Potential of Endophytic Microorganisms in Fermentative Processes Using Agro-Industrial Waste as Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruits
2.2. Agro-Industrial Residues
2.3. Isolation and Selection of Endophytic Microorganisms
2.3.1. Sanitization of Fruits
2.3.2. Isolation of Microorganisms
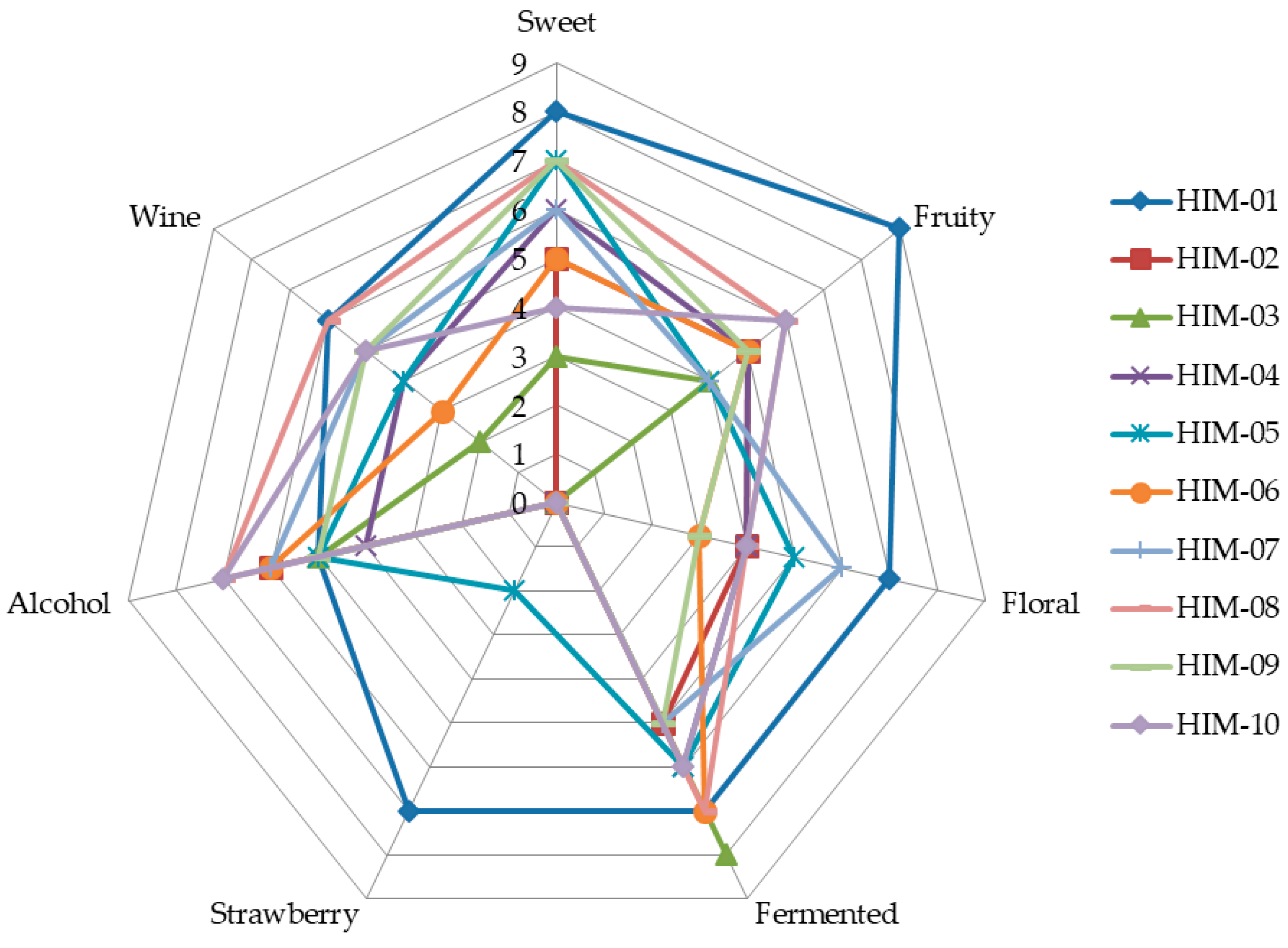
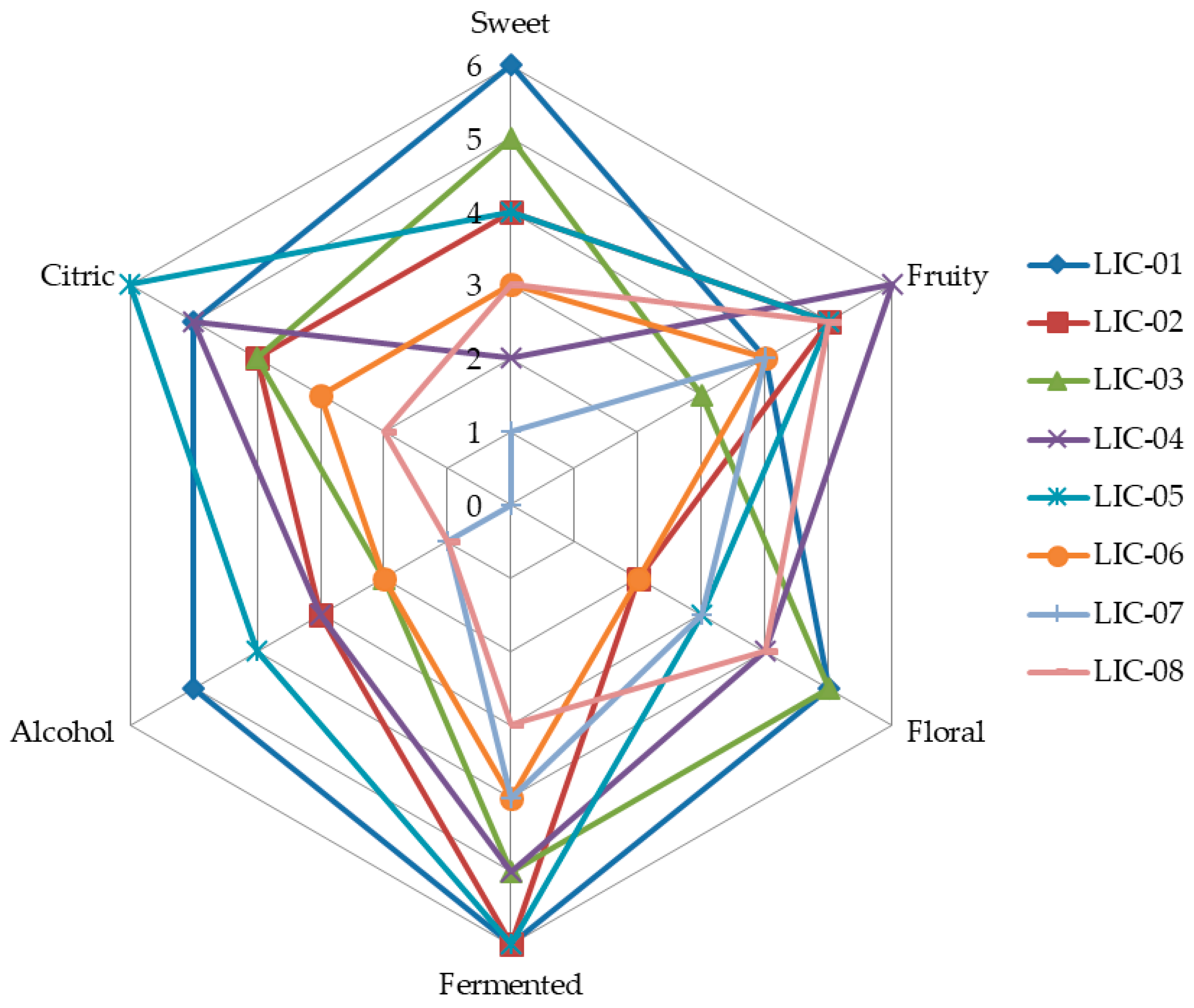
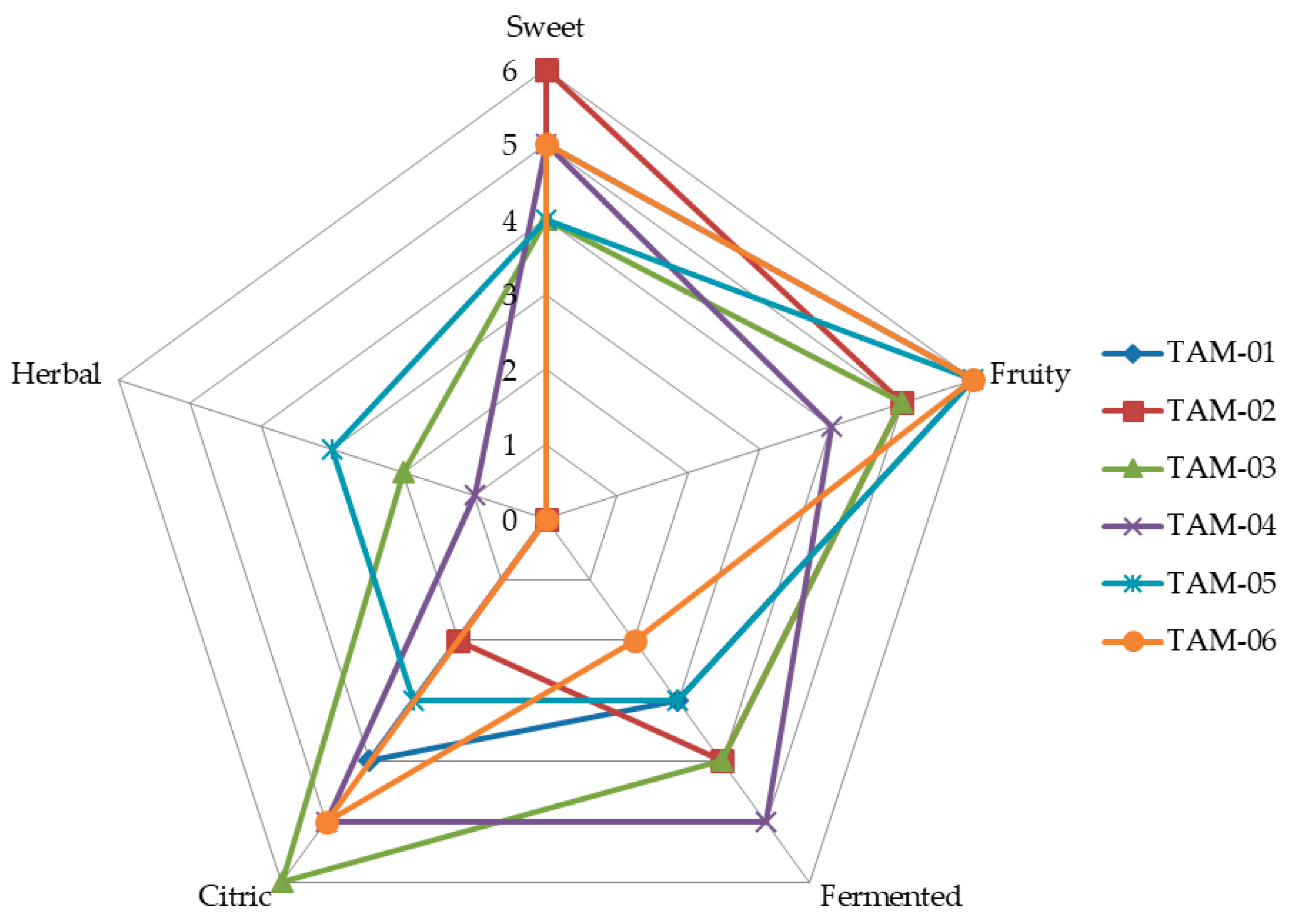
2.3.3. Selection of Endophytic Microorganisms Using Sensory Analysis
2.4. Identification of the Selected Microorganism
2.5. Fermentation of the Selected Strain with Agro-Industrial Residues
2.6. Analysis of Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Selection of Aroma-Producing Microorganisms
3.2. Identification of the HIM-01 Strain by MALDI-TOF-MS
3.3. Volatile Compounds Obtained from the Fermentation of Kloeckera Apiculata in Culture Medium Supplemented with Agro-Industrial Residues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandes, R.D.D.; dos Santos, R.A.R.; de Jesus, M.S.; Araujo, H.C.S.; Leite Neta, M.T.S.; Rajkumar, G.; Narain, N. Agro-Industrial Residues Used as Substrates for the Production of Bioaroma Compounds with Basidiomycetes: A Comprehensive Review. Fermentation 2024, 10, 23. [Google Scholar] [CrossRef]
- Bicas, J.L.; Silva, J.C.; Dionísio, A.P.; Pastore, G.M. Biotechnological production of bioflavors and functional sugars. Food Sci. Technol. 2010, 30, 7–18. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef]
- Maróstica Júnior, M.R.; Pastore, G.M. Biotransformação de limoneno: Uma revisão das principais rotas metabólicas. Quim. Nova 2007, 30, 382–387. [Google Scholar] [CrossRef]
- Medeiros, A.B.; Pandey, A.; Vandenberghe, L.P.; Pastore, G.M.; Soccol, C.R. Production and Recovery of Aroma Compounds Produced by Solid-State Fermentation Using Different Adsorbents. Food Technol. Biotechnol. 2006, 44, 47–51. [Google Scholar]
- Uenojo, M.; Pastore, G.M. β-carotene biotransformation to obtain aroma compounds. Food Sci. Technol. 2010, 30, 822–827. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Saikkonen, K. Forest structure and fungal endophytes. Fungal Biol. Rev. 2007, 21, 67–74. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, F.; Zhang, X.; Dai, X.; Dong, X.; Song, W. Endophytic Bacterial Diversity in Rice (Oryza sativa L.) Roots Estimated by 16S rDNA Sequence Analysis. Microb. Ecol. 2008, 55, 415–424. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.-H.; Xu, L.-H.; Li, W.-J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Thomas, P.; Kumari, S.; Swarna, G.K.; Prakash, D.P.; Dinesh, M.R. Ubiquitous presence of fastidious endophytic bacteria in field shoots and index-negative apparently clean shoot-tip cultures of papaya. Plant Cell Rep. 2007, 26, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, S.; Ferraro, V.; Alfonzo, A.; Settanni, L.; Ercolini, D.; Burruano, S.; Moschetti, G. Presence of endophytic bacteria in Vitis vinifera leaves as detected by fluorescence in situ hybridization. Ann. Microbiol. 2010, 60, 161–167. [Google Scholar] [CrossRef]
- Melnick, R.L.; Suárez, C.; Bailey, B.A.; Backman, P.A. Isolation of endophytic endospore-forming bacteria from Theobroma cacao as potential biological control agents of cacao diseases. Biol. Control 2011, 57, 236–245. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.F.; Samuels, G.J. Fungal endophytes of Spondias mombin leaves in Brazil. J. Basic Microbiol. 1999, 39, 131–135. [Google Scholar] [CrossRef]
- Pereira, J.O.; Vieira, M.L.C.; Azevedo, J.L. Endophytic fungi from Musa acuminata and their reintroduction into axenic plants. World J. Microbiol. Biotechnol. 1999, 15, 37–40. [Google Scholar] [CrossRef]
- Uenojo, M.; Pastore, G.M. Isolamento e seleção de microrganismos pectinolíticos a partir de resíduos provenientes de agroindústrias para produção de aromas frutais. Food Sci. Technol. 2006, 26, 509–515. [Google Scholar] [CrossRef][Green Version]
- Soccol, C.R.; Vandenberghe, L.P.S. Overview of applied solid-state fermentation in Brazil. Biochem. Eng. J. 2003, 13, 205–218. [Google Scholar] [CrossRef]
- Medeiros, A.B.; Pandey, A.; Freitas, R.J.; Christen, P.; Soccol, C.R. Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem. Eng. J. 2000, 6, 33–39. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Lartigue, M.-F.; Héry-Arnaud, G.; Haguenoer, E.; Domelier, A.-S.; Schmit, P.-O.; Van Der Mee-Marquet, N.; Lanotte, P.; Mereghetti, L.; Kostrzewa, M.; Quentin, R. Identification of Streptococcus agalactiae isolates from various phylogenetic lineages by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2009, 47, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Tekarslan-Sahin, S.H. Adaptive Laboratory Evolution of Yeasts for Aroma Compound Production. Fermentation 2022, 8, 372. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Chen, H.; Li, Z. Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods 2023, 12, 3501. [Google Scholar] [CrossRef]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing Revolution in Bacteriology: Routine Identification of Bacteria by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- Drevinek, M.; Dresler, J.; Klimentova, J.; Pisa, L.; Hubalek, M. Evaluation of sample preparation methods for MALDI-TOF MS identification of highly dangerous bacteria. Lett. Appl. Microbiol. 2012, 55, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Schumaker, S.; Borror, C.M.; Sandrin, T.R. Automating data acquisition affects mass spectrum quality and reproducibility during bacterial profiling using an intact cell sample preparation method with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 243–253. [Google Scholar] [CrossRef]
- Mamede, M.E.; Cardello, H.M.; Pastore, G.M. Evaluation of an aroma similar to that of sparkling wine: Sensory and gas chromatography analyses of fermented grape musts. Food Chem. 2005, 89, 63–68. [Google Scholar] [CrossRef]
- Mamede, M.E.; Pastore, G.M. Study of methods for the extraction of volatile compounds from fermented grape must. Food Chem. 2006, 96, 586–590. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef]
- Sandes, R.D.D.; De Jesus, M.S.; Araujo, H.C.S.; Dos Santos, R.A.R.; Nogueira, J.P.; Leite Neta, M.T.S.; Narain, N. The Production of Bioaroma by Auriporia aurulenta Using Agroindustrial Waste as a Substrate in Submerged Cultures. Fermentation 2023, 9, 593. [Google Scholar] [CrossRef]
- Lee, P.R.; Ong, Y.L.; Yu, B.; Curran, P.; Liu, S.Q. Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cerevisiae and Williopsis saturnus. Food Microbiol. 2010, 27, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Gatfield, I.L. Production of flavor and aroma compounds by biotechnology. Food Technol. 1988, 42, 110–122. [Google Scholar]
- Welsh, F. Overview of bioprocess flavor and fragrance production. In Bioprocess Production of Flavor, Fragrance, and Color Ingredients; Wiley & Sons Ltd.: Hoboken, NJ, USA, 1994; pp. 1–17. [Google Scholar]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.-a.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef]
- Adame-Soto, P.J.; Aréchiga-Carvajal, E.T.; López, M.G.; González-Herrera, S.M.; Moreno-Jiménez, M.R.; Urtiz-Estrada, N.; Rutiaga-Quiñones, O.M. Potential production of 2-phenylethanol and 2-phenylethylacetate by non-Saccharomyces yeasts from Agave durangensis. Ann. Microbiol. 2019, 69, 989–1000. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- de Lima, L.A.; Diniz, R.H.S.; de Queiroz, M.V.; Fietto, L.G.; da Silveira, W.B. Screening of Yeasts Isolated from Brazilian Environments for the 2-Phenylethanol (2-PE) Production. Biotechnol. Bioprocess Eng. 2018, 23, 326–332. [Google Scholar] [CrossRef]
- Romano, P.; Palla, G.; Caligiani, A.; Brandolini, V.; Maietti, A.; Salzano, G. Evaluation of stereoisomers of 2,3-butanediol and acetoin to differentiate Saccharomyces cerevisiae and Kloeckera apiculata wine strains. Biotechnol. Lett. 2000, 22, 1947–1951. [Google Scholar] [CrossRef]
- Bilbao, A.; Irastorza, A.; Dueñas, M.; Fernandez, K. The effect of temperature on the growth of strains of Kloeckera apiculata and Saccharomyces cerevisiae in apple juice fermentation. Lett. Appl. Microbiol. 1997, 24, 37–39. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Wang, F.; Leng, Y.; Liu, J.-K. Rare merosesquiterpenoids from basidiomycete Craterellus odoratus and their inhibition of 11β-hydroxysteroid dehydrogenases. Phytochemistry 2010, 71, 100–103. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between Varietal Amino Acid Profile of Grapes and Wine Aromatic Composition. Experiments with Model Solutions and Chemometric Study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Tapiero, C.; Cordonnier, R.E. Hydrolysis of grape monoterpenyl. beta.-D-glucosides by various. beta.-glucosidases. J. Agric. Food Chem. 1990, 38, 1232–1236. [Google Scholar] [CrossRef]
- Nunes, J.C.; Lago, M.G.; Castelo-Branco, V.N.; Oliveira, F.R.; Torres, A.G.; Perrone, D.; Monteiro, M. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 2016, 197, 881–890. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Pardo, M.E.S.; Cassellis, M.E.R.; Escobedo, R.M.; García, E.J. Chemical characterisation of the industrial residues of the pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar] [CrossRef]
- Sanchez, B.; Bautista, J. Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzyme Microb. Technol. 1988, 10, 315–318. [Google Scholar] [CrossRef]
- Ando, S.; Arai, I.; Kiyoto, K.; Hanai, S. Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae. J. Ferment. Technol. 1986, 64, 567–570. [Google Scholar] [CrossRef]
- Tran, A.V.; Chambers, R.P. Red oak wood derived inhibitors in the ethanol fermentation of xylose by Pichia stipitis CBS 5776. Biotechnol. Lett. 1985, 7, 841–845. [Google Scholar] [CrossRef]
- Clark, T.A.; Mackie, K.L. Fermentation inhibitors in wood hydrolysates derived from the softwood Pinus radiata. J. Chem. Technol. Biotechnol. 1984, 34, 101–110. [Google Scholar] [CrossRef]
- Erten, H. Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J. Microbiol. Biotechnol. 2002, 18, 377–382. [Google Scholar] [CrossRef]

| Fruits | Number of Yeasts Isolated | Number of Fungi Isolated |
|---|---|---|
| Strawberry | 10 | 3 |
| Lychee | 8 | 2 |
| Tangerine | 6 | 8 |
| N° | Compounds | IRCal | Area (%) | B.Q. * | B.B. * | Aroma b |
|---|---|---|---|---|---|---|
| 1 | Ethanol | 522 | 10.03 | x | alcoholic | |
| 2 | Methyl acetate | 560 | 10.72 | x | sweet, fruity | |
| 3 | 2-Methyl-1-propanol | 550 | 1.81 | x | ||
| 4 | Ethyl acetate | 640 | 11.14 | x | fruity | |
| 5 | Isobutyl acetate | 790 | 0.67 | x | fruity | |
| 6 | Hexanal | 800 | 0.47 | x | green | |
| 7 | Ethyl butanoate | 802 | 1.74 | x | fruity | |
| 8 | (Z)-3-Hexen-1-ol | 855 | 1.12 | x | ||
| 9 | 1-hexanol | 863 | 6.07 | herbs | ||
| 10 | isoamyl acetate | 877 | 5.95 | x | fruity | |
| 11 | 2-Methylbutyl acetate | 879 | 1.35 | x | ||
| 12 | Styrene | 890 | 0.63 | x | ||
| 13 | 2-Heptanol | 900 | 0.60 | x | citrus | |
| 14 | Methyl hexanoate | 925 | 0.56 | x | fruity | |
| 15 | α-Thujene | 934 | 0.54 | x | ||
| 16 | Benzaldehyde | 960 | 6.33 | x | ||
| 17 | β-Myrcene | 991 | 3.02 | spicy | ||
| 18 | β-Cymene | 1019 | 1.12 | x | ||
| 19 | (E)-Ocimene | 1029 | 0.84 | x | herbs | |
| 20 | Benzeneacetaldehyde | 1035 | 3.30 | x | green | |
| 21 | 3-Carene | 1038 | 4.47 | sweet | ||
| 22 | 4-(benzoylmethyl)-6-methyl-2H-1,4-benzoxazin-3-one | 1058 | 0.56 | x | ||
| 23 | Terpinolene | 1076 | 0.98 | x | herbs | |
| 24 | Methyl benzoate | 1082 | 6.51 | x | phenolic | |
| 25 | 2-Phenylethyl acetate | 1104 | 14.54 | roses | ||
| 26 | Ethyl benzoate | 1165 | 2.68 | x | mint | |
| 27 | Hexyl 2-methylpropanoate | 1189 | 2.26 |
| N° | Compounds | IRCal | Area (%) | B.Q. * | B.B. * | Aroma b |
|---|---|---|---|---|---|---|
| 1 | Ethanol | 529 | 2.96 | x | x | alcohol |
| 2 | Ethyl acetate | 644 | 7.89 | x | fruity | |
| 3 | Isoamyl alcohol | 775 | 1.26 | x | ||
| 4 | 2-Methyl-1-butanol | 777 | 0.78 | x | ||
| 5 | Isoamyl acetate | 877 | 4.34 | x | ||
| 6 | D-limonene | 1022 | 1.21 | x | citric | |
| 7 | Eucalyptol | 1024 | 0.53 | x | eucalyptus | |
| 8 | (E)-Ocimene | 1029 | 3.21 | x | ||
| 9 | 2-Phenylethanol | 1038 | 0.57 | x | floral | |
| 10 | α-Pyronene | 1109 | 0.55 | |||
| 11 | 2-Phenylethyl acetate | 1117 | 0.42 | x | floral, roses | |
| 12 | α-Copaene | 1259 | 0.87 | x | woody | |
| 13 | β-Caryophyllen | 1374 | 6.26 | x | sweet | |
| 14 | Aromandendrene | 1419 | 41.23 | x | woody | |
| 15 | Humulene | 1437 | 7.06 | x | woody | |
| 16 | Alloaromadendrene | 1451 | 4.55 | xx | woody | |
| 17 | γ-Muurolene | 1458 | 0.85 | x | herbal | |
| 18 | β-Selinene | 1474 | 1.22 | x | herbal | |
| 19 | α-Selinene | 1483 | 6.48 | x | Waxy | |
| 20 | δ-Cadinene | 1492 | 6.29 | x | herbal | |
| 21 | (+)-Ledene | 1521 | 0.87 | x | ||
| 22 | β-Guaiene | 1579 | 0.57 | x | balsamic |
| N° | Compounds | IRCal | Area (%) | B.Q. * | B.B. * | Aroma b |
|---|---|---|---|---|---|---|
| 1 | Isoamyl alcohol | 775 | 4.72 | x | fruity | |
| 2 | 2-Methyl-1-butanol | 776 | 5.10 | x | burned | |
| 3 | (E)-2-Methylcyclopentanol | 800 | 0.46 | x | ||
| 4 | 2,4-Dimethylheptane | 821 | 0.89 | x | ||
| 5 | Furfural | 832 | 37.09 | x | ||
| 6 | 2-Ethoxy-2-cyclohexenone | 841 | 9.97 | x | ||
| 7 | 1,3-Dimethylheptane | 863 | 1.67 | x | ||
| 8 | Isoamyl acetate | 877 | 18.45 | x | fruity | |
| 9 | 2-Methylbutyl acetate | 880 | 2.49 | x | fruity | |
| 10 | Methional | 906 | 0.45 | x | ||
| 11 | 2-Acetylfuran | 913 | 0.42 | x | ||
| 12 | Benzaldehyde | 961 | 1.89 | x | ||
| 13 | 2-Pentylfuran | 991 | 0.91 | x | ||
| 14 | 2-Furanmethanol | 995 | 0.80 | x | ||
| 15 | Ethyl hexanoate | 999 | 1.60 | fruity | ||
| 16 | 4-Methyldecane | 1007 | 0.99 | |||
| 17 | 3-(prop-2-enoyloxy)tetradecane | 1017 | 0.75 | x | ||
| 18 | Benzeneacetaldehyde | 1035 | 3.46 | x | green | |
| 19 | 1-Propyldecyl phenylacetate | 1040 | 0.71 | x | ||
| 20 | 1-Methylodecyl phenylacetate | 1043 | 0.56 | x | ||
| 21 | 2,6,6-trimethyl-bicyclo[3.1.1]hept-3-ylamine | 1091 | 0.37 | x | ||
| 22 | Ethyl octanoate | 1195 | 3.90 | wax | ||
| 23 | 2-Phenylethyl acetate | 1261 | 2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite Neta, M.T.S.; Sandes, R.D.D.; De Jesus, M.S.; Araujo, H.C.S.; Dos Santos, R.A.R.; Santana, L.C.L.D.A.; Narain, N. Potential of Endophytic Microorganisms in Fermentative Processes Using Agro-Industrial Waste as Substrates. Fermentation 2024, 10, 430. https://doi.org/10.3390/fermentation10080430
Leite Neta MTS, Sandes RDD, De Jesus MS, Araujo HCS, Dos Santos RAR, Santana LCLDA, Narain N. Potential of Endophytic Microorganisms in Fermentative Processes Using Agro-Industrial Waste as Substrates. Fermentation. 2024; 10(8):430. https://doi.org/10.3390/fermentation10080430
Chicago/Turabian StyleLeite Neta, Maria Terezinha Santos, Rafael Donizete Dutra Sandes, Mônica Silva De Jesus, Hannah Caroline Santos Araujo, Raquel Anne Ribeiro Dos Santos, Luciana Cristina Lins De Aquino Santana, and Narendra Narain. 2024. "Potential of Endophytic Microorganisms in Fermentative Processes Using Agro-Industrial Waste as Substrates" Fermentation 10, no. 8: 430. https://doi.org/10.3390/fermentation10080430
APA StyleLeite Neta, M. T. S., Sandes, R. D. D., De Jesus, M. S., Araujo, H. C. S., Dos Santos, R. A. R., Santana, L. C. L. D. A., & Narain, N. (2024). Potential of Endophytic Microorganisms in Fermentative Processes Using Agro-Industrial Waste as Substrates. Fermentation, 10(8), 430. https://doi.org/10.3390/fermentation10080430






