Online Monitoring of the Temperature and Relative Humidity of Recycled Bedding for Dairy Cows on Dairy Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strip Stacking Fermentation of Dairy Manure
2.3. Temperature and Relative Humidity Online Monitoring
2.4. Composition Measurement
2.5. Bacterial Content Measurement
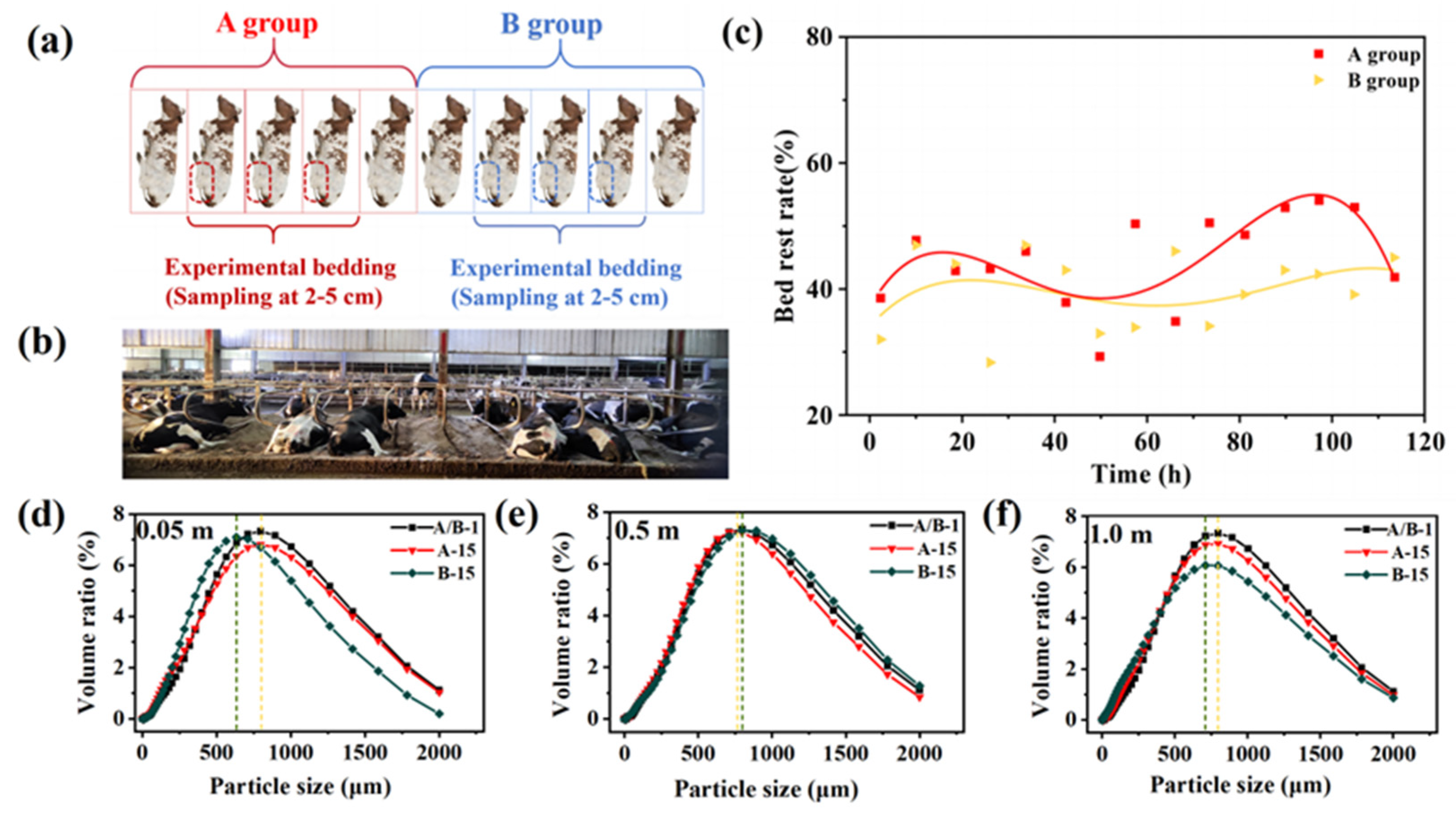
2.6. Bed Rest Rate Measurement
3. Results and Discussion
3.1. Temporal and Spatial Distribution of Temperature
3.2. Temporal and Spatial Distribution of Relative Humidity
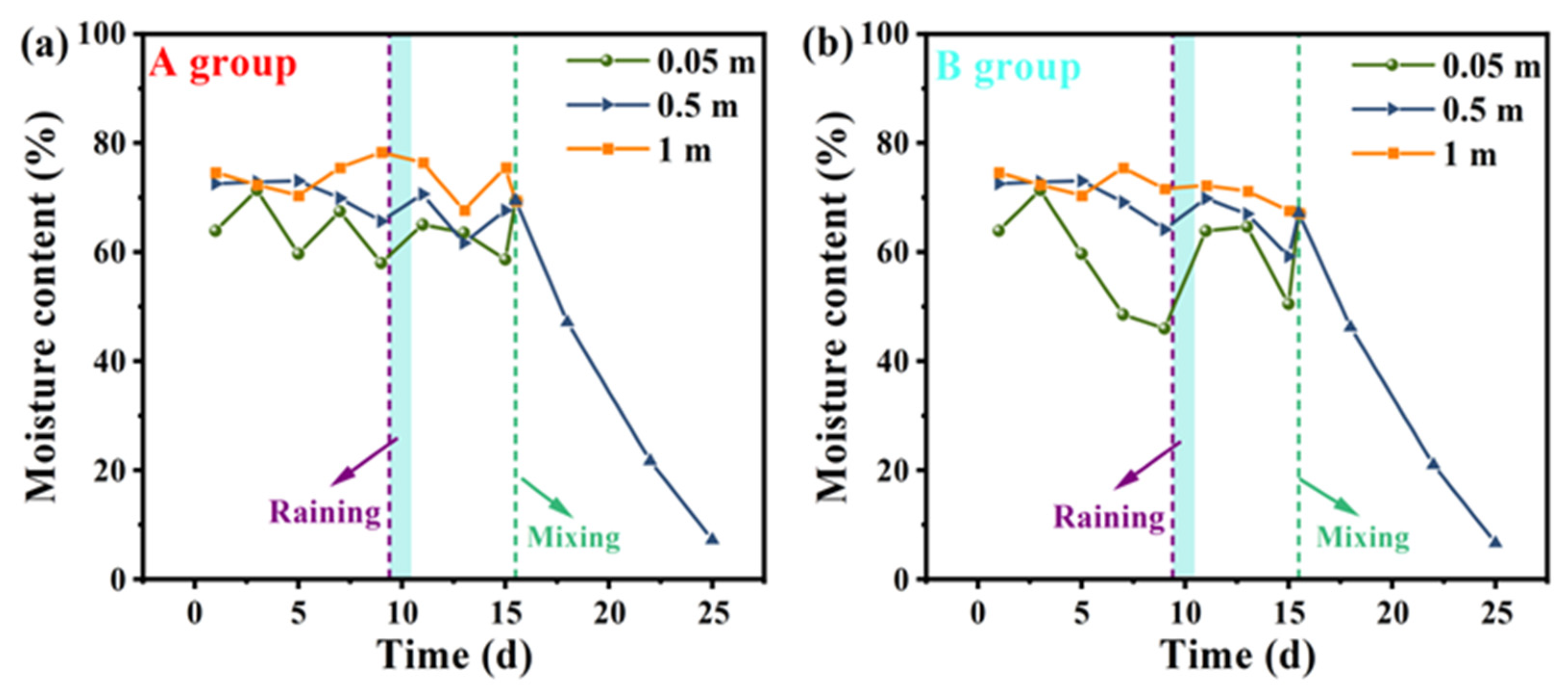
3.3. Moisture Content Analysis
3.4. Bactericidal Performance Evaluation
3.5. Composition Analysis
3.6. Bedding Bacteriostatic Performance
3.7. Bed Rest Rate Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, X.; Guo, L.; Li, C.; Liu, M.; Wu, G.; Jiang, G. The total biomass nitrogen reservoir and its potential of replacing chemical fertilizers in China. Renew. Sustain. Energy Rev. 2021, 135, 110215. [Google Scholar] [CrossRef]
- Li, Y.; Qi, C.; Zhang, Y.; Li, Y.; Wang, Y.; Li, G.; Luo, W. Anaerobic digestion of agricultural wastes from liquid to solid state: Performance and environ-economic comparison. Bioresour. Technol. 2021, 332, 125080. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Lee, M.R.F.; Ma, L.; Ledgard, S.; Oenema, O.; Velthof, G.L.; Ma, W.; Guo, M.; Zhao, Z.; Wei, S.; et al. Global environmental costs of China’s thirst for milk. Glob. Chang. Biol. 2018, 24, 2198–2211. [Google Scholar] [CrossRef] [PubMed]
- Adghim, M.; Abdallah, M.; Saad, S.; Shanableh, A.; Sartaj, M.; El Mansouri, A.E. Comparative life cycle assessment of anaerobic co-digestion for dairy waste management in large-scale farms. J. Clean. Prod. 2020, 256, 120320. [Google Scholar] [CrossRef]
- Wang, X.; Ledgard, S.; Luo, J.; Guo, Y.; Zhao, Z.; Guo, L.; Liu, S.; Zhang, N.; Duan, X.; Ma, L. Environmental impacts and resource use of milk production on the North China Plain, based on life cycle assessment. Sci. Total Environ. 2018, 625, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Tasistro, A.S.; Cabrera, M.L.; Ritz, C.W.; Kissel, D.E. Manipulating bedding materials and PLTTM to reduce NH(3) emissions from broiler manure. Bioresour. Technol. 2008, 99, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Zigo, F.; Sasáková, N.; Gregová, G.; Výrostková, J.; Ondrašovičová, S. Effects of Using an Alternative Bedding Composition on the Levels of Indicator Microorganisms and Mammary Health in Dairy Farm Conditions. Agriculture 2020, 10, 245. [Google Scholar] [CrossRef]
- Fernández, A.; Mainau, E.; Manteca, X.; Siurana, A.; Castillejos, L. Impacts of Compost Bedded Pack Barns on the Welfare and Comfort of Dairy Cows. Animals 2020, 10, 431. [Google Scholar] [CrossRef]
- Fávero, S.; Portilho, F.V.R.; Oliveira, A.C.R.; Langoni, H.; Pantoja, J.C.F. Factors associated with mastitis epidemiologic indexes, animal hygiene, and bulk milk bacterial concentrations in dairy herds housed on compost bedding. Livest. Sci. 2015, 181, 220–230. [Google Scholar] [CrossRef]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited review: Compost-bedded pack barns for dairy cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef]
- Bertocchi, L.; Fusi, F.; Angelucci, A.; Bolzoni, L.; Pongolini, S.; Strano, R.M.; Ginestreti, J.; Riuzzi, G.; Moroni, P.; Lorenzi, V. Characterization of hazards, welfare promoters and animal-based measures for the welfare assessment of dairy cows: Elicitation of expert opinion. Prev. Vet. Med. 2018, 150, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.J.; Leach, K.A.; Green, M.J.; Gibbons, J.; Ohnstad, I.C.; Black, D.H.; Payne, B.; Prout, V.E.; Breen, J.E. The impact of dairy cows’ bedding material and its microbial content on the quality and safety of milk—A cross sectional study of UK farms. Int. J. Food Microbiol. 2018, 269, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Šubová, E.; Sasáková, N.; Zigo, F.; Mindžáková, I.; Vargová, M.; Kachnič, J.; Laktičová, K.V. Amendment of Livestock Manure with Natural Zeolite-Clinoptilolite and Its Effect on Decomposition Processes during Composting. Agriculture 2021, 11, 980. [Google Scholar] [CrossRef]
- Ferraz, P.F.P.; Ferraz, G.A.E.S.; Leso, L.; Klopcic, M.; Barbari, M.; Rossi, G. Properties of conventional and alternative bedding materials for dairy cattle. J. Dairy Sci. 2020, 103, 8661–8674. [Google Scholar] [CrossRef] [PubMed]
- Kupczyński, R.; Bednarski, M.; Budny-Walczak, A.; Kociuba, W. Evaluation of Suitability of New Bedding Material Obtained after Straw Biogasification for Dairy Cows. Animals 2023, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence rate of pathogen-specific clinical mastitis on conventional and organic Canadian dairy farms. J. Dairy Sci. 2016, 99, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Wu, Y.; Zhang, D.; Xu, H.; Xing, X.; Qi, Z. Relationships among bedding materials, bedding bacterial composition and lameness in dairy cows. Anim. Biosci. 2021, 34, 1559. [Google Scholar] [CrossRef] [PubMed]
- Vargová, M.; Zigo, F.; Výrostková, J.; Farkašová, Z.; Rehan, I.F. Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F.O. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Owada, H.; Kobayashi, S.; Komatsu, N.; Takehara, K.; Ito, M.; Matsuda, K.; Sato, K.; Itabashi, H.; Sugimura, S.; et al. Cacao bean husk: An applicable bedding material in dairy free-stall barns. Asian-Australas. J. Anim. Sci. 2017, 30, 1048–1053. [Google Scholar] [CrossRef]
- Yin, H.; Fang, C.; He, X.; Yu, H.; Liang, Y.; Han, L.; Huang, G. Safety production and application of dairy bedding by membrane-covered aerobic fermentation: Insight into the evolution of mastitis pathogens and harmful gas emissions. J. Environ. Chem. Eng. 2023, 11, 110002. [Google Scholar] [CrossRef]
- Zeng, J.; Shen, X.; Sun, X.; Liu, N.; Han, L.; Huang, G. Spatial and temporal distribution of pore gas concentrations during mainstream large-scale trough composting in China. Waste Manag. 2018, 75, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sobte, H.F.M.; Buijs, S. Impact of paper bedding on lying behaviour and welfare in lactating dairy cows. Appl. Anim. Behav. Sci. 2021, 239, 105321. [Google Scholar] [CrossRef]
- Teixeira, D.L.; Villarroel, M.; María, G.A. Assessment of different organic beddings materials for fattening lamb. Small Rumin. Res. 2014, 119, 22–27. [Google Scholar] [CrossRef]
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Water addition, evaporation and water holding capacity of poultry litter. Sci. Total Environ. 2015, 538, 979–985. [Google Scholar] [CrossRef]
- Fregonesi, J.A.; Veira, D.M.; von Keyserlingk, M.A.G.; Weary, D.M. Effects of Bedding Quality on Lying Behavior of Dairy Cows. J. Dairy Sci. 2007, 90, 5468–5472. [Google Scholar] [CrossRef]
- Peltre, C.; Dignac, M.F.; Derenne, S.; Houot, S. Change of the chemical composition and biodegradability of the Van Soest soluble fraction during composting: A study using a novel extraction method. Waste Manag. 2010, 30, 2448–2460. [Google Scholar] [CrossRef]
- Robles, I.; Kelton, D.F.; Barkema, H.W.; Keefe, G.P.; Roy, J.P.; von Keyserlingk, M.A.G.; DeVries, T.J. Bacterial concentrations in bedding and their association with dairy cow hygiene and milk quality. Animal 2020, 14, 1052–1066. [Google Scholar] [CrossRef]
- Pabón-Pereira, C.P.; Hamelers, H.V.M.; Matilla, I.; van Lier, J.B. New Insights on the Estimation of the Anaerobic Biodegradability of Plant Material: Identifying Valuable Plants for Sustainable Energy Production. Processes 2020, 8, 806. [Google Scholar] [CrossRef]
- Hossain, M.S.; Nik Norulaini, N.A.; Banana, A.A.; Mohd Zulkhairi, A.R.; Ahmad Naim, A.Y.; Mohd Omar, A.K. Modeling the supercritical carbon dioxide inactivation of Staphylococcus aureus, Escherichia coli and Bacillus subtilis in human body fluids clinical waste. Chem. Eng. J. 2016, 296, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.-Q.; Jiang, B.; Mo, X.-B.; Du, J.-J.; Li, A.-X. Influence of temperature on the vaccine efficacy against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 521, 734943. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.L.; Todhunter, D.A.; Schoenberger, P.S. Bacterial Counts Associated with Recycled Newspaper Bedding. J. Dairy Sci. 1990, 73, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Okuyama, K.; Sato, M. Effect of culture temperature on high-voltage pulse sterilization of Escherichia coli. J. Electrost. 2002, 55, 227–235. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, Q.; Liu, Z.; Wang, D.; Liu, X.; He, D.; He, Y.; Li, Y.; Yang, J.; Duan, A. Insights into potassium permanganate reducing H2S generation from anaerobic fermentation of sludge. Chem. Eng. J. 2022, 430, 133150. [Google Scholar] [CrossRef]
- Amon, B.; Amon, T.; Boxberger, J.; Alt, C. Emissions of NH3, N2O and CH4 from dairy cows housed in a farmyard manure tying stall (housing, manure storage, manure spreading). Nutr. Cycl. Agroecosyst. 2001, 60, 103–113. [Google Scholar] [CrossRef]
- Das, K.; Keener, H.M. Moisture effect on compaction and permeability in composts. J. Environ. Eng. 1997, 123, 275–281. [Google Scholar] [CrossRef]
- Giambra, I.J.; Jahan, Y.; Yin, T.; Engel, P.; Weimann, C.; Brügemann, K.; König, S. Identification of Thermophilic Aerobic Sporeformers in Bedding Material of Compost-Bedded Dairy Cows Using Microbial and Molecular Methods. Animals 2021, 11, 2890. [Google Scholar] [CrossRef]
- Megiatto, J.D.; Silva, C.G.; Rosa, D.S.; Frollini, E. Sisal chemically modified with lignins: Correlation between fibers and phenolic composites properties. Polym. Degrad. Stab. 2008, 93, 1109–1121. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Akinmoladun, O.F.; Mpendulo, C.T. Enhancement of the Nutritional Composition and Antioxidant Activities of Fruit Pomaces and Agro-Industrial Byproducts through Solid-State Fermentation for Livestock Nutrition: A Review. Fermentation 2024, 10, 227. [Google Scholar] [CrossRef]
- Tang, M.; Wu, Z.; Li, W.; Shoaib, M.; Aqib, A.I.; Shang, R.; Yang, Z.; Pu, W. Effects of different composting methods on antibiotic-resistant bacteria, antibiotic resistance genes, and microbial diversity in dairy cattle manures. J. Dairy Sci. 2023, 106, 257–273. [Google Scholar] [CrossRef]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Sutaryo, S.; Sempana, A.N.; Mulya, R.M.; Sulistyaningrum, D.; Ali, M.S.; Damarjati, R.I.; Purbowati, E.; Adiwinarti, R.; Purnomoadi, A. Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure. Fermentation 2022, 8, 736. [Google Scholar] [CrossRef]
- Munksgaard, L.; Jensen, M.B.; Pedersen, L.J.; Hansen, S.W.; Matthews, L. Quantifying behavioural priorities—Effects of time constraints on behaviour of dairy cows, Bos taurus. Appl. Anim. Behav. Sci. 2005, 92, 3–14. [Google Scholar] [CrossRef]
- Norring, M.; Manninen, E.; de Passillé, A.M.; Rushen, J.; Munksgaard, L.; Saloniemi, H. Effects of Sand and Straw Bedding on the Lying Behavior, Cleanliness, and Hoof and Hock Injuries of Dairy Cows. J. Dairy Sci. 2008, 91, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.B.; Weary, D.M.; von Keyserlingk, M.A.G.; Beauchemin, K.A. Cow comfort in tie-stalls: Increased depth of shavings or straw bedding increases lying time. J. Dairy Sci. 2009, 92, 2684–2690. [Google Scholar] [CrossRef]
- Tucker, C.B.; Weary, D.M. Bedding on Geotextile Mattresses: How Much is Needed to Improve Cow Comfort? J. Dairy Sci. 2004, 87, 2889–2895. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Liu, K.; Li, Y.; Li, Z.; Zhao, T.; Zhao, P.; Qi, Y.; Li, M.; Wang, Z. Online Monitoring of the Temperature and Relative Humidity of Recycled Bedding for Dairy Cows on Dairy Farms. Fermentation 2024, 10, 346. https://doi.org/10.3390/fermentation10070346
Wei Y, Liu K, Li Y, Li Z, Zhao T, Zhao P, Qi Y, Li M, Wang Z. Online Monitoring of the Temperature and Relative Humidity of Recycled Bedding for Dairy Cows on Dairy Farms. Fermentation. 2024; 10(7):346. https://doi.org/10.3390/fermentation10070346
Chicago/Turabian StyleWei, Yong, Kun Liu, Yaao Li, Zhixing Li, Tianyu Zhao, Pengfei Zhao, Yayin Qi, Meiying Li, and Zongyuan Wang. 2024. "Online Monitoring of the Temperature and Relative Humidity of Recycled Bedding for Dairy Cows on Dairy Farms" Fermentation 10, no. 7: 346. https://doi.org/10.3390/fermentation10070346
APA StyleWei, Y., Liu, K., Li, Y., Li, Z., Zhao, T., Zhao, P., Qi, Y., Li, M., & Wang, Z. (2024). Online Monitoring of the Temperature and Relative Humidity of Recycled Bedding for Dairy Cows on Dairy Farms. Fermentation, 10(7), 346. https://doi.org/10.3390/fermentation10070346






