Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N. cadamba Leaf Powder
2.2. Extraction of NE
2.3. Stylo Silage Preparation
2.4. Microbial Counts and Chemical Composition Analysis
2.5. Bacterial Community Analyses
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Raw Stylo Materials Prior to Ensiling
3.2. Fermentation Quality and Microbial Population
3.3. Organic Acids and Nutritional Quality
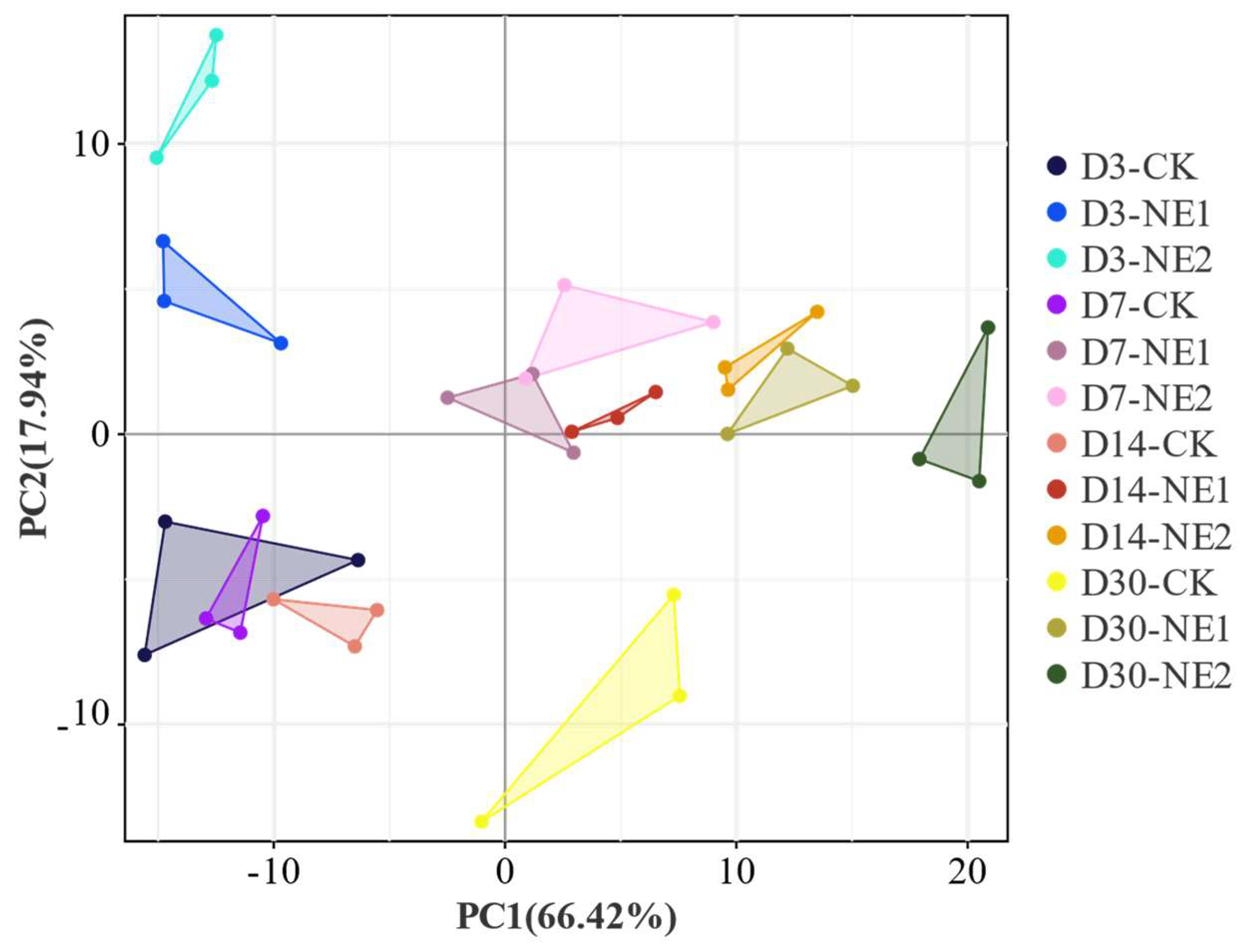
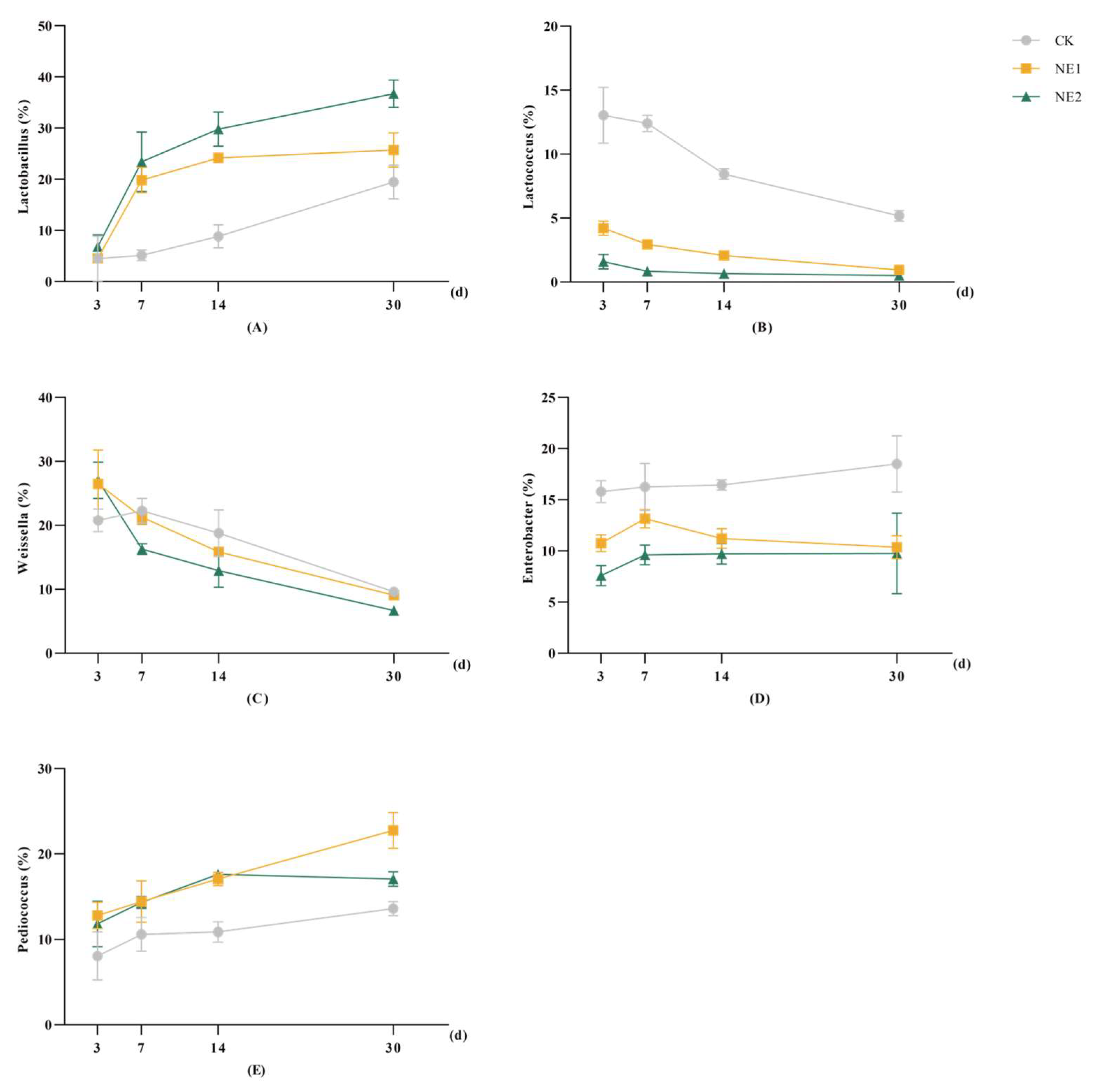
3.4. Microbial Community of Stylo Silage during Ensiling
4. Discussion
4.1. Characteristics of Raw Stylo Materials
4.2. Microbial Population and Quality of Fermentation in Stylo Silage
4.3. Organic Acids and Nitrogen Fractions of Stylo Silage
4.4. Bacterial Diversity of Stylo Silage
4.5. Bacterial Abundance in Stylo Silage
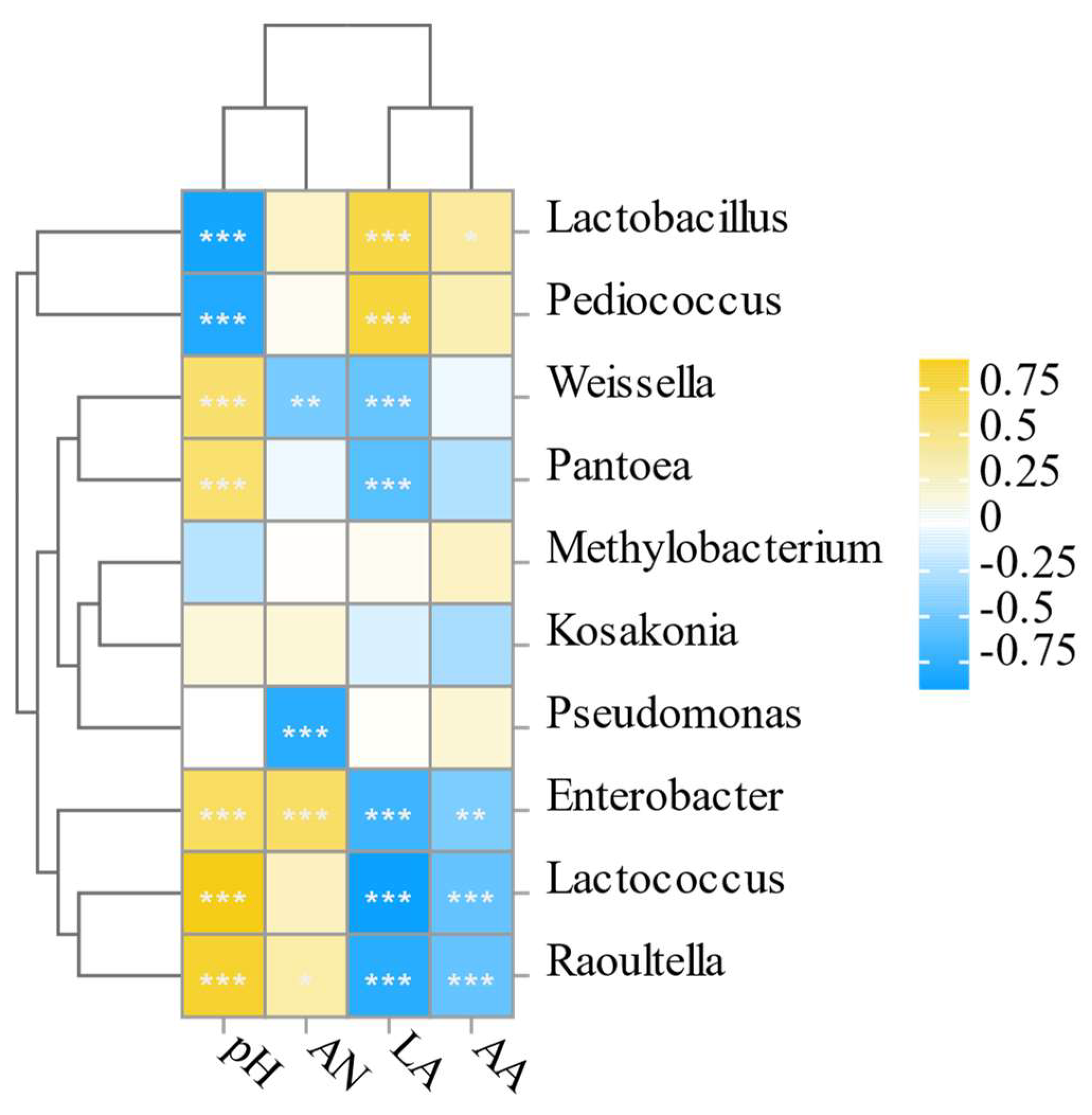
4.6. Analysis of the Relationship between the Microbial Community Structure and the Quality of Fermentation in Stylo Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, C.; Liu, P.; Wang, X.; Zhang, W.; He, L. Effects of phenyllactic acid on fermentation parameters, nitrogen fractions and bacterial community of high-moisture stylo silage. Fermentation 2023, 9, 572. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Lv, H.; Zhou, W.; Zhang, Q. Beneficial effects of tannic acid on the quality of bacterial communities present in high-moisture mulberry leaf and stylo silage. Front. Microbiol. 2020, 11, 586412. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Huang, L.; Tian, R.; Wu, J.; Tang, R.; Zhang, J. Fermentation quality and bacterial community of delayed filling stylo silage in response to inoculating lactic acid bacteria strains and inoculating time. Chemical and Biological Technologies in Agriculture 2023, 10, 44. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Zhang, J.; Shi, S.; Cai, Y. Characteristics of isolated lactic acid bacteria and their effectiveness to improve stylo (Stylosanthes guianensis Sw.) silage quality at various temperatures. Anim. Sci. J. 2012, 83, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Denek, N.; Can, A.; Avci, M.; Aksu, T.; Durmaz, H. The effect of molasses-based pre-fermented juice on the fermentation quality of first-cut lucerne silage. Grass Forage Sci. 2011, 66, 243–250. [Google Scholar] [CrossRef]
- Bao, J.; Wang, L.; Yu, Z. Effects of different moisture levels and additives on the ensiling characteristics and in vitro digestibility of Stylosanthes silage. Animals 2022, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, C.; Wang, Y.; Zhang, W.; He, L. Effect of tea polyphenols on the fermentation quality, protein preservation, antioxidant capacity and bacterial community of stylo silage. Front. Microbiol. 2022, 13, 993750. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Zi, X.; Cai, Y. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, Y.F.; Miranda, E.; Lopez, M.F.; Ordonez, P.E. Antibacterial activity of plant extracts against Streptococcus equi subsp. zooepidemicus isolates from guinea pigs with lymphadenitis in Ecuador. Heliyon 2024, 10, e25226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Q.; Wang, S. Effects of rosemary extract, grape seed extract and green tea polyphenol on the formation of N-nitrosamines and quality of western-style smoked sausage. J. Food Process Pres. 2020, 44, e14459. [Google Scholar] [CrossRef]
- Haris, Z.; Ahmad, I. Evaluation of antioxidant-rich bioactive plant extracts with special reference to Moringa oleifera Lam. for their interference to bacterial quorum sensing and biofilm. Phytomed. Plus 2024, 4, 100511. [Google Scholar] [CrossRef]
- Li, S.; Sui, M.; Wu, F.; Chen, X.; Chen, B.; Yao, L. Effects of Chinese herbal plant extracts on diarrhea rate, intestinal morphology, nutrient digestibility, and immunity of weaned piglets. Can. J. Anim. Sci. 2023, 103, 388–393. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Zou, X.; Ruan, S.; Kholif, A.E.; Hu, L.; Chen, X.; Zhou, W. Effects of Neolamarckia cadamba leaves extract on methanogenesis, microbial community in the rumen and digestibility of stylo silage. J. Clean. Prod. 2022, 369, 133338. [Google Scholar] [CrossRef]
- Adli, D.N.; Sugiharto, S.; Irawan, A.; Tribudi, Y.A.; Wibowo, S.; Azmi, A.; Sjofjan, O.; Jayanegara, A.; Tistiana, H.; Wahyono, T.; et al. The effects of herbal plant extract on the growth performance, blood parameters, nutrient digestibility and carcase quality of rabbits: A meta-analysis. Heliyon 2024, 10, e25724. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, D.; Lv, H.; Zhang, Q.; Zheng, P. Effect of ellagic acid on fermentation quality and bacterial community of stylo silage. Fermentation 2021, 7, 256. [Google Scholar] [CrossRef]
- Vaiciuliene, G.; Bakutis, B.; Jovaisiene, J.; Falkauskas, R.; Gerulis, G.; Bartkiene, E.; Klupsaite, D.; Klementaviciute, J.; Baliukoniene, V. Effects of Ethanol Extracts of Origanum vulgare and Thymus vulgaris on the Mycotoxin Concentrations and the Hygienic Quality of Maize (Zea mays L.) Silage. Toxins 2022, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; He, Z.; Xiao, Y.; Ouyang, K.; Wang, X.; Hu, X. Population structure and genetic diversity in the natural distribution of Neolamarckia cadamba in China. Genes 2023, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Rahman, S.; Ahmed, N.; Hossain, M.; Biswas, A.; Sarkar, S.; Banna, H.; Khatun, A.; Chowdhury, M.H.; Rahmatullah, M. Evaluation of Neolamarckia Cadamba (Roxb.) bosser leaf extract on glucose tolerance In glucose-induced hyperglycemic mice. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Dwevedi, A.; Sharma, K.; Sharma, Y.K. Cadamba: A miraculous tree having enormous pharmacological implications. Pharmacogn. Rev. 2015, 9, 107–113. [Google Scholar] [CrossRef]
- Mishra, A.; Maurya, S.K.; Singh, A.; Siddique, H.; Samanta, S.K.; Mishra, N. Neolamarckia cadamba (Roxb.) Bosser (Rubiaceae) extracts: Promising prospects for anticancer and antibacterial potential through in vitro and in silico studies. Med. Oncol. 2023, 40, 99. [Google Scholar] [CrossRef] [PubMed]
- Chandel, M.; Sharma, U.; Kumar, N.; Singh, B.; Kaur, S. Antioxidant activity and identification of bioactive compounds from leaves of Anthocephalus cadamba by ultra-performance liquid chromatography/electrospray ionization quadrupole time of flight mass spectrometry. Asian Pac. J. Trop. Med. 2012, 5, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Sahu, M.C.; Rath, S.; Paty, B.P.; Debata, N.K.; Padhy, R.N. Antimicrobial activity of medicinal plants used by aborigines of Kalahandi, Orissa, India against multidrug resistant bacteria. Asian Pac. J. Trop. Biomed. 2012, 2, S846–S854. [Google Scholar] [CrossRef]
- Khandelwal, V.; Bhatia, A.K.; Goel, A. Antimicrobial and antioxidant efficacy of aqueous extract of Anthocephalus cadamba leaves. J. Pure Appl. Microbiol. 2016, 10, 209. [Google Scholar]
- Rajesh, T.; Roy, A.K.; Erumalla, V.R.; Goli, D.; Basha, S.J. Development and evaluation of antimicrobial ointment formulation containing extracts of Ocimum sanctum, Anthocephalus cadamba, Allium sativum and Origanum vulgare. World J. Pharm. Res. 2014, 3, 398–422. [Google Scholar]
- Zi, X.; Liu, Y.; Chen, T.; Li, M.; Zhou, H.; Tang, J. Effects of Sucrose, Glucose and Molasses on Fermentation Quality and Bacterial Community of Stylo Silage. Fermentation 2022, 8, 191. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy. Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Köhler, B.; Taube, F.; Ostertag, J.; Thurner, S.; Kluß, C.; Spiekers, H. Dry-matter losses and changes in nutrient concentrations in grass and maize silages stored in bunker silos. Grass Forage Sci. 2019, 74, 274–283. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R. Determination of water-soluble carbohydrates in grass. J. Sci. Food Agric. 1964, 15, 395–398. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed. Sci. Tech. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, M.; Zhang, Y.; Xu, J.; Li, P.; Sun, H.; Xie, Y.; Dong, R.; Zheng, Y.; Chen, C. Effects of Different Cutting Stages and Additives on the Fermentation Quality and Microbial Community of Sudangrass (Sorghum sudanense Stapf.) Silages. Fermentation 2023, 9, 777. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Shi, S.; Sun, Q. The effects of wilting and storage temperatures on the fermentation quality and aerobic stability of stylo silage. Anim. Sci. J. 2011, 82, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Da, S.J.; Ribeiro, K.G.; Pereira, O.G.; Mantovani, H.C.; Cecon, P.R.; Pereira, R.C.; Silva, J.L. Nutritive value and fermentation quality of palisadegrass and stylo mixed silages. Anim. Sci. J. 2018, 89, 72–78. [Google Scholar] [CrossRef]
- Bureenok, S.; Sisaath, K.; Yuangklang, C.; Vasupen, K.; Schonewille, J.T. Ensiling characteristics of silages of Stylo legume (Stylosanthes guianensis), Guinea grass (Panicum maximum) and their mixture, treated with fermented juice of lactic bacteria, and feed intake and digestibility in goats of rations based on these silages. Small Ruminant Res. 2016, 134, 84–89. [Google Scholar] [CrossRef]
- Guyader, J.; Baron, V.; Beauchemin, K. Corn Forage Yield and Quality for Silage in Short Growing Season Areas of the Canadian Prairies. Agronomy 2018, 8, 164. [Google Scholar] [CrossRef]
- Fang, D.; Dong, Z.; Wang, D.; Li, B.; Shi, P.; Yan, J.; Zhuang, D.; Shao, T.; Wang, W.; Gu, M. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J. Appl. Microbiol. 2022, 132, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of lactobacillus spp. from an inoculant and of weissella and leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Stough, E.C.; McDonell, E.E.; Schmidt, R.J.; Hofherr, M.W.; Reich, L.J.; Klingerman, C.M. The effect of wide swathing on wilting times and nutritive value of alfalfa haylage. J. Dairy. Sci. 2010, 93, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy. Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Chen, Y.; Fu, C.; Long, W.; Xiao, X.; Liao, H.; Yang, Y. Bamboo lignocellulose degradation by gut symbiotic microbiota of the bamboo snout beetle Cyrtotrachelus buqueti. Biotechnol. Biofuels 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresource Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy. Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Kumai, S.; Zhang, J.; Benno, Y. Comparative studies of lactobacilli and enterococci associated with forage crops as silage inoculants. Anim. Sci. J. 1999, 70, 188–194. [Google Scholar] [CrossRef]
- Ren, F.; He, R.; Zhou, X.; Gu, Q.; Xia, Z.; Liang, M.; Zhou, J.; Lin, B.; Zou, C. Dynamic changes in fermentation profiles and bacterial community composition during sugarcane top silage fermentation: A preliminary study. Bioresource Technol. 2019, 285, 121315. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy. Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Bandla, N.; Südekum, K.; Gerlach, K. Review: Role of silage volatile organic compounds in influencing forage choice behavior and intake in ruminants. Anim. Feed. Sci. Tech. 2024, 307, 115853. [Google Scholar] [CrossRef]
- Ohshima, M.; McDonald, P. A review of the changes in nitrogenous compounds of herbage during ensilage. J. Sci. Food Agric. 1978, 29, 497–505. [Google Scholar] [CrossRef]
- He, L.; Chen, N.; Lv, H.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresource Technol. 2020, 295, 122255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, X.; Elsabagh, M.; Lin, B.; Wang, H. Effects of formic acid and corn flour supplementation of banana pseudostem silages on nutritional quality of silage, growth, digestion, rumen fermentation and cellulolytic bacterial community of Nubian black goats. J. Integr. Agric. 2021, 20, 2214–2226. [Google Scholar] [CrossRef]
- Henderson, N. Silage additives. Anim. Feed. Sci. Tech. 1993, 45, 35–56. [Google Scholar] [CrossRef]
- Qureshi, A.K.; Liew, S.Y.; Othman, N.A.; Awang, K. Phytochemical constituents from Neolamarckia cadamba (Roxb.) Bosser. Biochem. Syst. Ecol. 2021, 96, 104257. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresource Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- Miller, M.D.; Kokko, C.; Ballard, C.S.; Dann, H.M.; Fustini, M.; Palmonari, A.; Formigoni, A.; Cotanch, K.W.; Grant, R.J. Influence of fiber degradability of corn silage in diets with lower and higher fiber content on lactational performance, nutrient digestibility, and ruminal characteristics in lactating Holstein cows. J. Dairy. Sci. 2021, 104, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Pech Cervantes, A.A.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy. Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
- Li, S.; Bao, Y.; Lv, M.; Zhang, L.; Liu, L.; Liu, Y.; Lu, Q. Comparative Na+ and K+ profiling reveals microbial community assembly of alfalfa silage in different saline-alkali soils. Fermentation 2023, 9, 877. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela Saldinger, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biot. 2018, 102, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Ridwan, R.; Rusmana, I.; Widyastuti, Y.; Wiryawan, K.G.; Prasetya, B.; Sakamoto, M.; Ohkuma, M. Fermentation Characteristics and Microbial Diversity of Tropical Grass-legumes Silages. Asian-Australas. J. Anim. Sci. 2015, 28, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ke, W.; Lu, Q.; Gao, L.; Zhou, X.; Ma, C. Effects of Total Flavonoids from Taraxacum mongolicum Hand.-Mazz. on Fermentation Quality, Antioxidant Status and Microbial Community of Caragana korshinskii Kom. silage. Fermentation 2023, 9, 949. [Google Scholar] [CrossRef]
- Ke, W.C.; Yang, F.Y.; Undersander, D.J.; Guo, X.S. Fermentation characteristics, aerobic stability, proteolysis and lipid composition of alfalfa silage ensiled with apple or grape pomace. Anim. Feed. Sci. Tech. 2015, 202, 12–19. [Google Scholar] [CrossRef]
- Graf, K.; Ulrich, A.; Idler, C.; Klocke, M. Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 2016, 120, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 12, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Noori, F.; Etesami, H.; Najafi, Z.H.; Khoshkholgh-Sima, N.A.; Hosseini, S.G.; Alishahi, F. Mining alfalfa (Medicago sativa L.) nodules for salinity tolerant non-rhizobial bacteria to improve growth of alfalfa under salinity stress. Ecotoxicol. Environ. Saf. 2018, 162, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Tech. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Li, X.; MacAdam, J.W.; Zhang, Y. Interaction between plants and epiphytic lactic acid bacteria that affect plant silage fermentation. Front. Microbiol. 2023, 14, 1164904. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, H.; Xu, Y. Source tracking of prokaryotic communities in fermented grain of Chinese strong-flavor liquor. Int. J. Food Microbiol. 2017, 244, 27–35. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Lin, Y.; Xu, G.; Ni, K.; Yang, F. Effect of lactic acid bacteria and wheat bran on the fermentation quality and bacterial community of Broussonetia papyrifera silage. Chem. Biol. Technol. Agric. 2023, 10, 130. [Google Scholar] [CrossRef]

| Item | Stylo |
|---|---|
| DM (% FM) | 32.23 ± 0.30 |
| CP (% DM) | 10.74 ± 0.45 |
| NDF (% DM) | 62.38 ± 0.17 |
| ADF (% DM) | 47.01 ± 1.12 |
| WSC (% DM) | 1.79 ± 0.06 |
| Lactic acid bacteria (l g cfu·g−1 FM) | 4.52 ± 0.26 |
| Coliform (lg cfu·g−1 FM) | 4.80 ± 0.10 |
| Yeasts (lg cfu·g−1 FM) | 3.32 ± 0.11 |
| Molds (lg cfu·g−1 FM) | 2.98 ± 0.05 |
| Items | Treatments | Storage Days | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 30 | T | D | T × D | |||
| DM (% FM) | CK | 32.01 b | 31.98 c | 31.69 b | 32.32 | 0.86 | <0.01 | <0.01 | 0.03 |
| NE1 | 33.59 aA | 33.23 bA | 32.01 bB | 32.77 AB | |||||
| NE2 | 33.67 a | 34.23 a | 33.25 a | 33.10 | |||||
| DM loss (%) | CK | 0.31 aD | 0.61 aC | 0.96 aB | 1.97 aA | 0.59 | <0.01 | <0.01 | <0.01 |
| NE1 | 0.27 abD | 0.49 bC | 0.87 abB | 1.73 bA | |||||
| NE2 | 0.22 bD | 0.41 cC | 0.73 bB | 1.65 cA | |||||
| pH | CK | 5.42 aA | 5.25 aB | 5.25 aB | 5.09 aC | 0.28 | <0.01 | <0.01 | <0.01 |
| NE1 | 5.00 bA | 4.83 bB | 4.75 bBC | 4.71 bC | |||||
| NE2 | 5.06 bA | 4.69 cB | 4.56 cC | 4.58 cC | |||||
| Lactic acid bacteria (lg cfu·g−1 FM) | CK | 8.88 A | 8.40 cAB | 8.81 A | 8.25 bB | 0.35 | <0.01 | <0.01 | <0.01 |
| NE1 | 8.89 B | 9.41 aA | 8.81 BC | 8.57 aC | |||||
| NE2 | 8.86 B | 9.29 bA | 8.88 B | 8.59 aC | |||||
| Coliform (lg cfu·g−1 FM) | CK | 7.83 aA | 7.15 aB | 5.45 aC | 2.95 aD | 2.01 | <0.01 | <0.01 | <0.01 |
| NE1 | 7.26 bA | 5.76 bB | 3.71 bC | 2.36 bD | |||||
| NE2 | 6.73 cA | 5.45 bB | 2.83 cC | 2.20 bD | |||||
| Molds (lg cfu·g−1 FM) | CK | <2.00 | <2.00 | <2.00 | <2.00 | - | - | - | - |
| NE1 | <2.00 | <2.00 | <2.00 | <2.00 | |||||
| NE2 | <2.00 | <2.00 | <2.00 | <2.00 | |||||
| Yeasts (lg cfu·g−1 FM) | CK | <2.00 | <2.00 | <2.00 | <2.00 | - | - | - | - |
| NE1 | <2.00 | <2.00 | <2.00 | <2.00 | |||||
| NE2 | <2.00 | <2.00 | <2.00 | <2.00 | |||||
| Items | Treatments | Storage Days | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 30 | T | D | T × D | |||
| LA (% DM) | CK | 1.19 b | 1.09 c | 1.13 b | 1.21 b | 0.44 | <0.01 | <0.01 | 0.01 |
| NE1 | 1.47 aB | 1.49 bB | 2.06 aA | 2.20 aA | |||||
| NE2 | 1.63 aB | 1.77 aB | 2.06 aA | 2.23 aA | |||||
| AA (% DM) | CK | 0.12 cC | 0.35 cA | 0.19 cB | 0.17 cB | 0.13 | <0.01 | <0.01 | <0.01 |
| NE1 | 0.20 bC | 0.45 bA | 0.43 aA | 0.30 bB | |||||
| NE2 | 0.39 aB | 0.54 aA | 0.29 bC | 0.41 aB | |||||
| CP (% DM) | CK | 10.51 A | 10.36 A | 10.66 A | 9.92 B | 0.43 | 0.46 | <0.01 | 0.97 |
| NE1 | 10.34 | 10.15 | 10.55 | 9.92 | |||||
| NE2 | 10.28 AB | 10.03 AB | 10.70 A | 9.70 B | |||||
| TP (% DM) | CK | 7.73 A | 7.24 B | 6.98 B | 6.46 bC | 0.41 | 0.12 | <0.01 | 0.04 |
| NE1 | 7.62 A | 7.14 AB | 7.41 AB | 6.80 abB | |||||
| NE2 | 7.43 | 7.24 | 7.39 | 7.29 a | |||||
| NPN (% DM) | CK | 2.78 C | 3.12 BC | 3.68 aA | 3.47 AB | 0.45 | 0.02 | 0.01 | 0.14 |
| NE1 | 2.72 | 3.01 | 3.13 b | 3.13 | |||||
| NE2 | 2.85 AB | 2.79 AB | 3.32 abA | 2.41 B | |||||
| AN (% TN) | CK | 2.67 aD | 5.03 aC | 6.95 aB | 8.27 aA | 2.13 | <0.01 | <0.01 | <0.01 |
| NE1 | 1.37 bC | 3.04 bB | 3.94 bAB | 4.52 bA | |||||
| NE2 | 0.99 bD | 2.17 cC | 2.81 cB | 4.12 bA | |||||
| NDF (% DM) | CK | 60.55 aB | 61.72 AB | 60.49 B | 63.64 aA | 1.94 | <0.01 | <0.01 | 0.14 |
| NE1 | 60.27 aAB | 61.30 AB | 58.05 B | 62.00 abA | |||||
| NE2 | 57.45 bB | 59.79 A | 59.32 AB | 59.98 bA | |||||
| ADF (% DM) | CK | 48.86 aB | 50.32 AB | 50.90 aA | 49.05 aAB | 2.61 | <0.01 | <0.01 | <0.01 |
| NE1 | 46.62 abB | 50.23 A | 44.40 bB | 47.22 abB | |||||
| NE2 | 44.97 bBC | 49.05 A | 43.52 bC | 46.51 bAB | |||||
| Items | Sobs | Shannon | Simpson | Chao1 | Ace | Good’s Coverage | |
|---|---|---|---|---|---|---|---|
| Storage Days | Treatments | ||||||
| 3 | CK | 703 | 4.40 | 0.90 | 823 | 829 | 0.997 |
| NE1 | 812 | 4.36 | 0.88 | 903 | 918 | 0.998 | |
| NE2 | 770 | 4.03 | 0.85 | 914 | 921 | 0.998 | |
| 7 | CK | 604 | 4.21 | 0.89 | 717 | 711 | 0.998 |
| NE1 | 723 | 4.18 | 0.89 | 849 | 857 | 0.998 | |
| NE2 | 866 | 4.48 | 0.90 | 986 | 985 | 0.998 | |
| 14 | CK | 696 | 4.69 | 0.91 | 774 | 788 | 0.999 |
| NE1 | 699 | 4.38 | 0.90 | 800 | 804 | 0.998 | |
| NE2 | 825 | 4.42 | 0.89 | 901 | 896 | 0.999 | |
| 30 | CK | 549 | 4.52 | 0.91 | 617 | 617 | 0.999 |
| NE1 | 676 | 4.24 | 0.88 | 759 | 748 | 0.999 | |
| NE2 | 618 | 4.24 | 0.87 | 669 | 666 | 0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Chen, M.; Chen, D.; Zang, M.; Zhang, W.; Lin, X.; Han, H.; Zhang, Q. Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage. Fermentation 2024, 10, 347. https://doi.org/10.3390/fermentation10070347
Huang P, Chen M, Chen D, Zang M, Zhang W, Lin X, Han H, Zhang Q. Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage. Fermentation. 2024; 10(7):347. https://doi.org/10.3390/fermentation10070347
Chicago/Turabian StyleHuang, Peishan, Mengmeng Chen, Dekui Chen, Meiqi Zang, Weiling Zhang, Xiyue Lin, Hongyan Han, and Qing Zhang. 2024. "Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage" Fermentation 10, no. 7: 347. https://doi.org/10.3390/fermentation10070347
APA StyleHuang, P., Chen, M., Chen, D., Zang, M., Zhang, W., Lin, X., Han, H., & Zhang, Q. (2024). Effects of Neolamarckia cadamba Leaf Extract on Dynamic Fermentation Characteristics and Bacterial Community of Stylosanthes guianensis Silage. Fermentation, 10(7), 347. https://doi.org/10.3390/fermentation10070347






