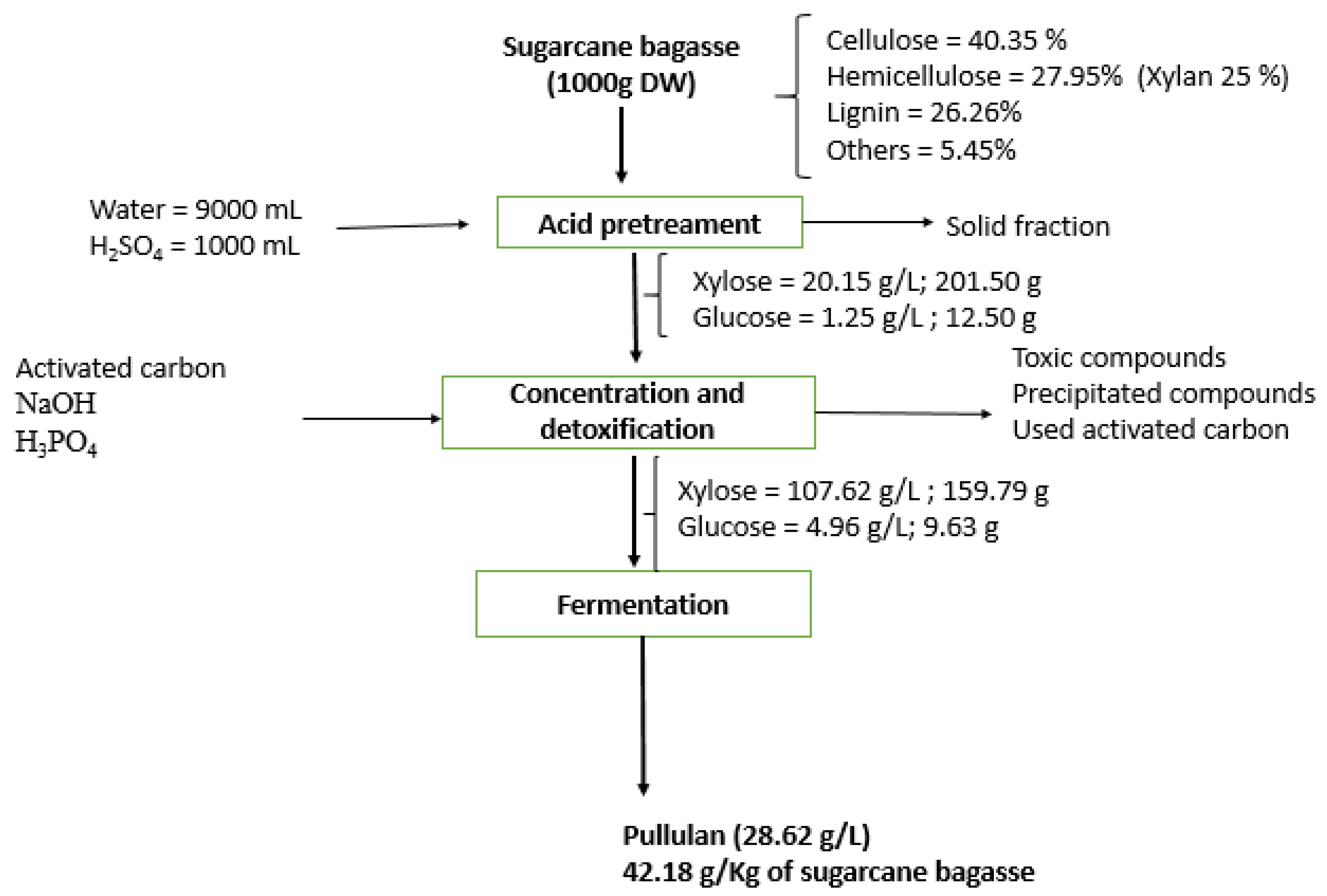
2.2. Hemicellulosic Hydrolysate Preparation
2.3. Microorganism and Inoculum Preparation
2.3.1. Production of Pullulan in Erlenmeyer Flasks
2.3.2. Production of Pullulan in Bubble Column Reactor in Batch Mode
2.4. Isolation and Purification of the Product
2.5. Analytical Methods
3. Results and Discussion
3.1. Hydrolysate Detoxification
3.2. Pullulan Production by Aureobasidium Pullulans in Erlenmeyer Flasks
3.3. Kinetics of Pullulan Production from Sugarcane Bagasse Hemicellulose Hydrolysate in a Bubble Column Reactor
3.4. Characterization of Exopolysaccharide Prepared with Sugarcane Bagasse Hemicellulose Hydrolysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barcelos, M.C.; Vespermann, K.A.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2019, 60, 1475–1495. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, H.; Gao, J.; Song, H.; Bai, W. High-level production of pullulan and its biosynthesis regulation in Aureobasidium pullulans BL06. Front. bioeng. Biotechnol. 2023, 11, 1131875. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; Resende, J.; Orsi, C.A.; Ahmed, M.A.; Lacerda, T.M.; Da Silva, S.S.; Santos, J.C. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int. J. Biol. Macromol. 2019, 127, 169–177. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan production in stirred tank reactor by a colour-variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. Technol. Appl. 2021, 2, 100086. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, C.; Zhang, G.; Wang, C.; Wei1, G. Enhanced β-glucan and pullulan production by Aureobasidium pullulans with zinc sulfate supplementation. Appl. Microbiol. Biot. 2020, 104, 1751–1760. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, S.; Ahmad, A.; Chen, L.; Bai, W. Ultra-highmolecular weight pullulan-based material with high deformability and shape-memory properties. Carbohydr. Polym. 2022, 295, 119836. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Hosseini, S.S. Production, optimization and characterization of pullulan from sesame seed oil cake as a new substrate by Aureobasidium pullulans. Carbohydr. Polym. Technol. Appl. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Chen, J.; Fang, Y.; Wu, L.; Xu, Y.; Wang, S. Production of pullulan from raw potato starch hydrolysates by a new strain of Auerobasidium pullulans. Int. J. Biol. Macromol. 2016, 82, 740–743. [Google Scholar] [CrossRef]
- Wang, D.; Ju, X.; Zhou, D.; Wei, G. Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresour. Technol. 2014, 164, 12–19. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Ma, S.J.; Chang, F.; Xue, W.J. Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz. J. Microbiol. 2017, 48, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, A.W.; Brown, F.; Farha, M.N.; Rosan, T.M.; Folberth, G.A.; Hayes, F.; Sitch, S. Impacts of ground-level ozone on sugarcane production. Sci. Total Environ. 2023, 904, 166817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Li, F.; Liu, M.; Zhang, X.; Guo, X.; Xiao, D. Production of pullulan from xylose and hemicellulose hydrolysate by Aureobasidium pullulans AY82 with pH control and DL-dithiothreitol addition. Biotechnol. Bioprocess Eng. 2014, 19, 282–288. [Google Scholar] [CrossRef]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess Biosyst. Eng. 2016, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Tengborg, C.; Stenberg, K.; Galbe, M.; Zacchi, G.; Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B. Comparison of SO2 and H2SO4 impregnation of softwood prior to steam pretreatment on ethanol production. Appl. Biochem. Biotechnol. 1998, 70, 3–15. [Google Scholar] [CrossRef]
- Chen, S.F.; Du, B.; Chambliss, K.C.; van Walsum, G.P. Pseudo reaction kinetics of organic degradation products in diluteacid-catalyzed corn stover pretreatment hydrolysates. Biotechnol. Bioeng. 2007, 98, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.E.; Roberto, I.E.C. Hydrolysate detoxification with activated charcoal for xylitol production by Candida guilliermondii. Biotechnol. Lett. 2001, 23, 1681–1684. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Rehman, M.S.U.; Terán-Hilares, R.; Khalid, S.; Han, J.I. Optimization of twin gear-based pretreatment of rice straw for bioethanol production. Energy Conv. Manag. 2017, 141, 120–125. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Gowthami, E.; Swathi, B.; Elakkiya, S.; Srivastava, S.N.; Ravikumar, R.; Gowdhaman, D.; Ponnusami, V. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydr. Polym. 2013, 92, 697–703. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Steyer, J.P.; Carrere, H. Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour. Technol. 2012, 104, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crops Prod. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Fonseca, B.G.; Puentes, J.G.; Mateo, S.; Sanchez, S.; Moya, A.J.; Roberto, I.C. Detoxification of rice straw and olive tree pruning hemicellulosic hydrolysates employing Saccharomyces cerevisiae and its effect on the ethanol production by Pichia stipitis. J. Agric. Food Chem. 2013, 61, 9658–9665. [Google Scholar] [CrossRef] [PubMed]
- Daza-Serna, L.; Masi, A.; Serna-Loaiza, S.; Pfnier, J.; Stark, G.; Mach, R.L.; Friedl, A. Detoxification strategy of wheat straw hemicellulosic hydrolysate for cultivating Trichoderma reesei: A contribution towards the wheat straw biorefinery. Biomass Conv. Bioref. 2023, 13, 16495–16509. [Google Scholar] [CrossRef]
- Youssef, F.; Roukas, T.; Biliaderis, C.G. Pullulan production by a non-pigmented strain of Aureobasidium pullulans using batch and fed-batch culture. Process Biochem. 1999, 34, 355–366. [Google Scholar] [CrossRef]
- Hilares, R.T.; Orsi, C.A.; Ahmed, M.A.; Marcelino, P.F.; Menegatti, C.R.; da Silva, S.S.; Dos Santos, J.C. Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour. Technol. 2017, 230, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz Oktay, B.; Bozdemir, M.T.; Özbaş, Z.Y. Evaluation of some agro-industrial wastes as fermentation medium for pullulan production by Aureobasidium pullulans AZ-6. Curr. Microbiol. 2022, 79, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, X.; Chen, C.; Chi, Z.; Zhang, Y.; Cui, Q.; Liu, Y.J. Robust production of pigment-free pullulan from lignocellulosic hydrolysate by a new fungus co-utilizing glucose and xylose. Carbohydr. Polym. 2020, 241, 116400. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolysates for use in fermentative process: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Santos, S.C.; Dionísio, S.R.; De Andrade, A.L.D.; Roque, L.R.; Da Costa, A.C.; Ienczak, J.L. Fermentation of xylose and glucose mixture in intensified reactors by Scheffersomyces stipitis to produce ethanol. IJBB 2015, 9, 539–544. [Google Scholar]
- Olivares-Marin, I.K.; Madrigal-Perez, L.A.; Canizal-Garcia, M.; García-Almendárez, B.E.; González-Hernández, J.C.; Regalado-Gonzalez, C. Interactions between carbon and nitrogen sources depend on RIM15 and determine fermentative or respiratory growth in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2018, 102, 4535–4548. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014, 15, 1–29. [Google Scholar] [CrossRef]
- Kennedy, I.I.D.E.; West, T.P. Effect of yeast extract addition to a mineral salts medium containing hydrolyzed plant xylan on fungal pullulan production. Z. für Naturforschung C 2018, 73, 319–323. [Google Scholar] [CrossRef]
- Guo, J.; Huang, S.; Chen, Y.; Guo, X.; Xiao, D. Heterologous expression of Spathaspora passalidarum xylose reductase and xylitol dehydrogenase genes improved xylose fermentation ability of Aureobasidium pullulans. Microbial Cell Factories 2018, 17, 64. [Google Scholar] [CrossRef]
- Cruz-Santos, M.M.; Antunes, F.A.; de Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.; Santos, J.C. Production and applications of Pullulan from Lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Ponnusami, V. Review on production, downstream processing and characterization of microbial pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Zamare, D.; Manikanta, A. Selection and utilization of agro-industrial waste for biosynthesis and hyper-production of pullulan: A review. Biosynthetic Technol. Environ. Chall. 2018, 89–103. [Google Scholar]
- Hamidi, M.; Kennedy, J.F.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Production optimization, characterization and gene expression of pullulan from a new strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2019, 138, 725–735. [Google Scholar] [CrossRef]
- Van den Eynde, K.; Boon, V.; Gaspar, R.C.; Fardim, P. Biofabrication of Functional Pullulan by Aureobasidium pullulans under the Effect of Varying Mineral Salts and Sugar Stress Conditions. Molecules 2023, 28, 2478. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.W.; Qin, Z.Y.; Xu, J.X.; Kong, B.H.; Liu, Q.; Wang, H. Preparation and characterization of pea protein isolate-pullulan blend electrospun nanofiber films. Int. J. Biol. Macromol. 2020, 157, 641–647. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Y.; Huang, J.; Lai, H.; Yuan, D.; Tang, X.; Yang, L. Self-organized thermo-responsive poly (lactic-co-glycolic acid)-graft-pullulan nanoparticles for synergistic thermo-chemotherapy of tumor. Carbohydr. Polym. 2020, 237, 116104. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.R.; Kumari, N.; Bhunia, B.K.; Sarkar, B.; Mandal, B.B.; Ghosh, A. Synthesis and characterization of a non-cytotoxic and biocompatible acrylamide grafted pullulan—Application in pH responsive controlled drug delivery. Int. J. Biol. Macromol. 2018, 120, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Pandey, A.; Kennedy, J.F. Hyper-production of pullulan from de-oiled rice bran by Aureobasidium pullulans in a stirred tank reactor and its characterization. Bioresour. Technol. Rep. 2020, 11, 100494. [Google Scholar] [CrossRef]
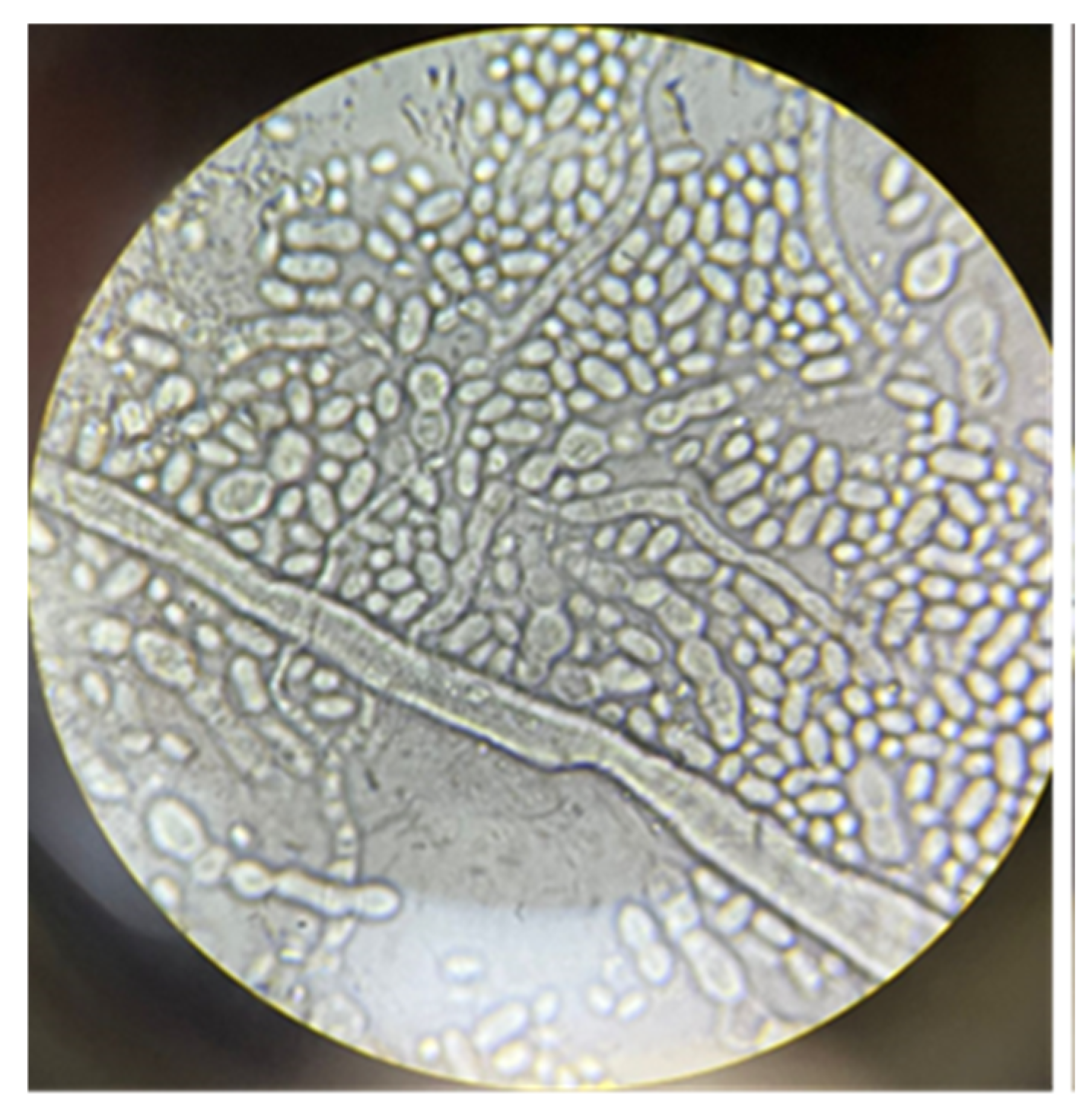



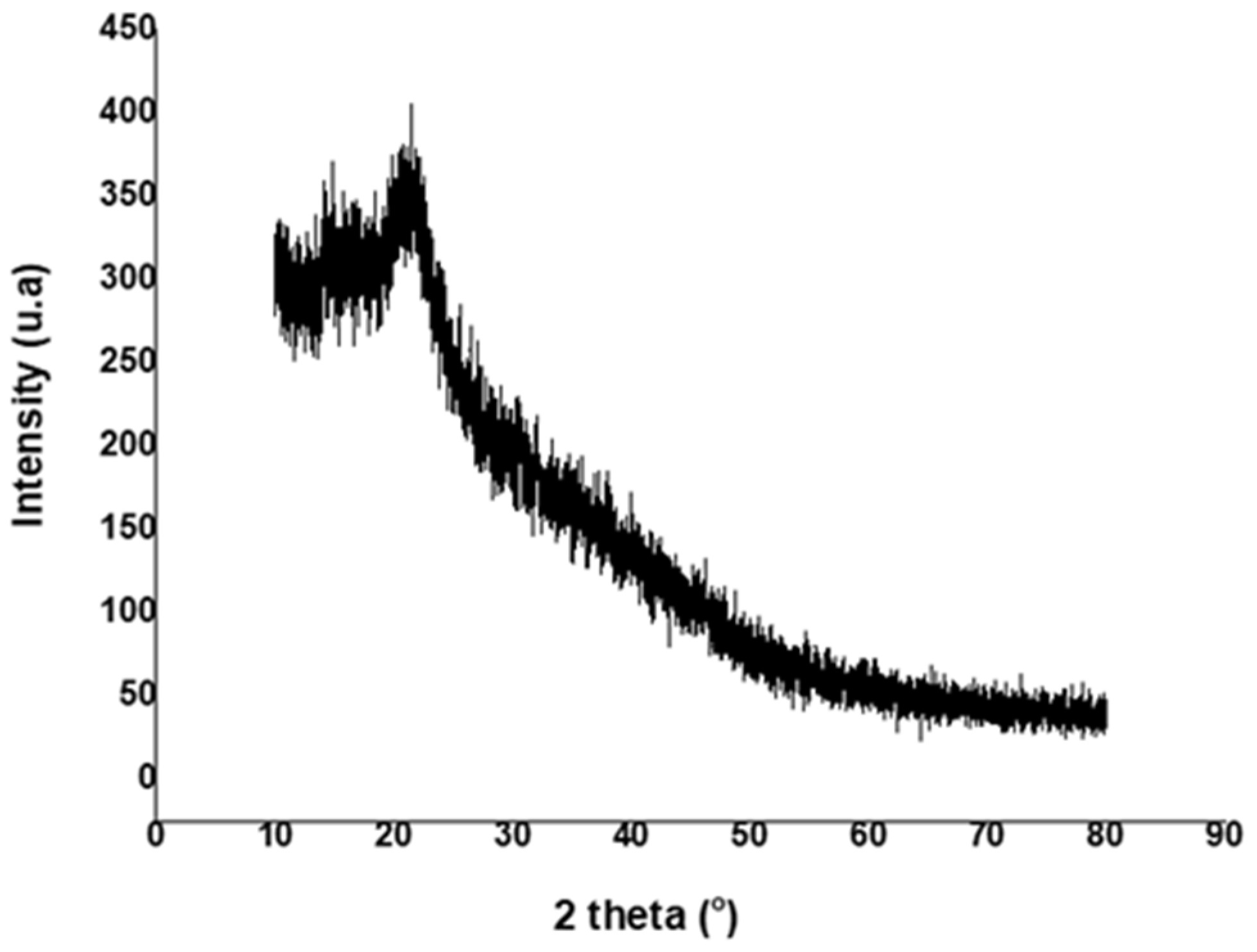
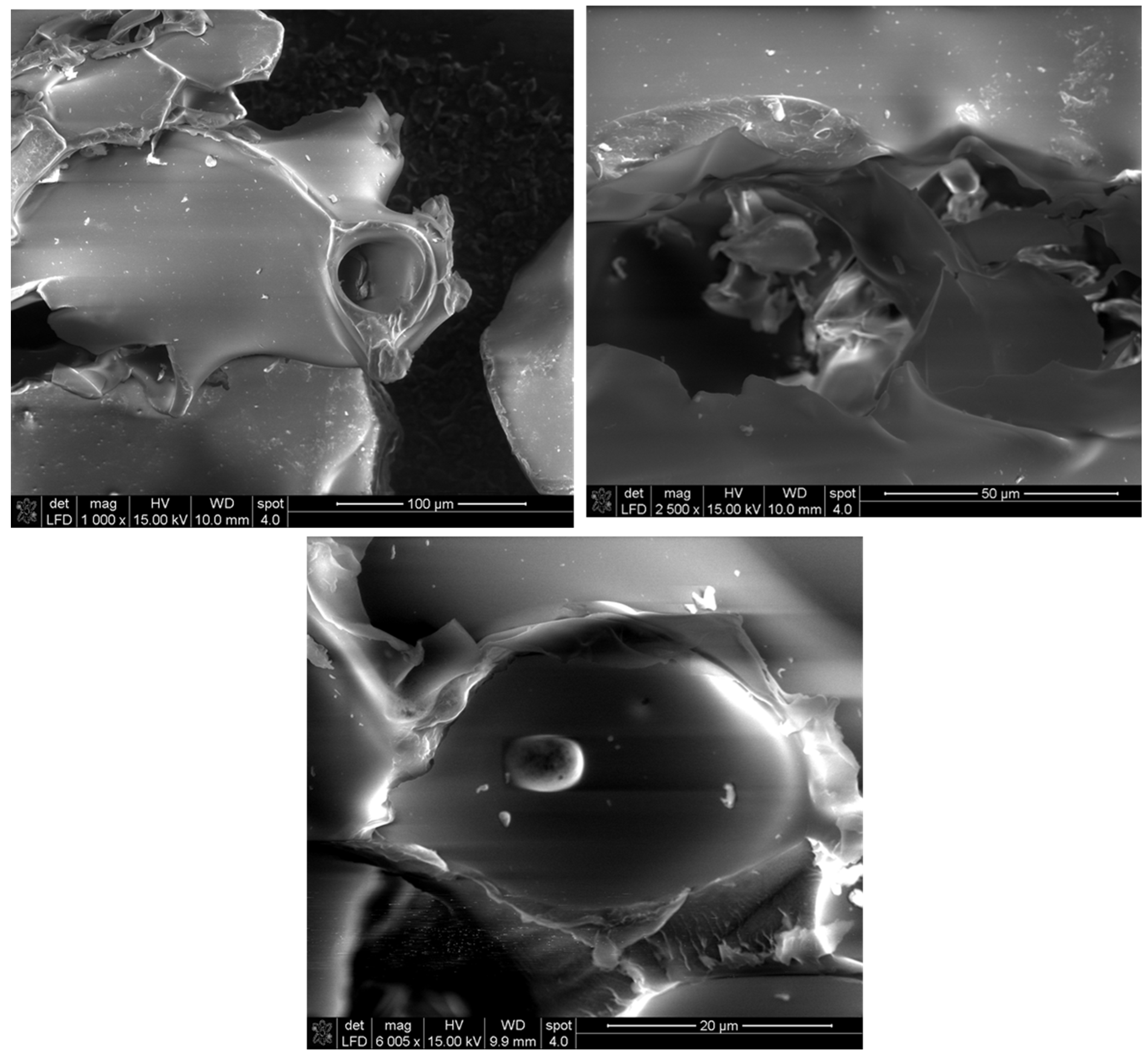
| Compound | Non Detoxified (g/L) | Detoxified (g/L) |
|---|---|---|
| D-glucose | 6.44 | 4.96 |
| D-xylose | 135.72 | 107.62 |
| Acetic acid | 1.201 | 1.744 |
| Furfural | 0.172 | 0.012 |
| 5-HMF | 0.208 | 0.101 |
| gallic acid | 0.036 | 0.015 |
| ferulic acid | 0.009 | 0.011 |
| pyrocatechol | 0.021 | 0.018 |
| 4-hydroxybenzoic acid | 0.009 | 0.007 |
| vanillic acid | 0.052 | 0.038 |
| Vanillin | 0.006 | 0.005 |
| syringaldehyde | 0.006 | Non detected |
| Carbon Source | Strain | Reactor | Pullulan (g/L) | References |
|---|---|---|---|---|
| sugarcane bagasse hemicellulosic hydrolysate | A. pullulans AY82 | 5 L fermentor | 12.65 g/L | [14] |
| xylose | A. pullulans AY82 | 5 L fermentor | 17.63 g/L | [14] |
| Sugarcane bagasse | A. pullulansLB83 | Bubble column reactor | 18.64 g/L | [3] |
| Sesame seed oil cake | A. pullulans KY767024 | 250 mL conical flasks | 51.4 g/L | [9] |
| Sugarcane bagasse Enzymatic hydrolysate | A. pullulans LB83 | Erlenmeyer flask | 20 g/L | [26] |
| Raw potato starch hydrolysates | A. pullulans CJ001 | Erlenmeyer flask | 36.17 g/L | [10] |
| Rice hull acid hydrolysate | A. pullulans ARH-1 | 3 L Stirred fermentator | 22.2 g/L | [11] |
| sugarcane bagasse hemicellulose hydrolysate | A. pullulans ATCC 42023 | Bubble column reactor | 28.62 g/L | This study |
| sugarcane bagasse hemicellulose hydrolysate | A. pullulans ATCC 42023 | 250 mL Erlenmeyer flask | 33.17 g/L | This study |
| Assignment | Pullulan Produced from Sugarcane Bagasse Hemicellulose Hydrolysate. Wavenumber (cm−1) | Commercial Pullulan Wavenumber (cm−1) |
|---|---|---|
| O–H stretching | 3395.72 | 3313.79 |
| C–H stretching | 2948.48 | 2907.52 |
| O–C–O stretching | 1607.63 | 1647.75 |
| C–O–H stretching | 1417.87 | 1336.78 |
| C–O stretching | 1092.69 | 998.22 |
| α-configuration | 795.09 | 666.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagne, R.F.T.; Cruz-Santos, M.M.; Antunes, F.A.F.; Shibukawa, V.P.; Miano, S.B.; Kenfack, J.A.A.; da Silva, S.S.; Ngomade, S.B.L.; Santos, J.C. Pullulan Production from Sugarcane Bagasse Hemicellulosic Hydrolysate by Aureobasidium pullulans ATCC 42023 inBubble Column Reactor. Fermentation 2024, 10, 322. https://doi.org/10.3390/fermentation10060322
Tagne RFT, Cruz-Santos MM, Antunes FAF, Shibukawa VP, Miano SB, Kenfack JAA, da Silva SS, Ngomade SBL, Santos JC. Pullulan Production from Sugarcane Bagasse Hemicellulosic Hydrolysate by Aureobasidium pullulans ATCC 42023 inBubble Column Reactor. Fermentation. 2024; 10(6):322. https://doi.org/10.3390/fermentation10060322
Chicago/Turabian StyleTagne, Rufis Fregue Tiegam, Mónica María Cruz-Santos, Felipe Antonio Fernandes Antunes, Vinícius Pereira Shibukawa, Sara Barboza Miano, Junie Albine Atangana Kenfack, Silvio Silvério da Silva, Serges Bruno Lemoupi Ngomade, and Júlio César Santos. 2024. "Pullulan Production from Sugarcane Bagasse Hemicellulosic Hydrolysate by Aureobasidium pullulans ATCC 42023 inBubble Column Reactor" Fermentation 10, no. 6: 322. https://doi.org/10.3390/fermentation10060322
APA StyleTagne, R. F. T., Cruz-Santos, M. M., Antunes, F. A. F., Shibukawa, V. P., Miano, S. B., Kenfack, J. A. A., da Silva, S. S., Ngomade, S. B. L., & Santos, J. C. (2024). Pullulan Production from Sugarcane Bagasse Hemicellulosic Hydrolysate by Aureobasidium pullulans ATCC 42023 inBubble Column Reactor. Fermentation, 10(6), 322. https://doi.org/10.3390/fermentation10060322








