The Effect of Covering Corn Silage with Tomato or Apple Pomace on Fermentation Parameters and Feed Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Standards
2.2. Preparation of Silage with Tomato or Apple Pomace
2.3. Chemical Analyses
2.4. OMD, ME and NEL Contents
2.5. In Vitro Gas Production
2.6. Silage Microbiological Analyses
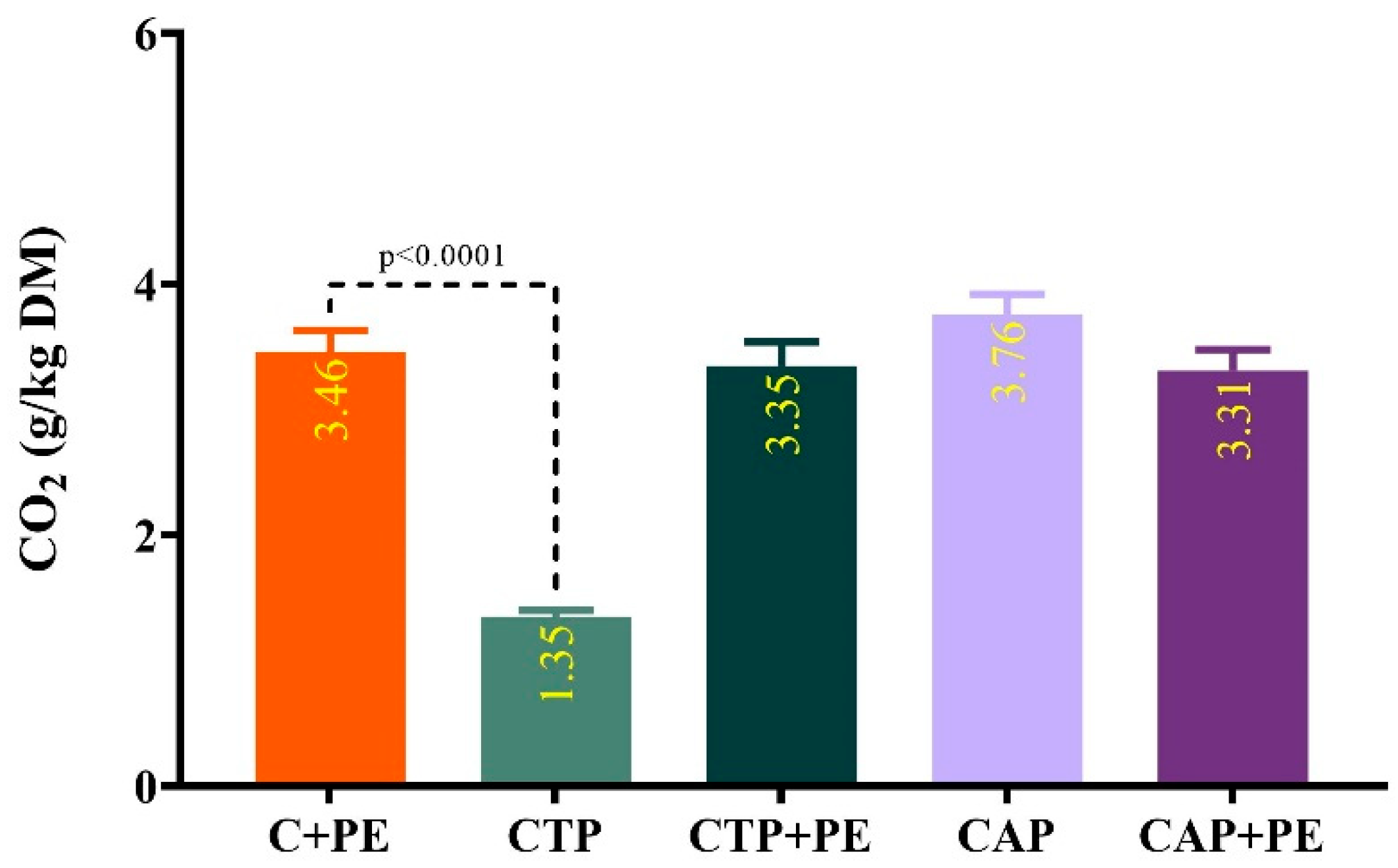
2.7. Determination of Aerobic Stability
2.8. Statistical Analysis
3. Results
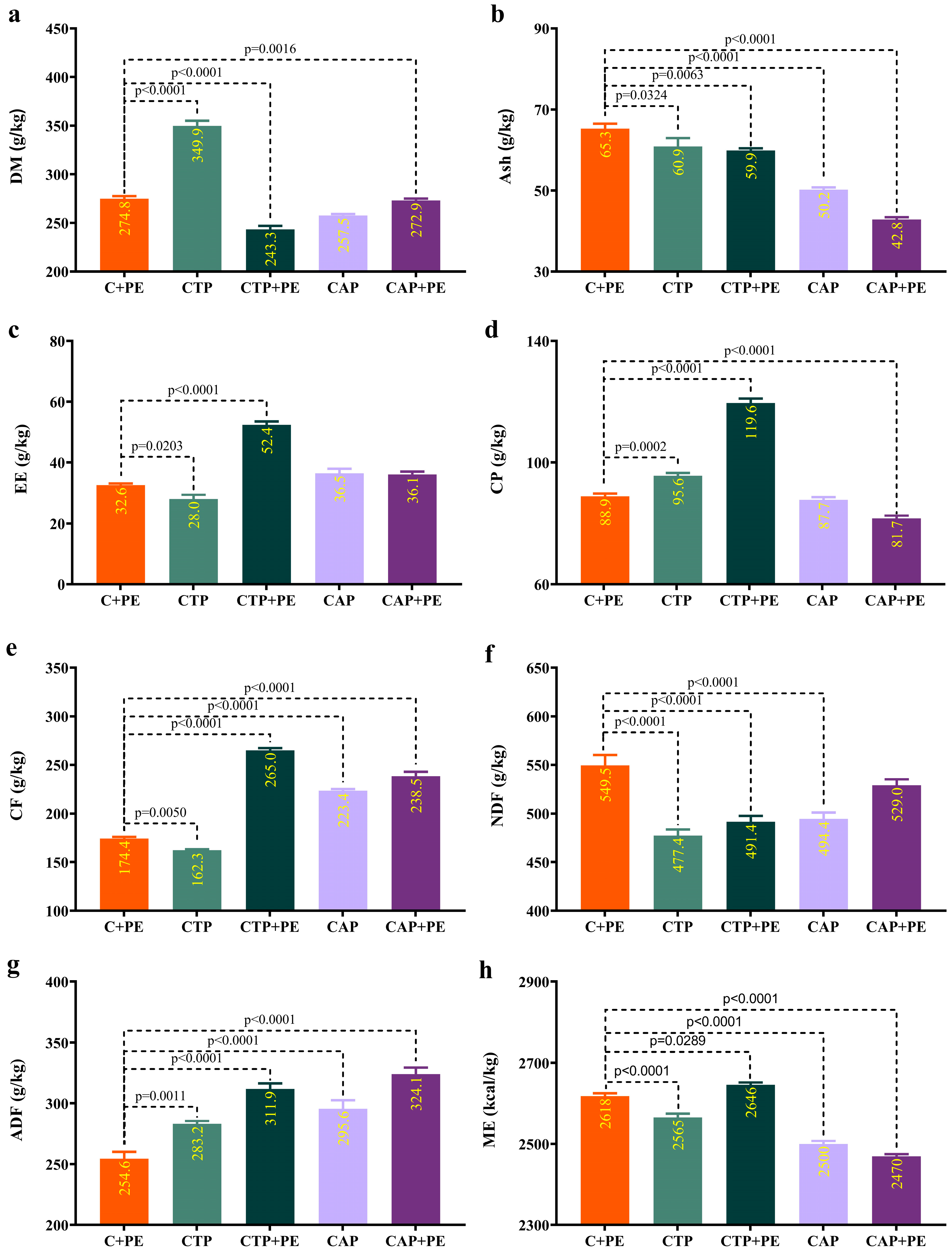
3.1. Nutritional Composition
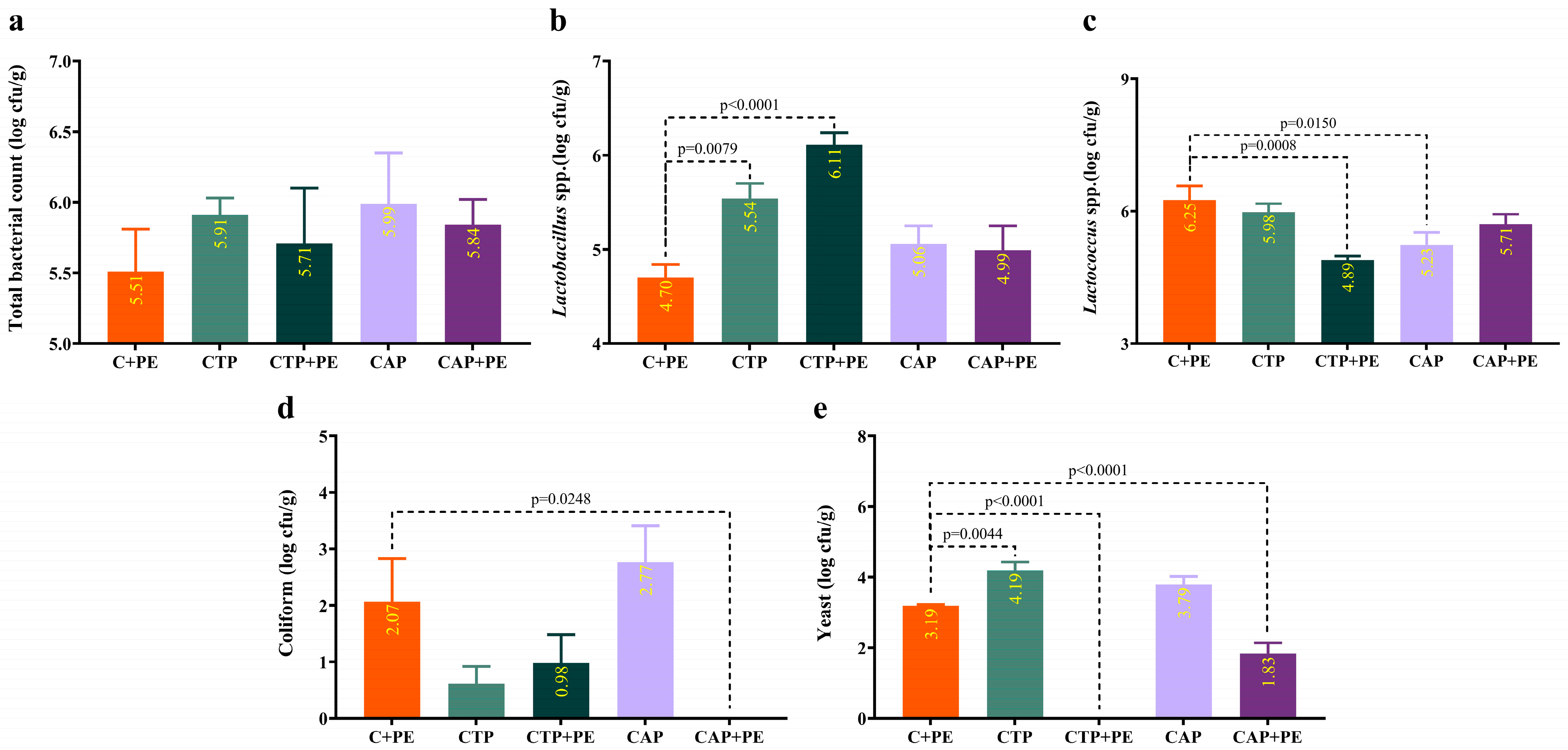
3.2. Bacterial and Yeast Count
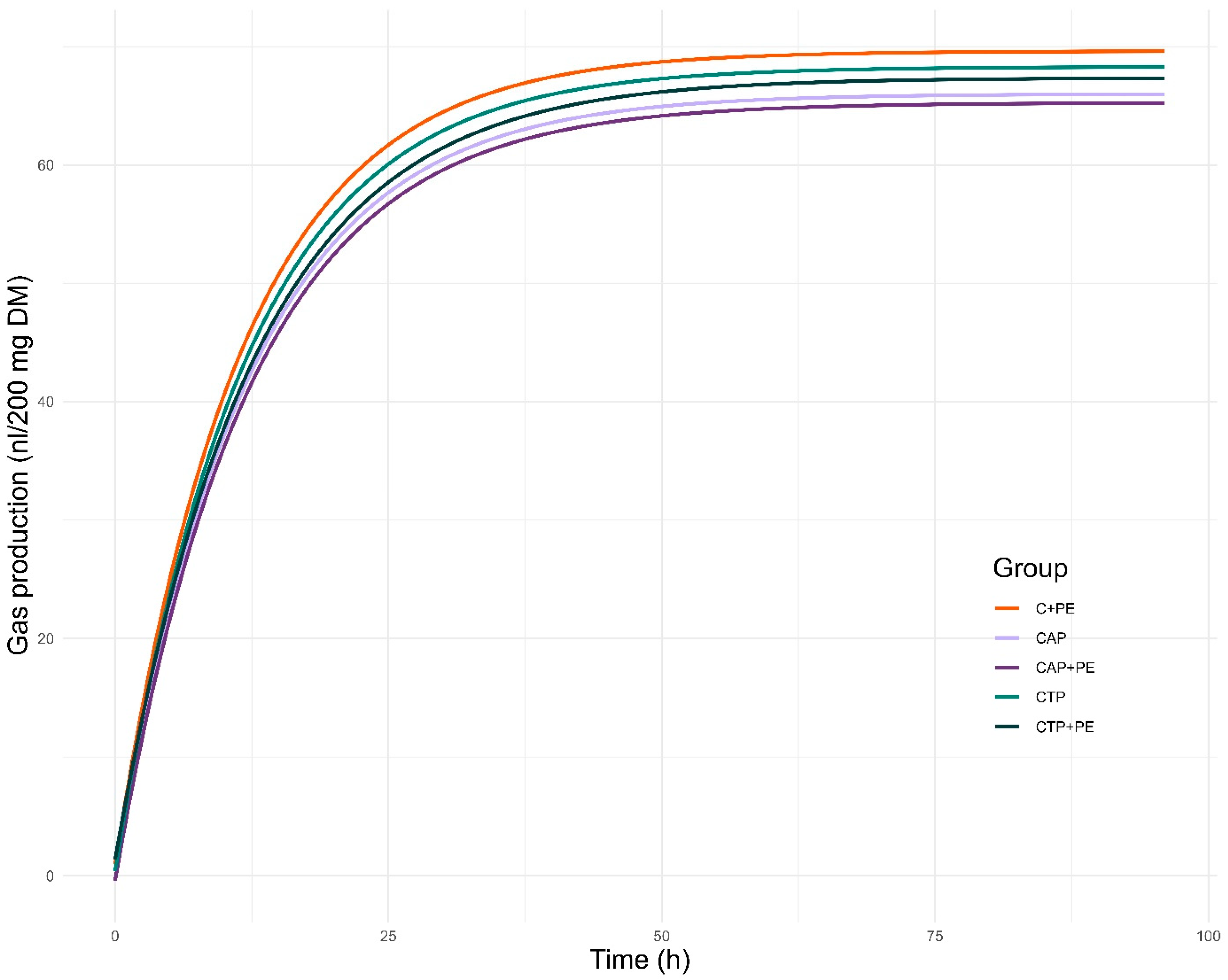
3.3. In Vitro Gas Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selli, F.; Eraslan, I.H.; Chowdhury, D. Sukumar, a International Competitiveness: Analysis of Turkish Animal Husbandry: An Empirical Study in Gap Region. Enterp. Risk Manag. 2010, 1, 100–114. [Google Scholar]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of Food and Kitchen Waste to Value-Added Products. J. Environ. Manag. 2019, 241, 619–630. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive Compounds in Food Waste: A Review on the Transformation of Food Waste to Animal Feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and Future Prospective of Lactic Acid Bacteria as Natural Additives for Silage Production—A Review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Çıtlak, H.; Kılıç, U. Innovative Approaches in Covering Materials Used in Silage Making. Int. Multiling. J. Sci. Technol. 2020, 5, 2528–9810. [Google Scholar]
- Schell, T.; Rico, A.; Vighi, M. Occurrence, Fate and Fluxes of Plastics and Microplastics in Terrestrial and Freshwater Ecosystems. Rev. Environ. Contam. Toxicol. 2020, 250, 1–43. [Google Scholar] [PubMed]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Approaching the Environmental Problem of Microplastics: Importance of WWTP Treatments. Sci. Total Environ. 2020, 740, 140016. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health Perspective of the Impacts of Microplastics on Animal, Human and Environmental Health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, H.M.; Gawad, R.M.A.; Mahmoud, A.E.M. Influence of Ration Containing Tomato Pomace Silage on Performance of Lactating Buffaloes and Milk Quality. Asian J. Anim. Vet. Adv. 2015, 10, 14–24. [Google Scholar] [CrossRef]
- Keklikçi, A.; Selçuk, Z. Determination of Digestibility of Tomato Pulp for Ruminants. Vet. Hekimler Derneği Derg. 2018, 89, 58–65. [Google Scholar]
- Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy of Sciences: Washington, DC, USA, 2001.
- Denek, N.; Can, A. Feeding Value of Wet Tomato Pomace Ensiled with Wheat Straw and Wheat Grain for Awassi Sheep. Small Rumin. Res. 2006, 65, 260–265. [Google Scholar] [CrossRef]
- Gadulrab, K.; Sidoruk, P.; Kozłowska, M.; Szumacher-Strabel, M.; Lechniak, D.; Kołodziejski, P.; Pytlewski, J.; Strzałkowska, N.; Horbańczuk, J.O.; Jóźwik, A. Effect of Feeding Dried Apple Pomace on Ruminal Fermentation, Methane Emission, and Biohydrogenation of Unsaturated Fatty Acids in Dairy Cows. Agriculture 2023, 13, 2032. [Google Scholar] [CrossRef]
- Bartel, I.; Koszarska, M.; Wysocki, K.; Kozłowska, M.; Szumacher-Strabel, M.; Cieślak, A.; Wyrwał, B.; Szejner, A.; Strzałkowska, N.; Horbańczuk, J.O. Effect of Dried Apple Pomace (DAP) as a Feed Additive on Antioxidant System in the Rumen Fluid. Int. J. Mol. Sci. 2022, 23, 10475. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace with and without Addition of a Lactiplantibacillus Plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in Vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and in Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Palangi, V. Effects of Processing Legume Forages with Organic Acids on In Vitro Gas Production, Rumen Fermantation and Methan Production; Ataturk Uni. Department of Animal Science: Erzurum, Turkey, 2019. [Google Scholar]
- Maggiolino, A.; Lorenzo, J.M.; Quiñones, J.; Latorre, M.A.; Blando, F.; Centoducati, G.; Dahl, G.E.; De Palo, P. Effects of Dietary Supplementation with Pinus Taeda Hydrolyzed Lignin on in Vivo Performances, in Vitro Nutrient Apparent Digestibility, and Gas Emission in Beef Steers. Anim. Feed Sci. Technol. 2019, 255, 114217. [Google Scholar] [CrossRef]
- Palangi, V.; Macit, M. Indictable mitigation of methane emission using some organic acids as additives towards a cleaner ecosystem. Waste Biomass Valorization 2021, 12, 4825–4834. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor in Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Sanz, S.; Olarte, C. Microbiological, Physicochemical and Sensory Characteristics of Cameros Cheese Packaged under Modified Atmospheres. Food Microbiol. 2000, 17, 407–414. [Google Scholar] [CrossRef]
- de Man, J.D.; Rogosa, D.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Terzaghi, B.E.; Sandine, W. Improved Medium for Lactic Streptococci and Their Bacteriophages. Appl. Microbiol. 1975, 29, 807–813. [Google Scholar] [CrossRef] [PubMed]
- AC07052752; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Presumptive Bacillus Cereus-Colony-Count Technique at 30 °C. ISO: Geneva, Switzerland, 2004.
- Hirsch, A.; Grinsted, E. 543. Methods for the Growth and Enumeration of Anaerobic Spore-Formers from Cheese, with Observations on the Effect of Nisin. J. Dairy Res. 1954, 21, 101–110. [Google Scholar] [CrossRef]
- Ashbell, G.; Weinberg, Z.G.; Azrieli, A.; Hen, Y.; Horev, B. A Simple System to Study the Aerobic Determination of Silages. Can. Agric. Eng. 1991, 34, 171–175. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.84; CRAN. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 4 January 2022).
- Türkgeldi, B.; Koç, F.; Lackner, M.; Okuyucu, B.; Okur, E.; Palangi, V.; Esen, S. Infrared Thermography Assessment of Aerobic Stability of a Total Mixed Ration: An Innovative Approach to Evaluating Dairy Cow Feed. Animals 2023, 13, 2225. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Piano, S.; Tabacco, E. Aerobic Stability of Maize Silage Stored under Plastic Films with Different Oxygen Permeability. J. Sci. Food Agric. 2014, 94, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.M.; Carvalho, B.F.; Bernardes, T.F.; Schwan, R.F.; da Silva Ávila, C.L. New Epiphytic Strains of Lactic Acid Bacteria Improve the Conservation of Corn Silage Harvested at Late Maturity. Anim. Feed Sci. Technol. 2021, 274, 114852. [Google Scholar] [CrossRef]
- Bueno, J.L.; Bolson, D.C.; Jacovaci, F.A.; Gomes, A.L.M.; Ribeiro, M.G.; Bueno, A.V.I.; Jobim, C.C.; Daniel, J.L.P. Storage length interacts with maturity to affect nutrient availability in unprocessed flint corn silage. Rev. Bras. Zootec. 2020, 49, e20190247. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Nussio, L.G.; do Amaral, R.C. Top Spoilage Losses in Maize Silage Sealed with Plastic Films with Different Permeabilities to Oxygen. Grass Forage Sci. 2012, 67, 34–42. [Google Scholar] [CrossRef]
- do Amaral, R.C.; Santos, M.C.; Daniel, J.L.P.; de Sá, A.; Bispo, Á.W.; Cabezas-Garcia, E.H.; Bernardes, T.F.; Nussio, L.G. The Influence of Covering Methods on the Nutritive Value of Corn Silage for Lactating Dairy Cows. Rev. Bras. Zootec. 2014, 43, 471–478. [Google Scholar] [CrossRef]
- Parra, C.S.; Bragatto, J.M.; Piran Filho, F.A.; Silva, S.M.S.; Tuzzi, B.F.; Jobim, C.C.; Daniel, J.L.P. Effect of Sealing Strategy on the Feeding Value of Corn Silage for Growing Dairy Heifers. J. Dairy Sci. 2021, 104, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Brusewitz, G.H.; Huhnke, R.L.; Barnes, E.M. Performance of Nutri-Shield in Protecting Bunker-Stored Silage. Appl. Eng. Agric. 1991, 7, 515–519. [Google Scholar] [CrossRef]
- Denoncourt, P.; Amyot, A.; Lacroix, M. Evaluation of Two Biodegradable Coatings on Corn Silage Quality. J. Sci. Food Agric. 2006, 86, 392–400. [Google Scholar] [CrossRef]
- Savoie, P.; Bernier-roy, M.; Pedneault, M.; Amyot, A. Evaluation of Apple Pulp and Peanut Butter as Alternative Bunker Silo Covers. Can. Biosyst. Eng. 2003, 45, 2–17. [Google Scholar]
- Tabacco, E.; Ferrero, F.; Borreani, G. Feasibility of Utilizing Biodegradable Plastic Film to Cover Corn Silage under Farm Conditions. Appl. Sci. 2020, 10, 2803. [Google Scholar] [CrossRef]
- Novinski, C.O.; Junges, D.; Schmidt, P.; Rossi Junior, P.; de Carvalho, J.P.G.; Teixeira, R.d.A. Methods of Lab Silos Sealing and Fermentation Characteristics and Aerobic Stability of Sugarcane Silage Treated with Microbial Additive. Rev. Bras. Zootec. 2012, 41, 264–270. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable Valorisation of Tomato Pomace: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E. Effects of Corn Silage Inoculants on Aerobic Stability. In Proceedings of the 2002 ASAE Annual Meeting; American Society of Agricultural and Biological Engineers, Jaipur, India, 18–20 October 2002; p. 1. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Hampshire, UK, 1991; ISBN 0948617225. [Google Scholar]
- Supel, P.; Kaszycki, P.; Kasperczyk, M.; Kacorzyk, P. Changes in Biochemical and Microbiological Quality of Silage Produced with the Use of Innovative Films. Agronomy 2022, 12, 2642. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, X.; Wang, W.; Li, J.; Dong, Z.; Zhao, J.; Shao, T. The effects of natamycin and hexanoic acid on the bacterial community, mycotoxins concentrations, fermentation profiles, and aerobic stability of high moisture whole-crop corn silage. Anim. Feed Sci. Technol. 2022, 286, 115250. [Google Scholar] [CrossRef]
- Driehuis, F.; Oude Elferink, S.; Van Wikselaar, P.G. Fermentation Characteristics and Aerobic Stability of Grass Silage Inoculated with Lactobacillus Buchneri, with or without Homofermentative Lactic Acid Bacteria. Grass Forage Sci. 2001, 56, 330–343. [Google Scholar] [CrossRef]
- Dai, T.; Wang, J.; Dong, D.; Yin, X.; Zong, C.; Jia, Y.; Shao, T. Effects of Brewers’ Spent Grains on Fermentation Quality, Chemical Composition and in Vitro Digestibility of Mixed Silage Prepared with Corn Stalk, Dried Apple Pomace and Sweet Potato Vine. Ital. J. Anim. Sci. 2022, 21, 198–207. [Google Scholar] [CrossRef]
- El-Araby, G.M. Processing Untraditional Formula from Snacks Using Apple and Tomato Pomace Powder as a Source of Dietary Fiber and Antioxidants. J. Food Dairy Sci. 2022, 13, 59–64. [Google Scholar] [CrossRef]
- Palangi, V. Identification of Ruminal Fermentation Curves of Some Legume Forages Using Particle Swarm Optimization. Animals 2023, 13, 1339. [Google Scholar] [CrossRef] [PubMed]
- Esen, S. Optimizing Ruminant Nutrition: Insights from a Comprehensive Analysis of Silage Composition and in Vitro Gas Production Dynamics Using Nonlinear Models. Biosystems 2023, 234, 105062. [Google Scholar] [CrossRef] [PubMed]
- Pirmohammadi, R.; Rouzbehan, Y.; Rezayazdi, K.; Zahedifar, M. Chemical Composition, Digestibility and in Situ Degradability of Dried and Ensiled Apple Pomace and Maize Silage. Small Rumin. Res. 2006, 66, 150–155. [Google Scholar] [CrossRef]




| a | b | c | a + b | |
|---|---|---|---|---|
| CPE | 0.95 ± 0.17 | 68.68 ± 0.30 | 0.086 ± 0.0004 | 69.63 ± 0.14 |
| CTP | 0.41 ± 0.10 | 67.89 ± 0.15 | 0.085 ± 0.0002 | 68.29 ± 0.15 **** |
| CTP+PE | 1.35 ± 0.25 | 66.01 ± 0.22 **** | 0.081 ± 0.0010 **** | 67.36 ± 0.12 **** |
| CAP | 0.38 ± 0.33 | 65.64 ± 0.27 **** | 0.082 ± 0.0011 ** | 66.02 ± 0.18 **** |
| CAP+PE | −0.41 ± 0.18 *** | 65.67 ± 0.13 **** | 0.082 ± 0.0007 *** | 65.26 ± 0.11 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünlü, H.B.; Canbolat, Ö.; Yerlikaya, O.; Esen, S.; Palangi, V.; Lackner, M. The Effect of Covering Corn Silage with Tomato or Apple Pomace on Fermentation Parameters and Feed Quality. Fermentation 2024, 10, 372. https://doi.org/10.3390/fermentation10070372
Ünlü HB, Canbolat Ö, Yerlikaya O, Esen S, Palangi V, Lackner M. The Effect of Covering Corn Silage with Tomato or Apple Pomace on Fermentation Parameters and Feed Quality. Fermentation. 2024; 10(7):372. https://doi.org/10.3390/fermentation10070372
Chicago/Turabian StyleÜnlü, Hayrullah Bora, Önder Canbolat, Oktay Yerlikaya, Selim Esen, Valiollah Palangi, and Maximilian Lackner. 2024. "The Effect of Covering Corn Silage with Tomato or Apple Pomace on Fermentation Parameters and Feed Quality" Fermentation 10, no. 7: 372. https://doi.org/10.3390/fermentation10070372









